Sugar-Sweetened Soft Drinks and Fructose Consumption Are Associated with Hyperuricemia: Cross-Sectional Analysis from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil)
Abstract
1. Introduction
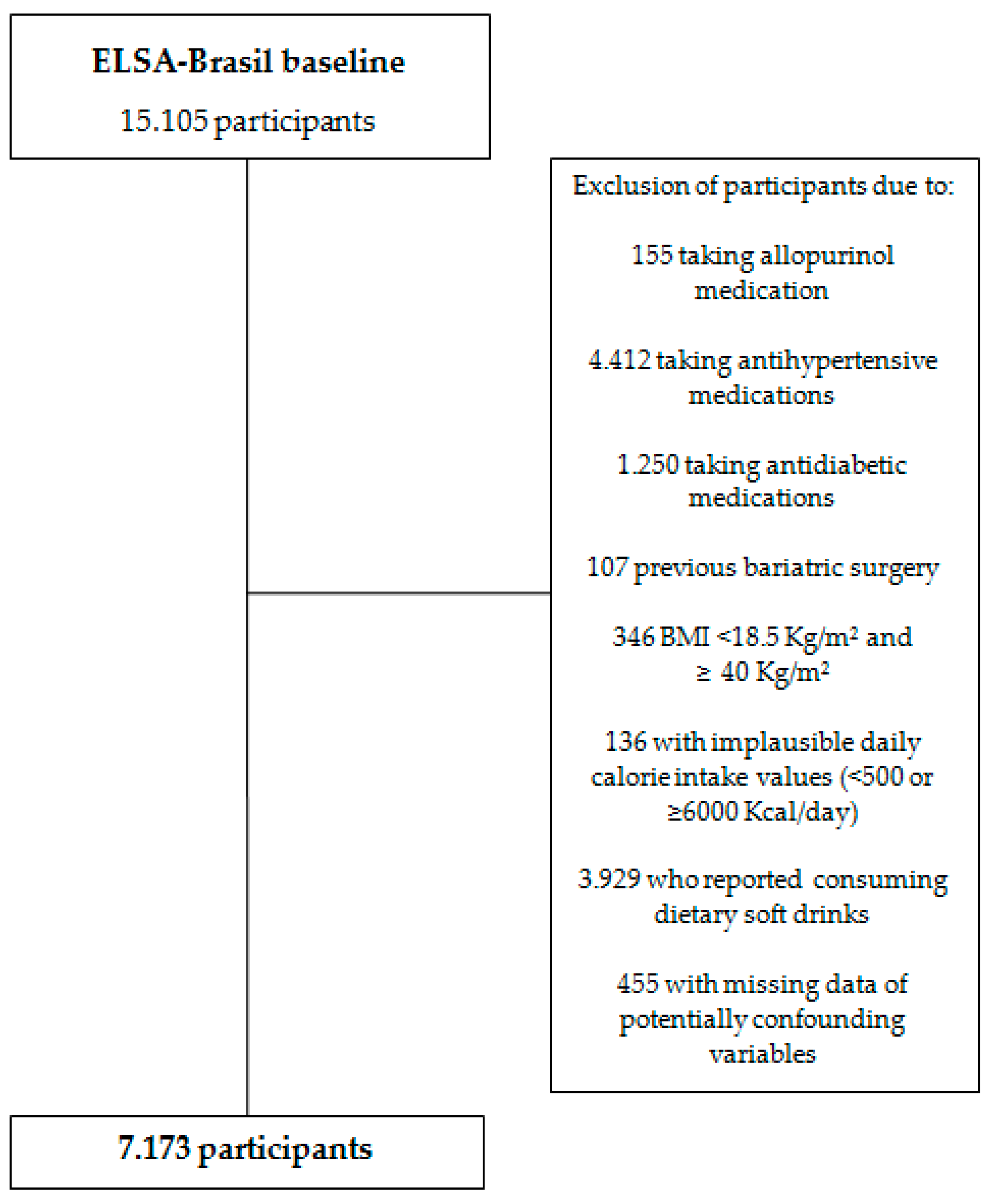
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ministério do Planejamento, Orçamento e Gestão, Instituto Brasileiro de Geografia e Estatística. Pesquisa De Orçamentos Familiares 2008–2009 Avaliação Nutricional da Disponibilidade Domiciliar de Alimentos no Brasil; IBGE: Rio de Janeiro, Brasil, 2010. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv47310.pdf (accessed on 10 April 2017).
- Levy-Costa, R.B.; Sichieri, R.; Pontes, N.S.; Monteiro, C.A. Household food availability in Brazil: Distribution and trends (1974–2003). Rev. Saude Publica 2005, 39, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Ministério do Planejamento, Orçamento e Gestão, Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares, 2008–2009: Análise do Consumo Alimentar Pessoal no Brasil; IBGE: Rio de Janeiro, Brasil, 2010. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv50063.pdf (accessed on 15 June 2017).
- Vartanian, L.R.; Schwartz, B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Ford, E.S.; Gao, X.; Choi, H.K. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Klag, M.J.; Mead, L.A.; Liang, K.Y.; Seidler, A.J.; Hochberg, M.C. Incidence and risk factors for gout in white men. JAMA 1991, 266, 3004–3007. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.H.; Zhu, Y.; Choi, H.K. The epidemiology of uric acid and fructose. Semin. Nephrol. 2011, 31, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hsieh, M.C.; Chang, S.J. Metabolic syndrome, diabetes and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhou, X.; Wang, J.; Wu, X.; Li, Y.; Wang, L.; Huang, H.; Li, J. Hyperuricemia and the Prognosis of Hypertensive Patients: A Systematic Review and Meta-Analysis. J. Clin. Hypertens. 2016, 18, 1191–1294. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.L.; Baldo, M.P.; Capingana, P.; Magalhães, P.; Dantas, E.M.; Molina, M.C.; Salaroli, L.B.; Morelato, R.L.; Mill, J.G. Gender Distribution of Serum Uric Acid and Cardiovascular Risk Factors: Population Based Study. Arq. Bras. Cardiol. 2012, 98, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Liu, S.; Curhan, G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005, 52, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.H.; See, L.C.; Huang, Y.C. Dietary factors associated with hyperuricemia in adults. Semin. Arthritis Rheum. 2008, 37, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zgaga, L.; Theodoratou, E.; Kyle, J.; Farrington, S.M.; Agakov, F.; Tenesa, A.; Walker, M.; McNeill, G.; Wright, A.F.; Rudan, I.; et al. The Association of Dietary Intake of Purine-Rich Vegetables, Sugar-Sweetened Beverages and Dairy with Plasma Urate, in a Cross-Sectional Study. PLoS ONE 2012, 7, e38123. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Curhan, G. Coffee consumption and risk of incident gout in women: The Nurses’ Health Study. Am. J. Clin. Nutr. 2010, 92, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Gao, X.; Curhan, G. Vitamin C intake and the risk of gout in men: A prospective study. Arch. Intern. Med. 2009, 169, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, L.; Qiao, N.; Choi, H.K.; Curhan, G.; Tucker, K.L.; Ascherio, A. Intake of Added Sugar and Sugar-Sweetened Drink and Serum Uric Acid Concentration in US Men and Women. Hypertension 2007, 50, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Chun, B.Y.; Park, P.S.; Choi, B.Y.; Kim, M.K.; Shin, M.H.; Lee, Y.H.; Shin, D.H.; Kim, S.K. Higher consumption of sugar-sweetened soft drinks increases the risk of hyperuricemia in Korean population: The Korean Multi-Rural Communities Cohort Study. Semin. Arthritis Rheum. 2014, 43, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Leon, J.; Denova-Gutiérrez, E.; Castañón-Robles, S.; Granados-García, V.; Talavera, J.O.; Rivera-Paredez, B.; Huitrón-Bravo, G.G.; Cervantes-Rodríguez, M.; Quiterio-Trenado, M.; Rudolph, S.E. Sweetened beverage consumption and the risk of hyperuricemia in Mexican adults: A cross-sectional study. BMC Public Health 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, B.T. Effect of oral fructose on urate production. Ann. Rheuim. 1974, 33, 276–280. [Google Scholar] [CrossRef]
- Bray, G.A. How bad is fructose? Am. J. Clin. Nutr. 2007, 86, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Block, G. Foods contributing to energy intake in the US: Data from NHANES III and NHANES 1999–2000. J. Food Compost. Anal. 2004, 17, 439–447. [Google Scholar] [CrossRef]
- Nakagawa, T.; Tuttle, K.R.; Short, R.A.; Johnson, R.J. Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat. Clin. Pract. Nephrol. 2005, 1, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.I.; Duncan, B.B.; Mill, J.G.; Lotufo, P.A.; Chor, D.; Barreto, S.M.; Aquino, E.M.; Passos, V.M.; Matos, S.M.; Molina, M.C.B. Cohort Profile: Longitudinal Study of Adult Health (ELSA-Brasil). Int. J. Epidemiol. 2015, 44, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Mill, J.G.; Pinto, K.; Griep, R.H.; Goulart, A.; Foppa, M.; Lotufo, P.A.; Maestri, M.K.; Ribeiro, A.L.; Andreão, R.V.; Dantas, E.M.; et al. Medical assessments and measurements in ELSA-Brasil. Rev. Saúde Publica 2013, 47, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chor, D.; Alves, M.G.; Giatti, L.; Cade, N.V.; Nunes, M.A.; Molina, M.C.B.; Benseñor, I.M.; Aquino, E.M.; Passos, V.; Santos, S.M.; et al. Questionário do ELSA-Brasil: Desafios na elaboração de instrumento multidimensional. Rev. Saúde Pública 2013, 47, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.C.B.; Bonseñor, I.M.; Cardoso, L.O.; Velasquez-Melendez, G.; Drehmer, M.; Pereira, T.S.S.; Faria, C.P.; Melere, C.; Manato, L.; Gomes, A.L.C.; et al. Reprodutibilidade e validade relativa do questionário de frequência alimentar do ELSA-Brasil. Cad. Saúde Pública 2013, 29, 379–389. [Google Scholar] [CrossRef]
- Fedeli, L.G.; Vidigal, P.G.; Leite, C.M.; Castilhos, C.D.; Pimentel, R.A.; Maniero, V.C.; Mill, J.G.; Lotufo, P.A.; Pereira, A.C.; Bensenor, I.M. Logística de coleta e transporte de material biológico e organização do laboratório central no ELSA-Brasil. Rev. Saúde Pública 2013, 47, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Earley, A.; Miskulin, D.; Lamb, E.J.; Levey, A.S.; Uhlig, K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: A systematic review. Ann. Intern. Med. 2012, 156, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality: The NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. J. Am. Med. Assoc. 2000, 283, 2404–2410. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Zanocco, J.A.; Nishida, S.K.; Passos, M.T.; Pereira, A.R.; Silva, M.S.; Pereira, A.B.; Kirsztajn, G.M. Race adjustment for estimating glomerular filtration rate is not always necessary. Nephron Extra 2012, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.V.; Gomes, E.C.; Chanan, J.; Carraro, M.A.; Camargo, E.G.; Soares, A.A.; Thomé, F.S.; Silveiro, S.P. Performance of CKD-EPI equation to estimate glomerular filtration rate as compared to MDRD equation in South Brazilian individuals in each stage of renal function. Clin. Chem. Lab. Med. 2014, 52, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist: Hip Ratio. In Obesity: Preventing and Managing the Global Epidemic: Report of a Who Consultation; World Health Organization: Geneva, Switzerland, 2010; pp. 9–11. [Google Scholar]
- Matsudo, S.; Araujo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. International physical activity questionnarie (IPAQ): Study of validity and reliability in Brazil. Rev. Bras. Ativ. Fis. Saude. 2011, 6, 6–18. [Google Scholar]
- Filho, F.F.R.; Mariosa, L.S.; Ferreira, S.R.G.; Zanella, M.T. Gordura Visceral e Síndrome Metabólica: Mais Que Uma Yesples Associação. Arq. Bras. Endocrinol. Metab. 2006, 50, 230–238. [Google Scholar] [CrossRef]
- Takahashi, S.; Yamamoto, T.; Tsutsumi, Z.; Moriwaki, Y.; Hada, T. Increased visceral fat accumulation in patients with primary gout. Adv. Exp. Med. Biol. 2000, 486, 131–134. [Google Scholar] [PubMed]
- Choi, H.K.; Willett, W.; Curhan, G. Fructose-Rich Beverages and Risk of Gout in Women. JAMA 2010, 304, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G. Fructose ingestion: Dose-dependent responses in health research. J. Nutr. 2009, 139, 1246S–1252S. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, A.; Snaith, M.L.; Scott, J.T. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br. Med. J. 1973, 1, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Curhan, G. Soft drinks, fructose consumption, and the risk of gout in men: Prospective cohort study. BMJ 2008, 336, 309. [Google Scholar] [CrossRef] [PubMed]
- Bomback, A.S.; Derebail, V.K.; Shoham, D.A.; Anderson, C.A.; Steffen, L.M.; Rosamond, W.D.; Kshirsagar, A.V. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010, 77, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Johnson, M.W.; Kirshenbaum, J.M.; Lopez, R.E. Effect of large oral doses of ascorbic acid on uric acid excretion by normal subjects. Clin. Pharmacol. Ther. 1981, 29, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Appel, L.J.; Choi, M.J.; Gelber, A.C.; Charleston, J.; Norkus, E.P.; Miller, E.R. The effects of vitamin C supplementation on serum concentrations of uric acid: Results of a randomized controlled trial. Arthritis Rheum. 2005, 52, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.H.; Kelley, W.N. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism 1972, 21, 713–721. [Google Scholar] [CrossRef]
- Mayes, P.A. Intermediary metabolism of fructose. Am. J. Clin. Nutr. 1993, 58, 754S–765S. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Wietlisbach, V.; Bovet, P.; Shamlaye, C.; Riesen, W.; Paccaud, F.; Burnier, M. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health 2004, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.G.; Juzwishin, K.D.; Eiriksdottir, G.; Gudnason, V.; Danesh, J. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: Prospective study and meta-analysis. PLoS Med. 2005, 2, e76. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Souza, A.M.; Duffey, K.J.; Sichieri, R.; Popkin, B.M. Beverage consumption in Brazil: Results from the first National Dietary Survey. Public Health Nutr. 2015, 18, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.N.; Wang, Y.C.; Wang, Y.; Gortmaker, S.L. Increasing consumption of sugar-sweetened beverages among US adults: 1988–1994 to 1999–2004. Am. J. Clin. Nutr. 2009, 89, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Moise, N.; Cifuentes, E.; Orozco, E.; Willett, W. Limiting the consumption of sugar sweetened beverages in Mexico’s obesogenic environment: A qualitative policy review and stakeholder analysis. J. Public Health Policy 2011, 32, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
| Variable | Male (n = 3.325) | Female (n = 3.848) | p-Value * |
|---|---|---|---|
| Age (years) | 0.045 | ||
| 35 to 44 | 996 (30) | 1064 (27.7) | |
| 45 to 54 | 1418 (42.6) | 1640 (46.2) | |
| 55 to 64 | 707 (21.3) | 912 (23.7) | |
| 65 to 74 | 204 (6.1) | 232 (6.0) | |
| Race | 0.441 | ||
| No white | 1690 (50.8) | 1948 (50.6) | |
| White | 1635 (49.2) | 1900 (49.4) | |
| Education | <0.001 | ||
| Elementary | 552 (16.6) | 309(8.0) | |
| Middle and high school | 1198 (36.0) | 1447 (37.6) | |
| College or higher | 1575 (47.4) | 2092 (54.4) | |
| Smoking | <0.001 | ||
| Non-smoker | 1782 (53.6) | 2442 (63.5) | |
| Former smoker | 1009 (30.3) | 887 (23.1) | |
| Smoker | 534 (16.1) | 519 (13.5) | |
| Alcohol consumption | <0.001 | ||
| Non-drinker | 145 (4.4) | 551 (14.3) | |
| Former drinker | 648 (19.5) | 729 (18.9) | |
| Current drinker | 2532 (76.2) | 2568(66.7) | |
| Physical activity during leisure time | <0.001 | ||
| Low | 2476 (74.5) | 3095 (80.4) | |
| Moderate | 481 (14.5) | 454 (11.8) | |
| High | 368 (11.1) | 299 (7.8) | |
| Waist circumference | <0.001 | ||
| Adequate | 1949 (58.6) | 1447 (37.6) | |
| Inadequate | 1376 (41.4) | 2401 (62.4) | |
| Hyperuricemia | <0.001 | ||
| Yes | 907 (27.3) | 335 (8.7) | |
| No | 2418(72.7) | 3513 (91.3) | |
| GFR (mL/min/1.73 m²) | 0.060 | ||
| <60 | 97 9 (2.92) | 86 (2.23) | |
| ≥60 | 3228 (97.08) | 3762 (97.77) | |
| Daily intake of fruits | <0.001 | ||
| Yes | 1477 (43.5) | 2371 (61.6) | |
| No | 1878 (56.5) | 1477 (38.4) | |
| Daily intake of vegetables | <0.001 | ||
| Yes | 1437 (43.2) | 2116 (55) | |
| No | 1888 (56.8) | 1732 (45) | |
| Use of vitamin supplements | <0.001 | ||
| Yes | 285 (8.6) | 550 (14.3) | |
| No | 3040 (91.4) | 3298 (85.7) | |
| Age (years) | 49 ± 8.5 | 50 ± 8.3 | 0.035 |
| Per capita income (US$) | 1745 ± 2276 | 3136 ± 2633 | <0.001 |
| Uric acid (mg/dL) | 6.3 ± 1.3 | 4.5 ± 1.0 | <0.001 |
| GFR (mL/min/1.73 m²) | 86 ± 14 | 89 ± 15 | <0.001 |
| Waist circumference (cm) | 92 ± 10 | 84 ± 11 | <0.001 |
| Dietary intake | |||
| Meat intake (g/day) | 205 ± 132 | 157 ± 115 | <0.001 |
| Seafood intake (g/day) | 57 ± 66 | 50 ± 60 | <0.001 |
| Dairy food intake (g/day) | 293 ± 278 | 340 ± 279 | <0.001 |
| Coffee intake (mL/day) | 146 ± 127 | 145 ± 125 | 0.847 |
| Sugar-sweetened soft drinks (mL/day) | 117 ± 212 | 58 ± 145 | <0.001 |
| Fruit juice (mL/day) | 44 ± 114 | 50 ± 119 | 0.050 |
| Total fructose (g/day) | 35 ± 20 | 32 ± 18 | <0.001 |
| Vitamin C (mg/day) | 351 ± 250 | 349 ± 239 | 0.707 |
| Total energy (kcal/day) | 2647 ± 814 | 2123 ± 710 | <0.001 |
| Male (n = 3.325) | Female (n = 3.848) | |||||
|---|---|---|---|---|---|---|
| Hyperuricemia | Hyperuricemia | |||||
| Variable | No | Yes | p-Value * | No | Yes | p-Value * |
| (n = 2418) | (n = 907) | (n = 3513) | (n = 335) | |||
| Age (years) | 0.101 | <0.001 | ||||
| 35 to 44 | 744 (74.7) | 252 (25.3) | 1014 (95.3) | 50 (4.7) | ||
| 45 to 54 | 1003 (70.7) | 415 (29.3) | 1507 (91.9) | 133 (8.1) | ||
| 55 to 64 | 515 (72.8) | 192 (27.2) | 796 (87.3) | 116 (12.7) | ||
| 65 to 74 | 156 (76.5) | 48 (23.5) | 196 (84.5) | 36 (15.5) | ||
| Race | 0.311 | 0.385 | ||||
| No white | 1242 (73.5) | 448 (26.5) | 1786 (91.7) | 162 (8.3) | ||
| White | 1176 (71.9) | 459 (28.1) | 1727 (90.9) | 173 (9.1) | ||
| Education | 0.005 | <0.001 | ||||
| Elementary | 383 (69.4) | 169 (30.6) | 265 (85.8) | 44 (14.2) | ||
| Middle and high school | 862 (72.0) | 336 (28.0) | 1320 (91.2) | 127 (8.8) | ||
| College or higher | 1173 (74.5) | 402 (25.5) | 1928 (92.9) | 164 (7.8) | ||
| Smoking | <0.001 | <0.001 | ||||
| Non-smoker | 1340 (75.2) | 442 (24.8) | 2267 (92.8) | 175 (7.2) | ||
| Former smoker | 677 (67.1) | 332 (32.9) | 778 (87.7) | 109 (12.3) | ||
| Smoker | 401 (75.1) | 133 (24.9) | 468 (90.2) | 51 (9.8) | ||
| Alcohol consumption | <0.001 | 0.885 | ||||
| Non-drinker | 121 (83.4) | 24 (16.6) | 506 (91.8) | 45 (8.2) | ||
| Former drinker | 510 (78.7) | 138 (21.3) | 663 (90.9) | 66 (9.1) | ||
| Current drinker | 1787 (70.6) | 745 (29.4) | 2344 (91.3) | 224 (8.7) | ||
| Physical activity during leisure time | 0.128 | 0.934 | ||||
| Low | 1779 (71.8) | 697 (28.2) | 2823 (91.2) | 272 (8.8) | ||
| Moderate | 358 (74.4) | 123 (25.6) | 416 (91.6) | 38 (8.4) | ||
| High | 281 (76.4) | 87 (23.6) | 274 (91.6) | 25 (8.4) | ||
| Waist circumference | <0.001 | <0.001 | ||||
| Adequate | 1573 (80.7) | 376 (19.3) | 1405 (97.1) | 42 (2.9) | ||
| Inadequate | 845 (61.4) | 531 (38.6) | 2108 (87.8) | 293 (12.2) | ||
| GFR (mL/min/1.73 m²) | <0.001 | <0.001 | ||||
| <60 | 43 (44.3) | 54 (55.7) | 58 (67.4) | 28 (32.6) | ||
| ≥60 | 2375 (73.6) | 853 (26.4) | 3455 (91.8) | 307 (8.2) | ||
| Daily intake of fruits | 0.104 | 0.118 | ||||
| Yes | 1073 (74.2) | 374 (25.8) | 2154 (90.8) | 217 (9.2) | ||
| No | 1345 (71.6) | 533 (28.4) | 1359 (92.0) | 118 (8.0) | ||
| Daily intake of vegetables | 0.479 | 0.023 | ||||
| Yes | 1036 (72.1) | 401 (27.9) | 1914 (90.5) | 202 (9.5) | ||
| No | 1382 (73.2) | 506 (26.8) | 1599 (92.3) | 133 (7.7) | ||
| Use of vitamin supplements | 0.224 | 0.042 | ||||
| Yes | 216 (75.8) | 89 (24.2) | 513 (93.3) | 37 (6.7) | ||
| No | 2202 (72.4) | 838 (27.6) | 3000 (91.0) | 298 (9.0) | ||
| Age (years) | 49 ± 9 | 50 ± 8 | 0.654 | 49 ± 8 | 53 ± 8 | <0.001 |
| Per capita income (US$) | 2803 ± 2324 | 2584 ± 2137 | 0.014 | 3159 ± 2633 | 2904 ± 2632 | 0.089 |
| Uric acid (mg/dL) | 5.6 ± 0.8 | 8 ± 0.9 | <0.001 | 4.3 ± 0.8 | 6.6 ± 0.6 | <0.001 |
| GFR (mL/min/1.73 m²) | 88 ± 14 | 81 ± 15 | <0.001 | 89 ± 14 | 80 ± 15 | <0.001 |
| Waist circumference (cm) | 90 ± 10 | 97 ± 10 | <0.001 | 83 ± 10 | 94 ± 11 | <0.001 |
| Dietary intake | ||||||
| Meat intake (g/day) | 201 ± 132 | 215 ± 131 | 0.005 | 168 ± 116 | 165 ± 107 | 0.751 |
| Seafood intake (g/day) | 57 ± 66 | 59 ± 65 | 0.716 | 49 ± 58 | 59 ± 71 | 0.004 |
| Dairy food intake (g/day) | 306 ± 281 | 256 ± 264 | <0.001 | 344 ± 279 | 298 ± 265 | 0.004 |
| Coffee intake (mL/day) | 142 ± 125 | 156 ± 130 | 0.005 | 145 ± 126 | 146 ± 119 | 0.942 |
| Sugar-sweetened soft drinks (mL/day) | 110 ± 205 | 140 ± 230 | <0.001 | 57 ± 146 | 63 ± 130 | 0.502 |
| Fruit juice (mL/day) | 44 ± 116 | 41 ± 107 | 0.464 | 49 ± 119 | 46 ± 116 | 0.593 |
| Total fructose (g/day) | 34 ± 19 | 35 ± 21 | 0.989 | 32 ± 17 | 36 ± 20 | 0.001 |
| Vitamin C (mg/day) | 348 ± 245 | 358 ± 260 | 0.293 | 348 ± 239 | 353 ± 229 | 0.742 |
| Total energy (kcal/day) | 2643 ± 810 | 2658 ± 826 | 0.626 | 2112 ± 706 | 2128 ± 743 | 0.909 |
| Variable | n | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Male (n = 3.325) | ||||
| Sugar-sweetened soft drinks (servings/day) | ||||
| 0 | 933 | 1.00 | 1.00 | 1.00 |
| >0 to<0,1 | 666 | 1.26 (1.00–1.60) | 1.21 (1.00–1.55) | 1.25 (1.00–1.60) |
| ≥0.1 to<1 | 1382 | 1.60 (1.31–1.96) | 1.54 (1.25–1.90) | 1.62 (1.30–2.01) |
| ≥1 | 344 | 1.84 (1.39–2.43) | 1.74 (1.30–2.34) | 1.89 (1.39–2.57) |
| p* | <0.001 | <0.001 | <0.001 | |
| Fruit juice (servings/day) | ||||
| 0 | 2.506 | 1.00 | 1.00 | 1.00 |
| >0 to<0.1 | 187 | 0.89 (0.62–1.27) | 0.86 (0.60–1.25) | 0.86 (0.59–1.25) |
| ≥0.1 to ≤0.9 | 498 | 1.13 (0.90–1.41) | 1.17 (0.93–1.48) | 1.24 (0.97–1.57) |
| ≥1 | 134 | 1.09 (0.73–1.62) | 1.08 (0.71–1.64) | 1.29 (0.84–1.98) |
| p* | 0.604 | 0.452 | 0.441 | |
| Total fructose (g/day) | ||||
| Quartile 1 (14 ± 4) | 805 | 1.00 | 1.00 | 1.00 |
| Quartile 2 (25 ± 3) | 774 | 0.82 (0.65–1.03) | 0.81 (0.64–1.02) | 0.84 (0.66–1.07) |
| Quartile 3 (36 ± 4) | 835 | 0.77 (0.62–0.96) | 0.78 (0.62–0.98) | 0.86 (0.67–1.11) |
| Quartile 4 (60 ± 17) | 911 | 1.04 (0.85–1.29) | 1.11 (0.89–1.39) | 1.30 (1.00–1.68) |
| p* | 0.005 | 0.004 | 0.002 | |
| Female (n = 3.848) ** | ||||
| Sugar-sweetened soft drinks (servings/day) | ||||
| 0 | 1660 | 1.00 | 1.00 | 1.00 |
| >0 to <0.1 | 1083 | 1.08 (0.80–1.44) | 1.01 (0.74–1.37) | 1.00 (0.74–1.17) |
| ≥0.1 to <1 | 947 | 1.70 (1.28–2.26) | 1.59 (1.18–2.13) | 1.61 (1.18–2.18) |
| ≥1 | 158 | 1.34 (0.74–2.41) | 1.16 (0.63–2.13) | 1.14 (0.61–2.13) |
| p* | 0.142 | 0.090 | 0.080 | |
| Fruit juice (servings/day) | ||||
| 0 | 2747 | 1.00 | 1.00 | 1.00 |
| >0 to <0.1 | 287 | 0.73 (0.44–1.20) | 0.77 (0.46–1.28) | 0.80 (0.48–1.33) |
| ≥0.1 to <1 | 666 | 0.81 (0.58–1.12) | 0.84 (0.60–1.17) | 0.87 (0.61–1.22) |
| ≥1 | 148 | 1.00 (0.55–1.18) | 0.96 (0.52–1.77) | 0.91 (0.49–1.70) |
| p* | 0.442 | 0.634 | 0.717 | |
| Total fructose (g/day) | ||||
| Quartile 1 (15 ± 4) | 988 | 1.00 | 1.00 | 1.00 |
| Quartile 2 (26 ± 3) | 1019 | 1.21 (0.86–1.71) | 1.26 (0.89–1.79) | 1.32 (0.92–1.89) |
| Quartile 3 (35 ± 4) | 959 | 1.42 (1.01–1.98) | 1.43 (1.01–2.02) | 1.48 (1.03–2.14) |
| Quartile 4 (60 ± 16) | 882 | 1.48 (1.05–2.06) | 1.41 (1.00–2.00) | 1.47 (1.00–2.20) |
| p* | 0.786 | 0.584 | 0.470 | |
| Variable | n | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | ||
| Male (n = 3.325) | ||||
| Sugar-sweetened soft drinks (servings/day) | ||||
| 0 | 933 | 0.00 | 0.00 | 0.00 |
| >0 to <0.1 | 666 | 0.15 (0.02–0.28) | 0.10 (−0.01–0.22) | 0.11 (0.00–0.23) |
| ≥0.1 to <1 | 1382 | 0.35 (0.24–0.46) | 0.27 (0.17–0.38) | 0.29 (0.18–0.39) |
| ≥1 | 344 | 0.39 (0.22–0.55) | 0.28 (0.13–0.44) | 0.30 (0.15–0.46) |
| p* | <0.001 | <0.001 | <0.001 | |
| Fruit juice (servings/day) | ||||
| 0 | 2.506 | 0.00 | 0.00 | 0.00 |
| >0 to <0.1 | 187 | −0.00 (−0.20–0.19) | −0.06 (−0.18–0.17) | 0.01 (−0.17–0.19) |
| ≥0.1 to <1 | 498 | 0.00 (−0.12–0.13) | 0.03 (−0.08–0.15) | 0.06 (−0.05–0.18) |
| ≥1 | 134 | 0.03 (−0.19–0.26) | 0.04 (−0.17–0.25) | 0.14 (0.06–0.35) |
| p* | 0.970 | 0.967 | 0.948 | |
| Total fructose (g/day) | ||||
| Quartile 1 (14 ± 4) | 805 | 0.00 | 0.00 | 0.00 |
| Quartile 2 (25 ± 3) | 774 | −0.01 (−0.14–0.11) | −0.00 (−0.12–0.11) | 0.01 (−0.11–0.13) |
| Quartile 3 (36 ± 4) | 835 | −0.03 (−0.15–0.09) | 0.01 (−0.10–0.12) | 0.06 (−0.06–0.18) |
| Quartile 4 (60 ± 17) | 911 | 0.08 (−0.04–0.20) | 0.10 (−0.00–0.22) | 0.20 (0.05–0.32) |
| p* | 0.300 | 0.413 | 0.022 | |
| Female (n = 3.848) ** | ||||
| Sugar-sweetened soft drinks (servings/day) | ||||
| 0 | 1660 | 0.00 | 0.00 | 0.00 |
| >0 to <0.1 | 1083 | 0.11 (0.03–0.19) | 0.08 (0.01–0.16) | 0.08 (0.00–0.15) |
| ≥0.1 to <1 | 947 | 0.19 (0.11–0.27) | 0.13 (0.05–0.21) | 0.12 (0.04–0.20) |
| ≥1 | 158 | 0.28 (0.12–0.45) | 0.16 (0.00–0.31) | 0.15 (0.00–0.31) |
| p* | <0.001 | 0.012 | 0.013 | |
| Fruit juice (servings/day) | ||||
| 0 | 2747 | 0.00 | 0.00 | 0.00 |
| >0 to <0.1 | 287 | −0.06 (−0.16–0.03) | −0.00 (−0.09–0.08) | −0.06 (−0.18–0.04) |
| ≥0.1 to <1 | 666 | −0.07 (0.14–0.00) | −0.03 (−0.10–0.03) | −0.07 (−0.15–0.00) |
| ≥1 | 148 | −0.05 (−0.16–0.05) | −0.02 (−0.13–0.07) | −0.04 (−0.190.11) |
| p* | 0.235 | 0.519 | 0.488 | |
| Total fructose (g/day) | ||||
| Quartile 1 (15 ± 4) | 988 | 0.00 | 0.00 | 0.00 |
| Quartile 2 (26 ± 3) | 1019 | 0.01 (−0.07–0.10) | 0.00 (−0.07–0.09) | 0.01 (−0.06–0.10) |
| Quartile 3 (35 ± 4) | 959 | 0.06 (−0.02–0.15) | 0.04 (−0.04–0.12) | 0.05 (−0.02–0.14) |
| Quartile 4 (60 ± 16) | 882 | 0.07 (−0.01–0.17) | 0.03 (−0.05–0.11) | 0.04 (−0.06–0.13) |
| p* | 0.873 | 0.806 | 0.667 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siqueira, J.H.; Mill, J.G.; Velasquez-Melendez, G.; Moreira, A.D.; Barreto, S.M.; Benseñor, I.M.; Molina, M.D.C.B. Sugar-Sweetened Soft Drinks and Fructose Consumption Are Associated with Hyperuricemia: Cross-Sectional Analysis from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutrients 2018, 10, 981. https://doi.org/10.3390/nu10080981
Siqueira JH, Mill JG, Velasquez-Melendez G, Moreira AD, Barreto SM, Benseñor IM, Molina MDCB. Sugar-Sweetened Soft Drinks and Fructose Consumption Are Associated with Hyperuricemia: Cross-Sectional Analysis from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutrients. 2018; 10(8):981. https://doi.org/10.3390/nu10080981
Chicago/Turabian StyleSiqueira, Jordana Herzog, José Geraldo Mill, Gustavo Velasquez-Melendez, Alexandra Dias Moreira, Sandhi Maria Barreto, Isabela Martins Benseñor, and Maria Del Carmen Bisi Molina. 2018. "Sugar-Sweetened Soft Drinks and Fructose Consumption Are Associated with Hyperuricemia: Cross-Sectional Analysis from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil)" Nutrients 10, no. 8: 981. https://doi.org/10.3390/nu10080981
APA StyleSiqueira, J. H., Mill, J. G., Velasquez-Melendez, G., Moreira, A. D., Barreto, S. M., Benseñor, I. M., & Molina, M. D. C. B. (2018). Sugar-Sweetened Soft Drinks and Fructose Consumption Are Associated with Hyperuricemia: Cross-Sectional Analysis from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutrients, 10(8), 981. https://doi.org/10.3390/nu10080981





