Chia Seed Does Not Improve Cognitive Impairment in SAMP8 Mice Fed with High Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment of Animals
2.3. Glucose and Insulin Tolerance Tests
2.4. Morris Water Maze (MWM) Test
2.5. Brain Tissue Collection and Preservation
2.6. Western Blot Analysis
2.7. Aβ42 and Microglia Immune-Staining
2.8. Statistical Analysis
3. Results
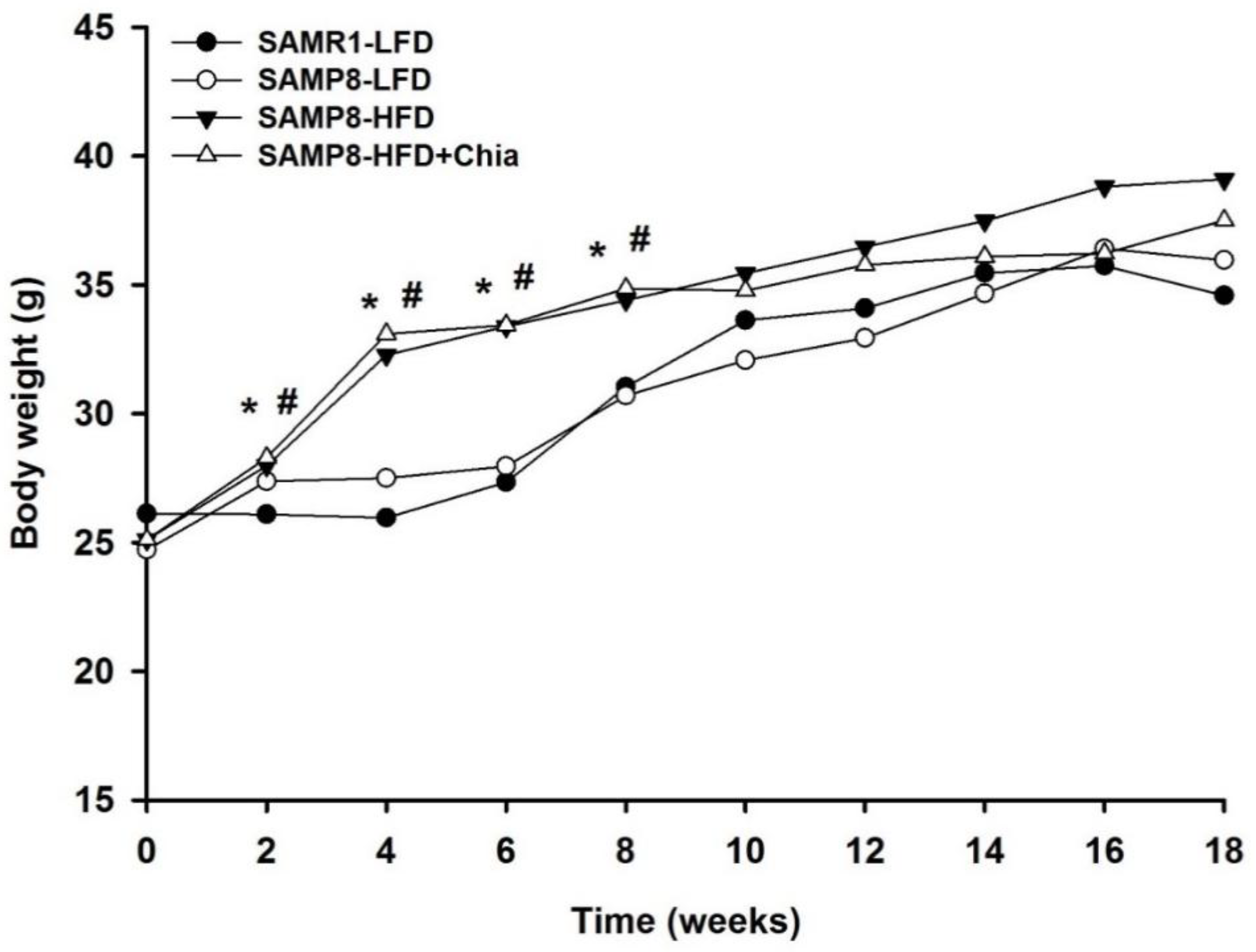
3.1. Body Weight
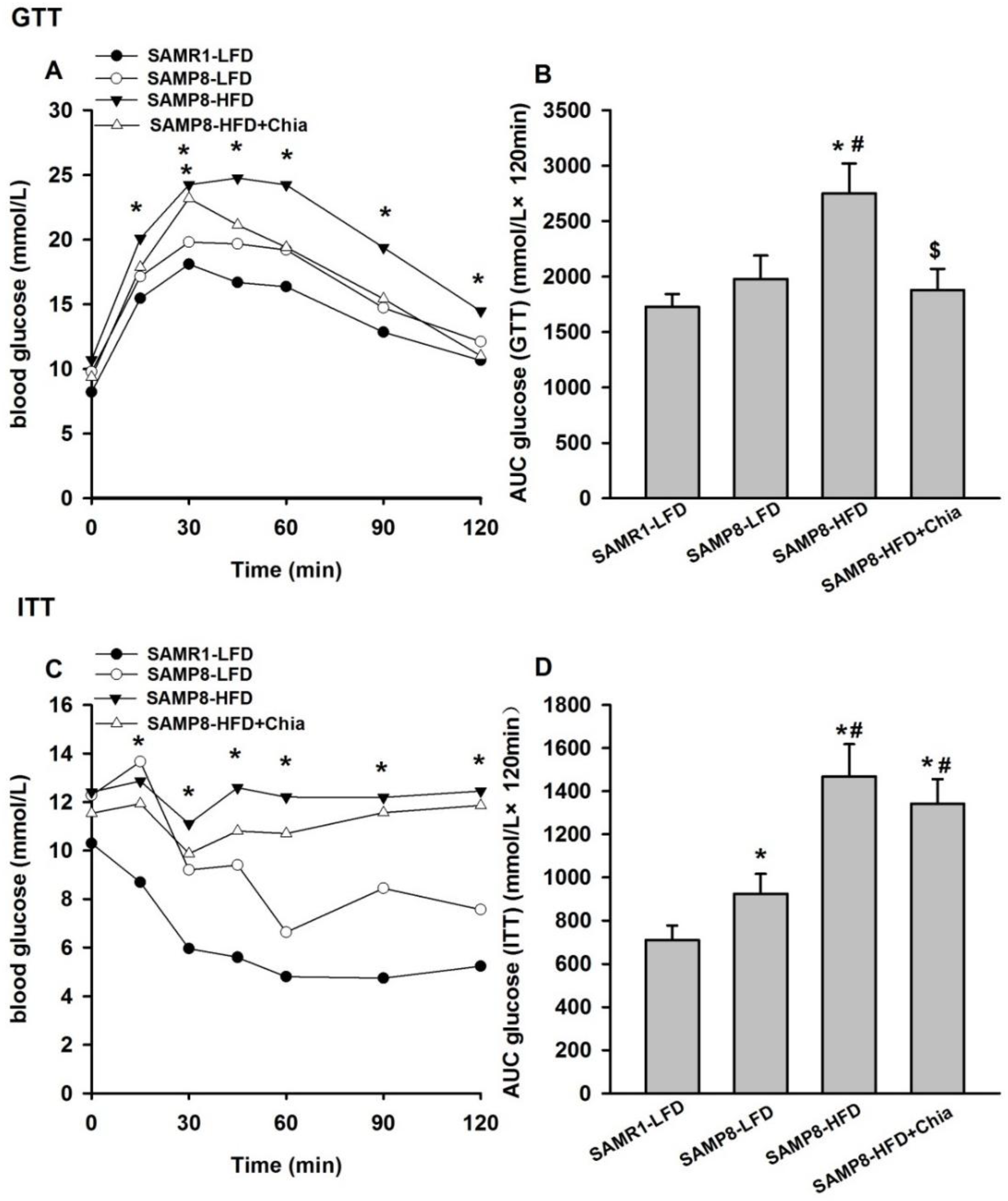
3.2. Glucose and Insulin Tolerance Test
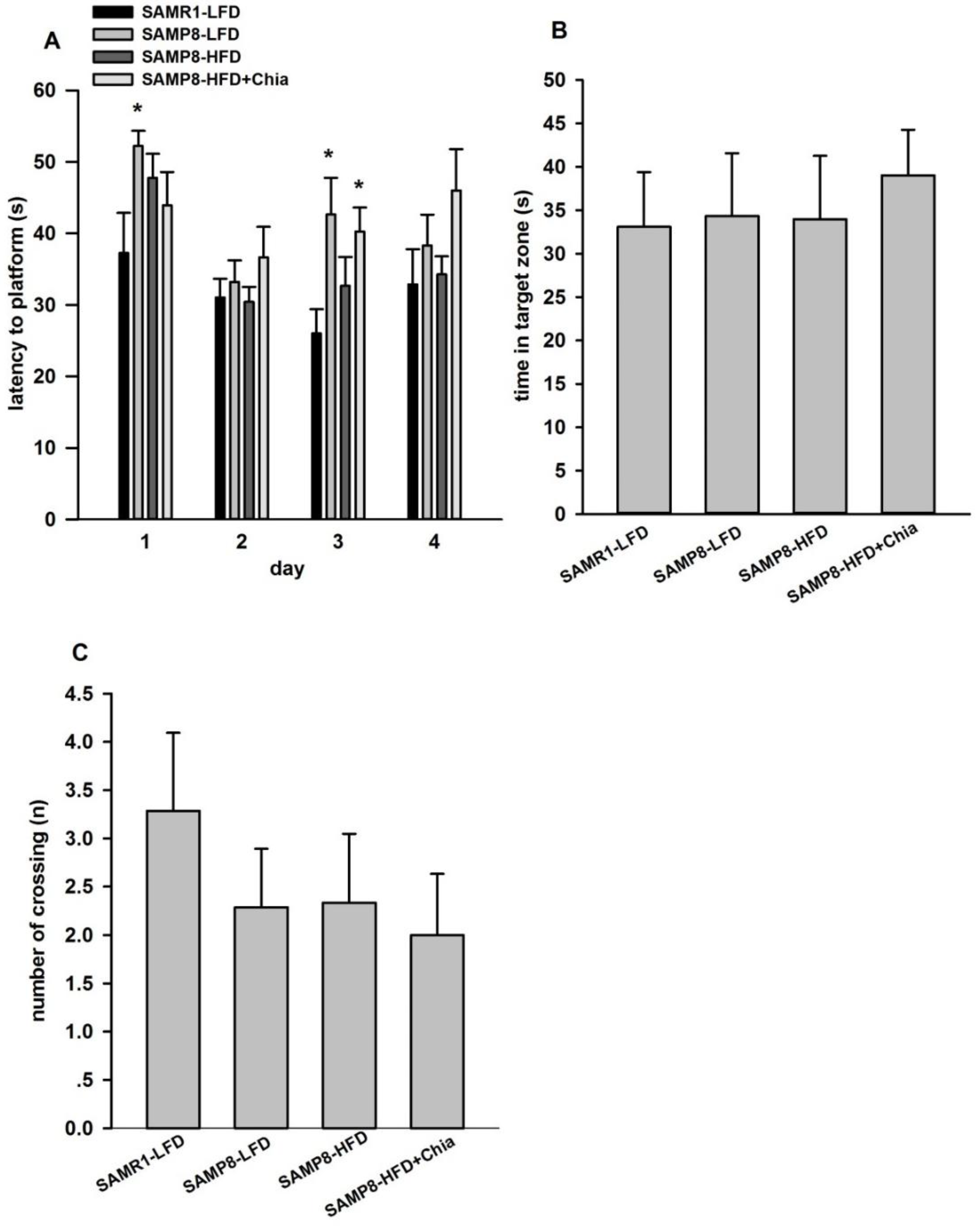
3.3. Cognitive Function via MWM Test
3.4. Protein Expression Related to Aβ Pathology
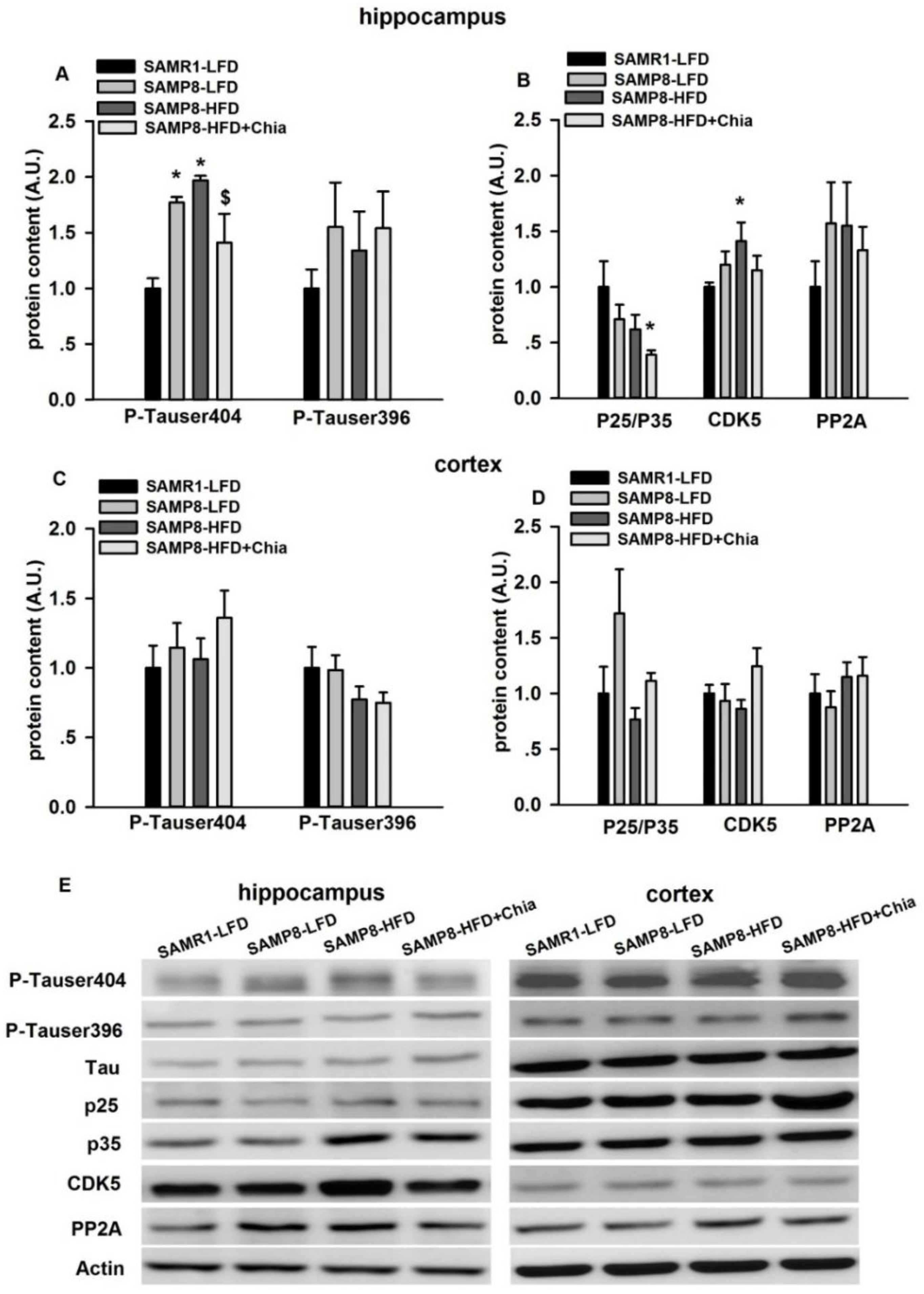
3.5. Tau Phosphorylation and Tau Kinases Protein Expression
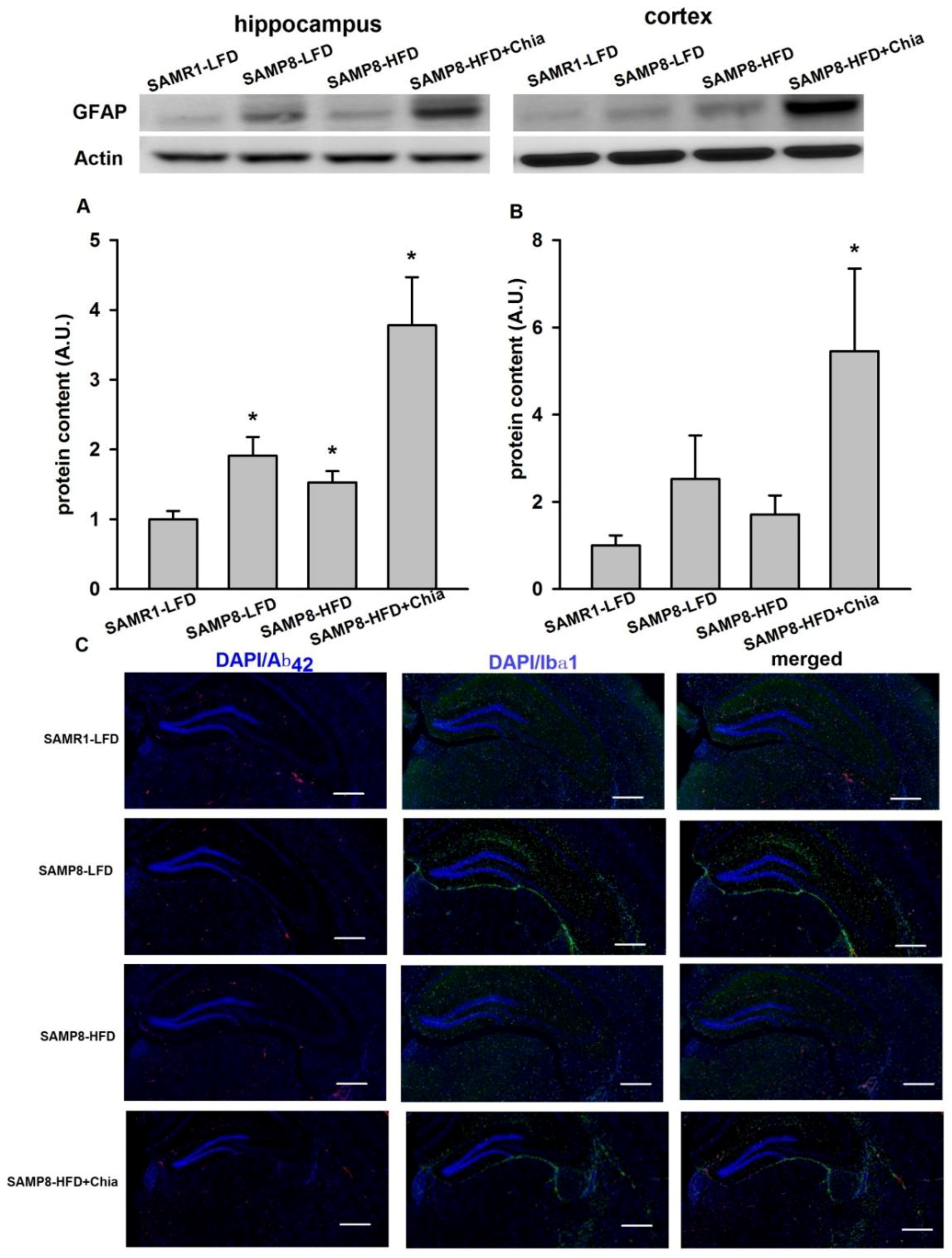
3.6. GFAP Protein Expression and Immune-Staining for Aβ42 + Ibα-1
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Letra, L.; Santana, R.; Seica, I. Obesity as a risk factor for Alzheimer’s disease: The role of adipocytokines. Metab. Brain Dis. 2014, 29, 563–568. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M. Relationships between diabetes and cognitive impairment. Endocrinol. Metab. Clin. N. Am. 2014, 43, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.J.; Hone, E.; Foster, J.K.; Sunram-Lea, S.I.; Gnjec, A.; Fuller, S.J.; Nolan, D.; Gandy, S.E.; Martins, R.N. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol. Psychiatry 2006, 11, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Loreto, A.; Cobos, A. Olga DiazJosé Miguel Aguilera Chia Seed (Salvia hispanica): An Ancient Grain and a New Functional Food. Food Rev. Int. 2013, 29, 394–408. [Google Scholar] [CrossRef]
- Coates, W. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind. Crops Prod. 2011, 34, 1366–1371. [Google Scholar] [CrossRef]
- Vuksan, V.; Jenkins, A.L.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A.L.; Bazinet, R.P.; Au-Yeung, F.; Zurbau, A.; Ho, H.V.; et al. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Jenkins, A.L.; Dias, A.G.; Lee, A.S.; Jovanovski, E.; Rogovik, A.L.; Hanna, A. Reduction in postprandial glucose excursion and prolongation of satiety: Possible explanation of the long-term effects of whole grain Salba (Salvia hispanica L.). Eur. J. Clin. Nutr. 2010, 64, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Lee, A.S.; Jovanovski, E.; Jenkins, A.L.; Desouza, V.; Vuksan, R. Effect of whole and ground Salba seeds (Salvia hispanica L.) on postprandial glycemia in healthy volunteers: A randomized controlled, dose-response trial. Eur. J. Clin. Nutr. 2013, 67, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Tovar, A.R.; Aguilar-Salinas, C.A.; Medina-Vera, I.; Gil-Zenteno, L.; Hernandez-Viveros, I.; Lopez-Romero, P.; Ordaz-Nava, G.; Canizales-Quinteros, S.; Guillen Pineda, L.E.; et al. A dietary pattern including nopal, chia seed, soy protein, and oat reduces serum triglycerides and glucose intolerance in patients with metabolic syndrome. J. Nutr. 2012, 142, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Marineli Rda, S.; Moura, C.S.; Moraes, E.A.; Lenquiste, S.A.; Lollo, P.C.; Morato, P.N.; Amaya-Farfan, J.; Marostica, M.R., Jr. Chia (Salvia hispanica L.) enhances HSP, PGC-1alpha expressions and improves glucose tolerance in diet-induced obese rats. Nutrition 2015, 31, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Chicco, A.G.; D’Alessandro, M.E.; Hein, G.J.; Oliva, M.E.; Lombardo, Y.B. Dietary chia seed (Salvia hispanica L.) rich in alpha-linolenic acid improves adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br. J. Nutr. 2009, 101, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.K.; Waanders, J.; Ward, L.; Brown, L. Lipid redistribution by alpha-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J. Nutr. Biochem. 2012, 23, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Nieman, D.C.; Sha, W.; Xie, G.; Qiu, Y.; Jia, W. Supplementation of milled chia seeds increases plasma ALA and EPA in postmenopausal women. Plant. Foods Hum. Nutr. 2012, 67, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Whitham, D.; Sievenpiper, J.L.; Jenkins, A.L.; Rogovik, A.L.; Bazinet, R.P.; Vidgen, E.; Hanna, A. Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: Results of a randomized controlled trial. Diabetes Care 2007, 30, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Cayea, E.J.; Austin, M.D.; Henson, D.A.; McAnulty, S.R.; Jin, F. Chia seed does not promote weight loss or alter disease risk factors in overweight adults. Nutr. Res. 2009, 29, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yan, P.; Zhang, S.; Nie, S.; Huang, F.; Han, H.; Deng, Q.; Huang, Q.; Yang, W.; Wu, H.; et al. Chronic alpha-linolenic acid treatment alleviates age-associated neuropathology: Roles of PERK/eIF2alpha signaling pathway. Brain Behav. Immun. 2016, 57, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Choi, J.M.; Lee, J.; Lee, M.H.; Lee, S.; Cho, E.J. Effects of Vegetable Oils with Different Fatty Acid Compositions on Cognition and Memory Ability in Abeta25-35-Induced Alzheimer’s Disease Mouse Model. J. Med. Food 2016. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Farr, S.A.; Flood, J.F. Antibody to amyloid beta protein alleviates impaired acquisition, retention, and memory processing in SAMP8 mice. Neurobiol. Learn. Mem. 2002, 78, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Canudas, A.M.; Gutierrez-Cuesta, J.; Rodriguez, M.I.; Acuna-Castroviejo, D.; Sureda, F.X.; Camins, A.; Pallas, M. Hyperphosphorylation of microtubule-associated protein tau in senescence-accelerated mouse (SAM). Mech. Ageing Dev. 2005, 126, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.Y. Okuma Age-related defects in lifespan and learning ability in SAMP8 mice. Neurobiol. Aging 1999, 20, 111–115. [Google Scholar] [CrossRef]
- Mehla, J.; Chauhan, B.C.; Chauhan, N.B. Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J. Alzheimers Dis. 2014, 39, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G. Toward a representational hypothesis of the role of hippocampal synaptic plasticity in spatial and other forms of learning. Cold Spring Harb. Symp. Quant. Biol. 1990, 55, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Thrush, A.B.; Legare, M.; Frier, B.C.; Sutherland, L.N.; Williams, D.B.; Wright, D.C. Epinephrine-mediated regulation of PDK4 mRNA in rat adipose tissue. Am. J. Physiol. Cell. Physiol. 2010, 299, C1162–C1170. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, L.; Zhang, Z.; Xu, J.; Wan, Z.; Qin, L. Resistin induces lipolysis and suppresses adiponectin secretion in cultured human visceral adipose tissue. Regul. Pept. 2014, 194–195, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, L.; Han, S.; Qin, L.; Chen, N. Zhongxiao Wan Treadmill running and rutin reverse high fat diet induced cognitive impairment in diet induced obese mice. J. Nutr. Health Aging 2015, 1–6. [Google Scholar] [CrossRef]
- Metcalfe, M.J.M.; Figueiredo-Pereira, E. Relationship between tau pathology and neuroinflammation in Alzheimer's disease. Mt. Sin. J. Med. 2010, 77, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.N.; Zukerberg, L.; Nikolic, M.; de la Monte, S.; Dikkes, P.; Tsai, L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999, 402, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005, 22, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Palomera-Avalos, V.; Grinan-Ferre, C.; Puigoriol-Ilamola, D.; Camins, A.; Sanfeliu, C.; Canudas, A.M.; Pallas, M. Resveratrol Protects SAMP8 Brain under Metabolic Stress: Focus on Mitochondrial Function and Wnt Pathway. Mol. Neurobiol. 2017, 54, 1661–1676. [Google Scholar] [CrossRef] [PubMed]
- Herrup, K. Reimagining Alzheimer;s disease—An age-based hypothesis. J. Neurosci. 2010, 30, 16755–16762. [Google Scholar] [CrossRef] [PubMed]
- Hook, V.; Schechter, I.; Demuth, H.U.; Hook, G. Alternative pathways for production of beta-amyloid peptides of Alzheimer's disease. Biol. Chem. 2008, 389, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Llovera, R.E.; de Tullio, M.; Alonso, L.G.; Leissring, M.A.; Kaufman, S.B.; Roher, A.E.; Gay, G.D.; Morelli, L.; Castano, E.M. The catalytic domain of insulin-degrading enzyme forms a denaturant-resistant complex with amyloid beta peptide: Implications for Alzheimer disease pathogenesis. J. Biol. Chem. 2008, 283, 17039–17048. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lemaire, H.G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.H.; Multhaup, G.; Beyreuther, K.; Muller-Hill, B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987, 325, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.V.; Lee, M. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 2011, 286, 15317–15331. [Google Scholar] [CrossRef] [PubMed]
- Hochgrafe, K.; Sydow, A.; Mandelkow, E.M. Regulatable transgenic mouse models of Alzheimer disease: Onset, reversibility and spreading of Tau pathology. FEBS J. 2013, 280, 4371–4381. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; He, S.S.; Wang, X.; Duan, Q.H.; Grundke-Iqbal, I.; Iqbal, K.; Wang, J. Levels of nonphosphorylated and phosphorylated tau in cerebrospinal fluid of Alzheimer’s disease patients: An ultrasensitive bienzyme-substrate-recycle enzyme-linked immunosorbent assay. Am. J. Pathol. 2002, 160, 1269–1278. [Google Scholar] [CrossRef]
- Rogers, J.; Webster, S.; Lue, L.F.; Brachova, L.; Civin, W.H.; Emmerling, M.; Shivers, B.; Walker, D.; McGeer, P. Inflammation and Alzheimer’s disease pathogenesis. Neurobiol. Aging 1996, 17, 681–686. [Google Scholar] [CrossRef]
- Yasuno, F.; Kosaka, J.; Ota, M.; Higuchi, M.; Ito, H.; Fujimura, Y.; Nozaki, S.; Takahashi, S.; Mizukami, K.; Asada, T.; et al. Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment-dementia converters measured by positron emission tomography with [(1)(1)C]DAA1106. Psychiatry Res. 2012, 203, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.E.; Ince, P.G.; Lace, G.; Forster, G.; Shaw, P.J.; Matthews, F.; Savva, G.; Brayne, C.; Wharton, S.B. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol. Aging 2010, 31, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Pomilio, C.; Pavia, P.; Gorojod, R.M.; Vinuesa, A.; Alaimo, A.; Galvan, V.; Kotler, M.L.; Beauquis, J.; Saravia, F. Glial alterations from early to late stages in a model of Alzheimer’s disease: Evidence of autophagy involvement in Abeta internalization. Hippocampus 2016, 26, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.; Jin, F.; Henson, D.A.; Kennerly, K.; Shanely, R.A.; Ore, B.; Su, M.; Schwartz, S. Chia seed supplementation and disease risk factors in overweight women: A metabolomics investigation. J. Altern. Complement. Med. 2012, 18, 700–708. [Google Scholar] [CrossRef] [PubMed]

| Fatty Acid Composition | g/100 g of Total Fatty Acid Content (N = 3) |
|---|---|
| C14:0 | 2.06 ± 0.38 |
| C16:0 | 2.06 ± 0.40 |
| C18:0 | 0.95 ± 0.22 |
| C18:1 n-9 | 1.9 ± 0.14 |
| C18:2 n-6 | 5.55 ± 0.37 |
| C18:3 n-3 | 19.5 ± 0.26 |
| C20:0 | 0.08 ± 0.0 |
| Total n-3 PUFA | 19.6 ± 0.52 |
| Total n-6 PUFA | 5.78 ± 0.24 |
| Total n-9 PUFA | 1.95 ± 0.25 |
| Total SFA | 3.18 ± 0.16 |
| Total MUFA | 2.02 ± 0.44 |
| Total PUFA | 25.2 ± 0.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rui, Y.; Lv, M.; Chang, J.; Xu, J.; Qin, L.; Wan, Z. Chia Seed Does Not Improve Cognitive Impairment in SAMP8 Mice Fed with High Fat Diet. Nutrients 2018, 10, 1084. https://doi.org/10.3390/nu10081084
Rui Y, Lv M, Chang J, Xu J, Qin L, Wan Z. Chia Seed Does Not Improve Cognitive Impairment in SAMP8 Mice Fed with High Fat Diet. Nutrients. 2018; 10(8):1084. https://doi.org/10.3390/nu10081084
Chicago/Turabian StyleRui, Yehua, Menglian Lv, Jie Chang, Jiaying Xu, Liqiang Qin, and Zhongxiao Wan. 2018. "Chia Seed Does Not Improve Cognitive Impairment in SAMP8 Mice Fed with High Fat Diet" Nutrients 10, no. 8: 1084. https://doi.org/10.3390/nu10081084
APA StyleRui, Y., Lv, M., Chang, J., Xu, J., Qin, L., & Wan, Z. (2018). Chia Seed Does Not Improve Cognitive Impairment in SAMP8 Mice Fed with High Fat Diet. Nutrients, 10(8), 1084. https://doi.org/10.3390/nu10081084





