Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approvement
2.2. Study Participants
2.3. Study Design
2.4. Sample Collection
2.5. Sample Preparation and NMR Spectroscopy Analysis
2.6. Pre-Processing and Statistical Analyses
2.7. Metabolite Identification
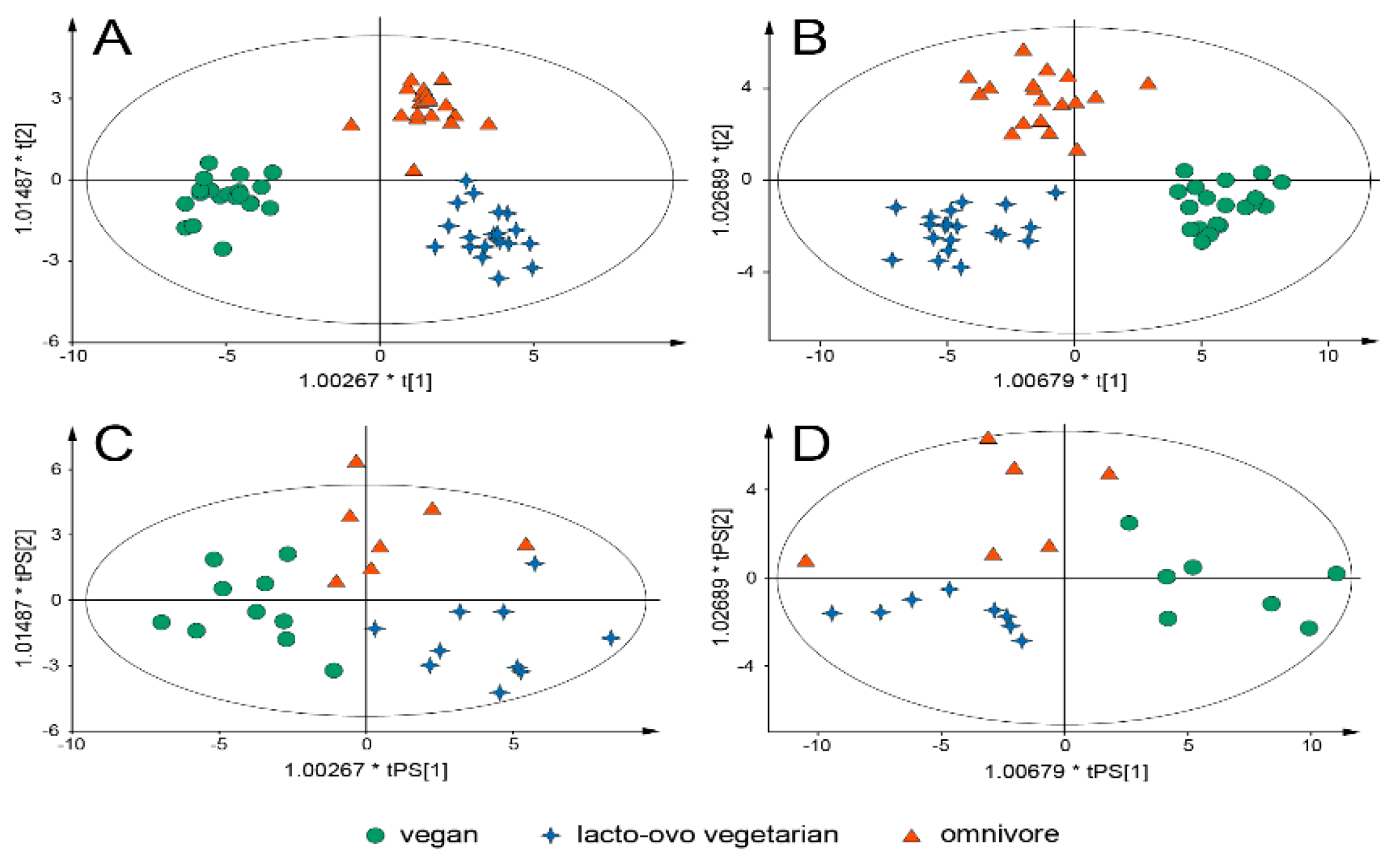
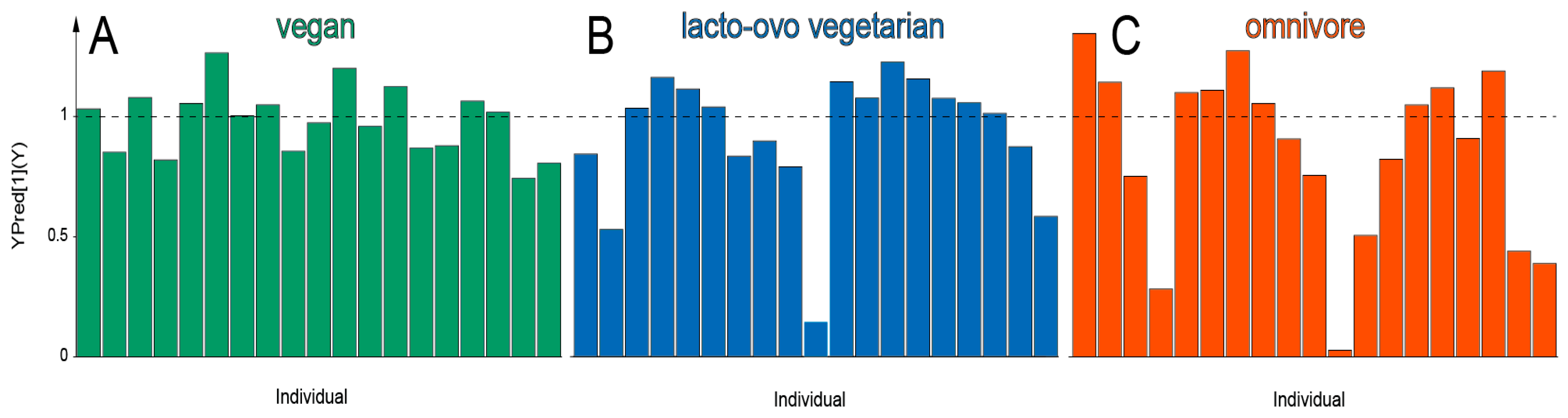
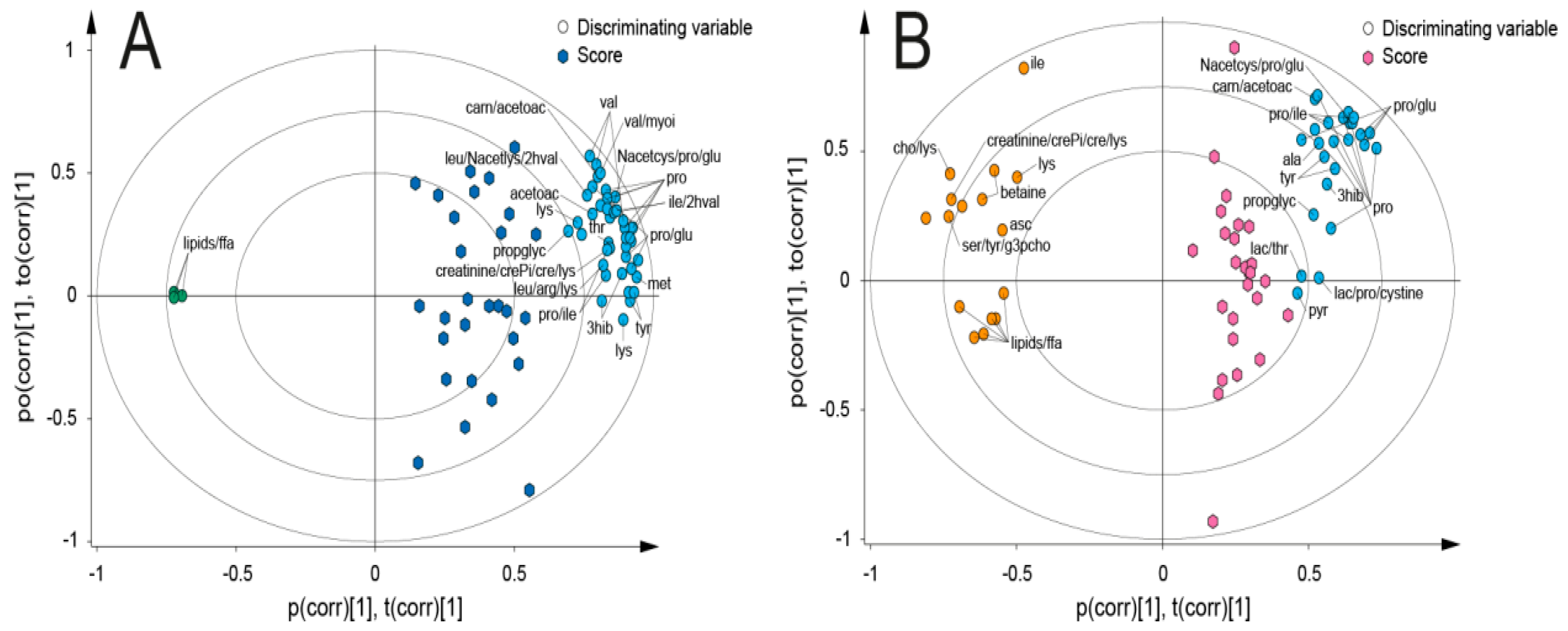
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schuit, A.J.; van Loon, A.J.M.; Tijhuis, M.; Ocké, M.C. Clustering of lifestyle risk factors in a general adult population. Prev. Med. 2002, 35, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Larsson, C.L.; Klock, K.S.; Nordrehaug Astrom, A.; Haugejorden, O.; Johansson, G. Lifestyle-related characteristics of young low-meat consumers and omnivores in sweden and norway. J. Adolesc. Health 2002, 31, 190–198. [Google Scholar] [CrossRef]
- Baines, S.; Powers, J.; Brown, W.J. How does the health and well-being of young australian vegetarian and semi-vegetarian women compare with non-vegetarians? Public Health Nutr. 2007, 10, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Panagiotakos, D.B.; Pitsavos, C.; Tsetsekou, E.; Fappa, E.; Papageorgiou, C.; Stefanadis, C. Eating habits in relations to anxiety symptoms among apparently healthy adults. A pattern analysis from the attica study. Appetite 2008, 51, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; Dragsted, L.O.; Draper, J.; Rappaport, S.M.; van der Hooft, J.J.; Wishart, D.S. The food metabolome: A window over dietary exposure. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.B.; Rinnan, A.; Manach, C.; Poulsen, S.K.; Pujos-Guillot, E.; Larsen, T.M.; Astrup, A.; Dragsted, L.O. Untargeted metabolomics as a screening tool for estimating compliance to a dietary pattern. J. Proteome Res. 2014, 13, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Draper, C.F.; Vassallo, I.; Di Cara, A.; Milone, C.; Comminetti, O.; Monnard, I.; Godin, J.P.; Scherer, M.; Su, M.; Jia, W.; et al. A 48-hour vegan diet challenge in healthy women and men induces a branch-chain amino acid related, health associated, metabolic signature. Mol. Nutr. Food Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pellis, L.; van Erk, M.J.; van Ommen, B.; Bakker, G.C.; Hendriks, H.F.; Cnubben, N.H.; Kleemann, R.; van Someren, E.P.; Bobeldijk, I.; Rubingh, C.M.; et al. Plasma metabolomics and proteomics profiling after a postprandial challenge reveal subtle diet effects on human metabolic status. Metabolomcs 2012, 8, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Karimpour, M.; Surowiec, I.; Wu, J.; Gouveia-Figueira, S.; Pinto, R.; Trygg, J.; Zivkovic, A.M.; Nording, M.L. Postprandial metabolomics: A pilot mass spectrometry and nmr study of the human plasma metabolome in response to a challenge meal. Anal. Chim. Acta 2016, 908, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Fazelzadeh, P.; Hangelbroek, R.W.J.; Joris, P.J.; Schalkwijk, C.G.; Esser, D.; Afman, L.; Hankemeier, T.; Jacobs, D.M.; Mihaleva, V.V.; Kersten, S.; et al. Weight loss moderately affects the mixed meal challenge response of the plasma metabolome and transcriptome of peripheral blood mononuclear cells in abdominally obese subjects. Metabolomics 2018, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Ministers, Nordic Council Ministers. Nordic Nutrition Recommendations, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014; Available online: https://books.google.com.hk/books?hl=zhTW&lr=&id=9_MblCPv5GcC&oi=fnd&pg=PA9&dq=Nordic+nutrition+recommendations+2012:+Integrating+nutrition+and+physical+activity%EF%BC%9B2014&ots=M7h_ndbEcZ&sig=5xbHmrVGfrkkYeerPXaw5cfcZT0&redir_esc=y#v=onepage&q&f=falseg (accessed on 15 July 2018).
- Trygg, J. O2-pls for qualitative and quantitative analysis in multivariate calibration. J. Chemom. 2002, 16, 283–293. [Google Scholar] [CrossRef]
- Jonsson, P.; Wuolikainen, A.; Thysell, E.; Chorell, E.; Stattin, P.; Wikstrom, P.; Antti, H. Constrained randomization and multivariate effect projections improve information extraction and biomarker pattern discovery in metabolomics studies involving dependent samples. Metabolomics 2015, 11, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. Hmdb 4.0: The human metabolome database for 2018. Nucl. Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. The metabolic signature associated with the western dietary pattern: A cross-sectional study. Nutr. J. 2013, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the epic-oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Lombard, K.A.; Olson, A.L.; Nelson, S.E.; Rebouche, C.J. Carnitine status of lactoovovegetarians and strict vegetarian adults and children. Am. J. Clin. Nutr. 1989, 50, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicova-Kudlackova, M.; Simoncic, R.; Bederova, A.; Babinska, K.; Beder, I. Correlation of carnitine levels to methionine and lysine intake. Physiol. Res. 2000, 49, 399–402. [Google Scholar] [PubMed]
- Demarquoy, J.; Georges, B.; Rigault, C.; Royer, M.-C.; Clairet, A.; Soty, M.; Lekounoungou, S.; Le Borgne, F. Radioisotopic determination of l-carnitine content in foods commonly eaten in western countries. Food Chem. 2004, 86, 137–142. [Google Scholar] [CrossRef]
- Cederblad, G. Effect of diet on plasma carnitine levels and urinary carnitine excretion in humans. Am. J. Clin. Nutr. 1987, 45, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010, 84, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.; Lohman, T.; Wang, Z.; Going, S. Human Body Composition, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2005. [Google Scholar]
- Brosnan, J.T.; da Silva, R.P.; Brosnan, M.E. The metabolic burden of creatine synthesis. Amino Acids 2011, 40, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Ericson, U.; Almgren, P.; Nilsson, J.; Magnusson, M.; Fernandez, C.; Melander, O. Postprandial levels of branch chained and aromatic amino acids associate with fasting glycaemia. J. Amino Acids 2016, 2016, 8576730. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLOS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed]
- Zar, T.; Graeber, C.; Perazella, M.A. Recognition, treatment, and prevention of propylene glycol toxicity. Semin. Dial. 2007, 20, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D. Current trends and challenges in sample preparation for global metabolomics using liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 1523–1548. [Google Scholar] [CrossRef] [PubMed]
- Stringer, K.A.; Younger, J.G.; McHugh, C.; Yeomans, L.; Finkel, M.A.; Puskarich, M.A.; Jones, A.E.; Trexel, J.; Karnovsky, A. Whole blood reveals more metabolic detail of the human metabolome than serum as measured by 1 h-nmr spectroscopy: Implications for sepsis metabolomics. Shock 2015, 44, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Nordlund, E.; Mattila, I.; Katina, K.; Aura, A.M.; Kolehmainen, M.; Oresic, M.; Mykkanen, H.; Poutanen, K. Postprandial differences in the plasma metabolome of healthy finnish subjects after intake of a sourdough fermented endosperm rye bread versus white wheat bread. Nutr. J. 2011, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Mullner, E.; Poutanen, K.; Mykkanen, H.; Moazzami, A.A. Metabolic changes in serum metabolome in response to a meal. Eur. J. Nutr. 2017, 56, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Svelander, C.; Undeland, I.; Pinto, R.; Sandberg, A.S. Herring and beef meals lead to differences in plasma 2-aminoadipic acid, beta-alanine, 4-hydroxyproline, cetoleic acid, and docosahexaenoic acid concentrations in overweight men. J. Nutr. 2015, 145, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.L.; Barr, S.I. Diets and selected lifestyle practices of self-defined adult vegetarians from a population-based sample suggest they are more ‘health conscious’. Int. J. Behav. Nutr. Phys. Act. 2005, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.N.; Thorogood, M.; Mann, J.I.; Key, T.J. The oxford vegetarian study: An overview. Am. J. Clin. Nutr. 1999, 70, 525s–531s. [Google Scholar] [CrossRef] [PubMed]
- Hlebowicz, J.; Wickenberg, J.; Fahlstrom, R.; Bjorgell, O.; Almer, L.O.; Darwiche, G. Effect of commercial breakfast fibre cereals compared with corn flakes on postprandial blood glucose, gastric emptying and satiety in healthy subjects: A randomized blinded crossover trial. Nutr. J. 2007, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Goetze, O.; Steingoetter, A.; Menne, D.; van der Voort, I.R.; Kwiatek, M.A.; Boesiger, P.; Weishaupt, D.; Thumshirn, M.; Fried, M.; Schwizer, W. The effect of macronutrients on gastric volume responses and gastric emptying in humans: A magnetic resonance imaging study. Am. J. Physiol. Gastrointest Liver Physiol. 2007, 292, G11–G17. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, F.; Benini, L.; Del Rio, D.; Casiraghi, C.; Pellegrini, N.; Scazzina, F.; Jenkins, D.J.; Vantini, I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am. J. Clin. Nutr. 2006, 83, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Sheflin, A.M.; Melby, C.L.; Carbonero, F.; Weir, T.L. Linking dietary patterns with gut microbial composition and function. Gut Microbes 2016. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The long-term stability of the human gut microbiota. Science 2013. [Google Scholar] [CrossRef] [PubMed]
| Model Set | Prediction Set 1 | |||
|---|---|---|---|---|
| Males (n = 11) | Females (n = 9) | Males (n = 5) | Females (n = 7) | |
| Characteristics | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Age (year) | 27.0 ± 6.6 | 25.9 ± 10.1 | 33.2 ± 13.2 | 31.9 ± 8.2 |
| Height (cm) | 184.1 ± 5.5 | 168.4 ± 4.7 | 183.0 ± 4.4 | 170.9 ± 3.8 |
| Body weight (kg) | 79.0 ± 11.0 | 62.0 ± 5.2 | 74.5 ± 4.4 | 60.0 ± 5.4 |
| BMI (kg/m2) | 23.3 ± 2.5 | 21.9 ± 1.9 | 22.2 ± 0.7 | 20.5 ± 1.6 |
| Fat mass (%) | 14.7 ± 5.4 | 23.5 ± 3.8 | 15.1 ± 3.0 | 21.8 ± 5.5 |
| Breakfast 1 | |||||
|---|---|---|---|---|---|
| Vegan | Lacto-Ovo Vegetarian | Omnivore | |||
| Food | g | Food | g | Food | g |
| Rye bred | 90 | Rye bred | 90 | Rye bred | 90 |
| Cashew nut butter | 22 | Hard cheese 28% | 24 | Liver pâté | 25 |
| Soy yoghurt blueberries | 100 | Fruit yoghurt 1.7% | 100 | Smoked ham | 30 |
| Olive oil 2 | 2 | Cottage cheese 4% | 47 | Egg | 54 |
| Lentils green (dry weight) 2 | 11 | Butter and margarine mix 75% | 12 | Butter and margarine mix 75% | 12 |
| Red bell pepper 2 | 9 | Apple | 20 | Red bell pepper | 22 |
| Green bell pepper | 25 | Tomato | 25 | Cucumber | 20 |
| Banana | 30 | Tea | 150 | Red caviar | 10 |
| Tea | 150 | Milk (1.5%) | 51 | Tea | 150 |
| Oat milk | 50 | Milk (1.5%) | 51 | ||
| Models | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VE 1 Breakfast | LOV 2 Breakfast | OM 3 Breakfast | LOV vs. VE Breakfast | LOV vs. OM Breakfast | ||||||||||
| Characteristic foods | Soy-yoghurt, cashew butter, lentils | Yoghurt, hard cheese, cottage cheese | Liver pâté, smoked ham, egg | |||||||||||
| Metabolite | Δconc. | p-value 4 | Δconc. | p-value | Δconc. | p-value | LOV Δconc. | VE Δconc. | p-value | Fold change | LOV Δconc. | OM Δconc. | p-value | Fold change |
| 3-Hydroxyisobutyrate | ↑ | 0.0001 | ↑ | 0.0004 | ↑ | <0.0001 | 1.83 | ↑ | 0.004 | 1.28 | ||||
| Acetate | ↓ | 0.0009 | ↓ | <0.0001 | ↓ | 0.0002 | ||||||||
| Acetoacetate | ↑ | <0.0001 | 1.45 | |||||||||||
| Acetone | ↓ | 0.02 | ||||||||||||
| Alanine | ↑ | 0.0004 | ↑ | 0.03 | 1.11 | |||||||||
| Glucose (alfa, beta) | ↓ | 0.03 | ↓ | 0.0006 | ||||||||||
| Arginine & Lysine 5 | ↑ | 0.003 | ↑ | <0.0001 | ↑ | 0.0002 | ||||||||
| Ascorbate | ↑ | 0.02 | 0.88 | |||||||||||
| Asparagine | ↑ | 0.02 | ||||||||||||
| Betaine | ↑ | 0.0003 | ↑ | <0.0001 | ↑ | 0.02 | 0.84 | |||||||
| Carnitine & Acetoacetate 5 | ↑ | <0.0001 | ↑ | 0.0002 | ↑ | <0.0001 | 1.24 | ↑ | 0.007 | 1.18 | ||||
| Choline | ↑ | 0.0001 | ↑ | 0.003 | 0.80 | |||||||||
| Creatinine | ↓ | 0.009 | ||||||||||||
| Creatinine & Creatine & Creatine phosphate 5 | ↓ | 0.004 | ↑ | <0.0001 | 1.12 | ↑ | 0.003 | 0.93 | ||||||
| Isoleucine | ↑ | 0.0001 | ↑ | <0.0001 | 1.21 | ↑ | 0.08 | 0.90 | ||||||
| Lactate | ↑ | 0.06 | 1.22 | |||||||||||
| Leucine | ↓ | 0.05 | ↑ | <0.0001 | 1.31 | |||||||||
| Leucine & Arginine 5 | ↓ | 0.09 | ↑ | 0.002 | ↑ | 0.002 | ↑ | <0.0001 | 1.48 | |||||
| Lipids/FFA | ↑ | <0.0001 | ↑ | 0.002 | ↑ | <0.0001 | 0.69 | ↑ | 0.003 | 0.76 | ||||
| Lysine | ↑ | 0.0002 | ↑ | <0.0001 | 1.51 | ↑ | 0.3 | 0.94 | ||||||
| Mannose | ↓ | 0.0004 | ↓ | <0.0001 | ↓ | <0.0001 | ||||||||
| Methionine | ↓ | 0.001 | ↑ | 0.0002 | ↑ | <0.0001 | 1.65 | |||||||
| myo-Inositol | ↑ | 0.0007 | ↑ | 0.0006 | ||||||||||
| N-Acetylcysteine & Proline & Glutamate 5 | ↑ | <0.0001 | ↑ | <0.0001 | 1.37 | ↑ | 0.005 | 1.37 | ||||||
| O-Phosphocholine & 3-Hydroxybutyrate 5 | ↓ | 0.005 | ||||||||||||
| Ornithine | ↑ | 0.0001 | ↑ | <0.0001 | ↑ | 0.0001 | ||||||||
| Proline | ↑ | <0.0001 | ↑ | 0.0001 | ↑ | <0.0001 | 1.41 | ↑ | 0.003 | 1.29 | ||||
| Proline & Glutamate & Unknown 5 | ↑ | 0.1 | ↑ | <0.0001 | ↑ | 0.0001 | ↑ | <0.0001 | 1.76 | ↑ | 0.003 | 1.48 | ||
| Propylene glycol | ↑ | <0.0001 | ↑ | 0.0004 | 1.42 | ↑ | 0.02 | 1.38 | ||||||
| Pyruvate | ↑ | 0.2 | 1.28 | |||||||||||
| Serine & Tyrosine 5 | ↑ | 0.001 | 0.86 | |||||||||||
| Succinic acid | ↓ | 0.0005 | ↑ | 0.2 | 1.26 | |||||||||
| Threonine | ↓ | 0.004 | ↑ | <0.0001 | 1.33 | |||||||||
| Tyrosine | ↑ | <0.0001 | ↑ | 0.0003 | ↑ | <0.0001 | 1.42 | ↑ | 0.005 | 1.13 | ||||
| Valine | ↑ | <0.0001 | 0.0001 | ↑ | <0.0001 | 1.22 | ||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rådjursöga, M.; Lindqvist, H.M.; Pedersen, A.; Karlsson, B.G.; Malmodin, D.; Ellegård, L.; Winkvist, A. Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets. Nutrients 2018, 10, 1063. https://doi.org/10.3390/nu10081063
Rådjursöga M, Lindqvist HM, Pedersen A, Karlsson BG, Malmodin D, Ellegård L, Winkvist A. Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets. Nutrients. 2018; 10(8):1063. https://doi.org/10.3390/nu10081063
Chicago/Turabian StyleRådjursöga, Millie, Helen M. Lindqvist, Anders Pedersen, B. Göran Karlsson, Daniel Malmodin, Lars Ellegård, and Anna Winkvist. 2018. "Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets" Nutrients 10, no. 8: 1063. https://doi.org/10.3390/nu10081063
APA StyleRådjursöga, M., Lindqvist, H. M., Pedersen, A., Karlsson, B. G., Malmodin, D., Ellegård, L., & Winkvist, A. (2018). Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets. Nutrients, 10(8), 1063. https://doi.org/10.3390/nu10081063





