Acute Effects of High-Intensity Interval and Moderate-Intensity Continuous Exercise on GLP-1, Appetite and Energy Intake in Obese Men: A Crossover Trial
Abstract
1. Introduction
2. Material and Methods
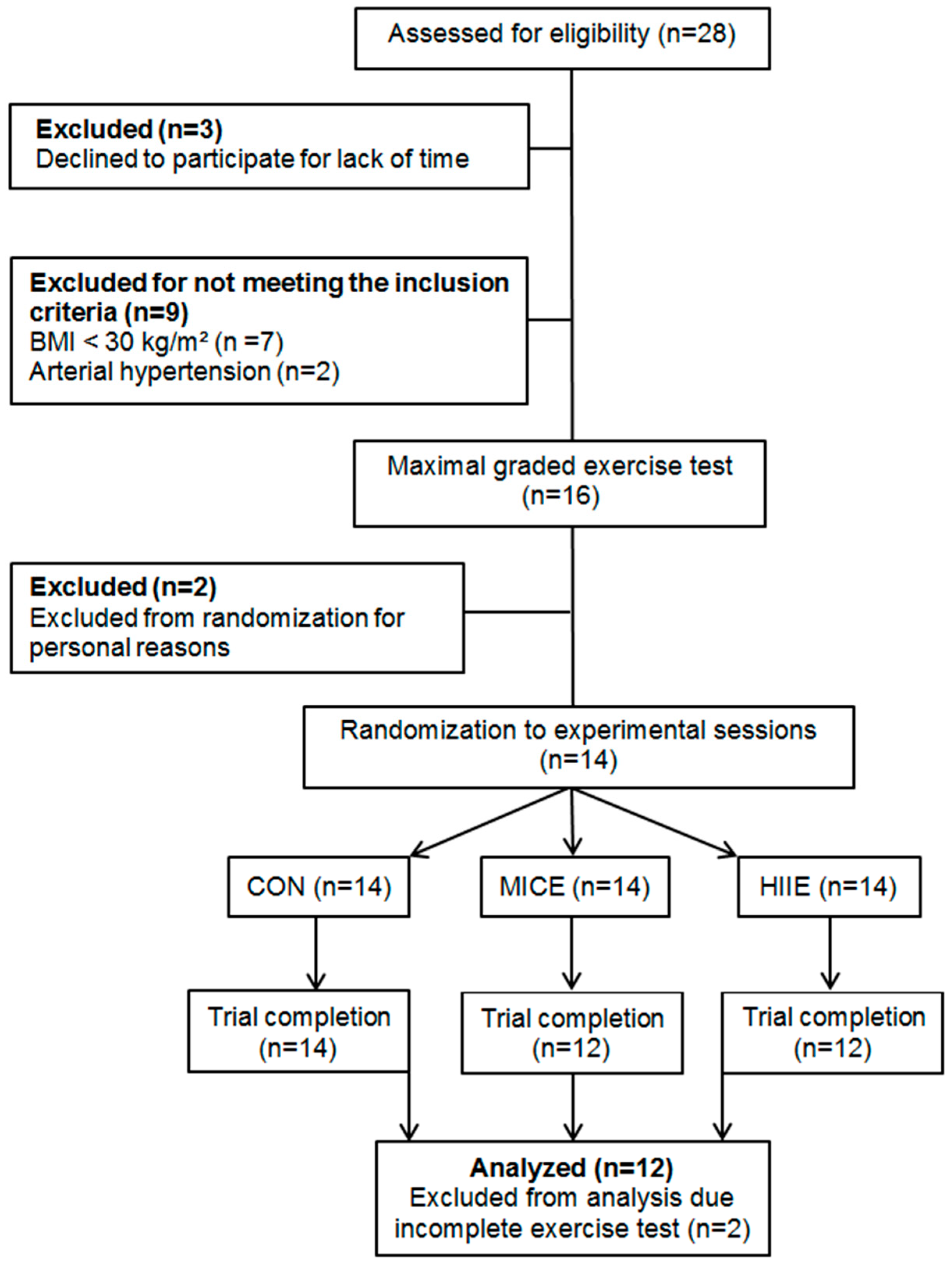
2.1. Study Design and Subjects
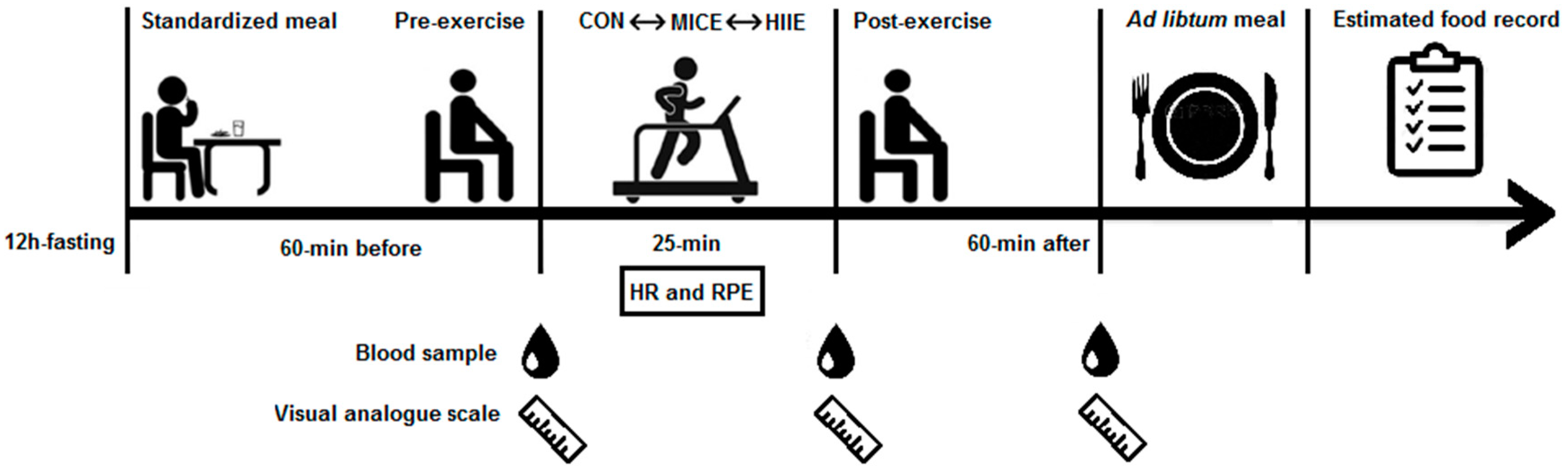
2.2. Procedures
2.3. Anthropometric and Body Composition Assessment
2.4. Biochemical Measurements
2.5. Maximal Graded Exercise Test
2.6. Standardized Meal
2.7. Experimental Sessions
2.8. Subjective Appetite Perception
2.9. Ad Libitum Meal
2.10. Estimated Food Record
3. Statistical Analysis
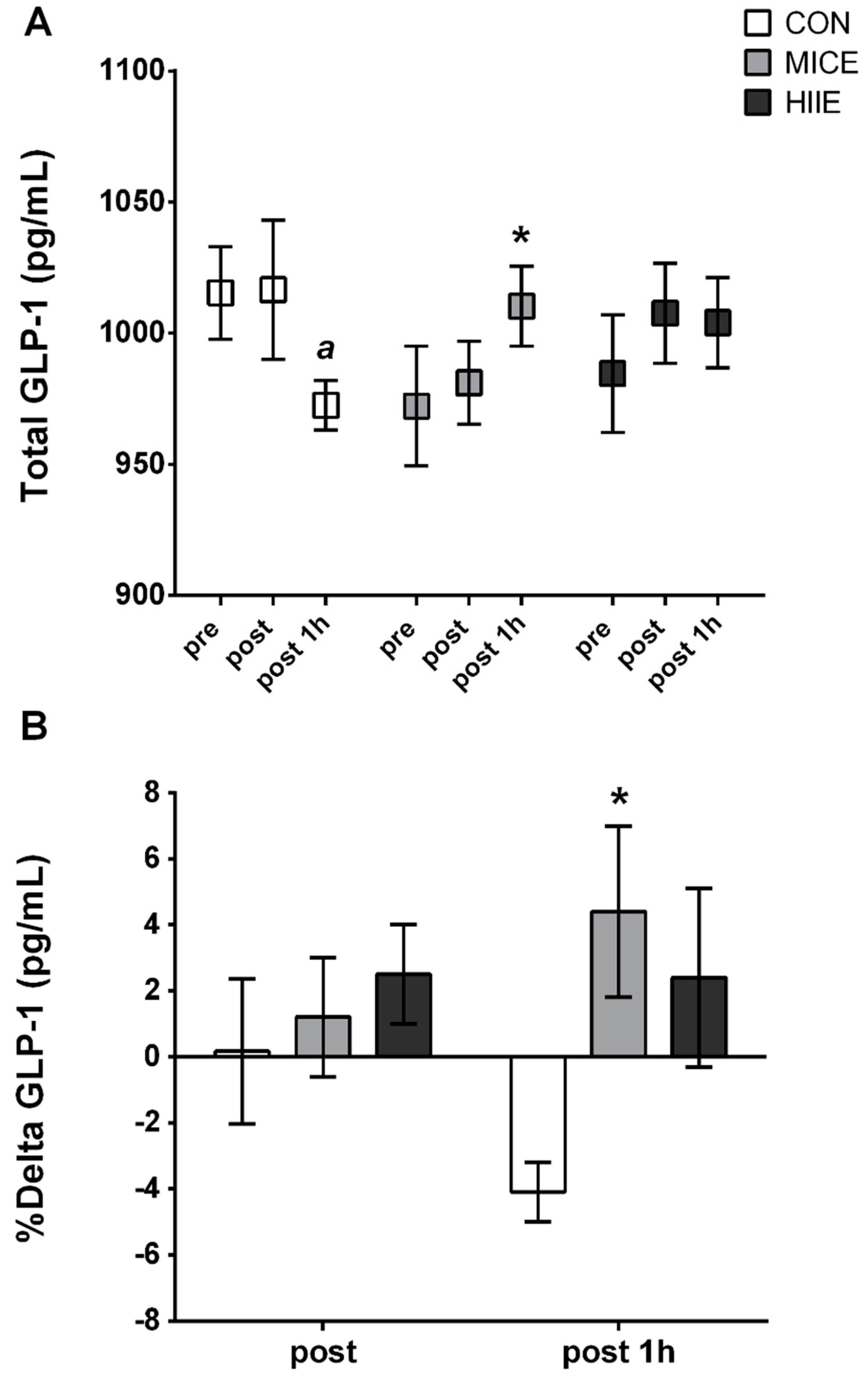
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Food | Amount (g or mL) | Energy (Kcal) | CHO (g) | PTN (g) | LIP (g) |
|---|---|---|---|---|---|
| Apple | 120 g | 82.8 | 19.9 | 0.2 | 0.2 |
| Banana | 180 g | 156.4 | 36.5 | 2.2 | 0.2 |
| Fruit Juice | 200 mL | 107.6 | 26 | 0.9 | 0 |
| Whole Yogurt | 170 g | 126.5 | 9.1 | 6.8 | 7 |
| Potato chips | 27 g | 153 | 13 | 1.7 | 10.5 |
| Chocolate | 40 g | 191.2 | 24 | 2.2 | 9.6 |
| Toast | 20 g | 77 | 13.2 | 3.1 | 1.3 |
| Boiled egg | 150 g | 211.7 | 0.9 | 20 | 14.3 |
| Fruit jelly | 30 g | 74.3 | 18.5 | 0 | 0 |
| Butter | 20 g | 148.7 | 0 | 0.1 | 16.5 |
| Total | 957 | 1329 | 161.2 | 37.2 | 59.6 |
| Total (% macronutrients) | - | - | 48.5 | 11.2 | 40.3 |
References
- Heymsfield, M.D.; Thomas, A.W. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases. Switzerland. 2014. Available online: http://www.who.int/nmh/ publications/ncd-status-report-2014/en/ (accessed on 18 December 2017).
- Ross, R.; Dagnone, D.; Jones, P.J.; Smith, H.P.; Hudson, R.; Janssen, I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann. Intern. Med. 2000, 133, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Trussardi, A.P.F.; Lopes, A.L.; Fernandes, P.R.; Reischak-Oliveira, A.; Friedman, R. Impact of weight loss with or without exercise on abdominal fat and insulin resistance in obese individuals: A randomised clinical trial. Br. J. Nutr. 2013, 110, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444. [Google Scholar] [CrossRef] [PubMed]
- Tang-Christensen, M.; Vrang, N.; Larsen, P.J. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 42–47. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Burley, V.J.; Blundell, J.E. Exercise-induced suppression of appetite: Effects on food intake and implications for energy balance. Eur. J. Clin. Nutr. 1994, 48, 715–724. [Google Scholar] [PubMed]
- Broom, D.R.; Stensel, D.J.; Bishop, N.C.; Burns, S.F.; Miyashita, M. Exercise induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007, 8, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.Y.; Wallman, K.E.; Fairchild, T.J.; Guelfi, K.J. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int. J. Obes. 2014, 38, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Karra, E.; Batterham, R.L.; Stensel, D.J. Appetite, energy intake, and PYY3-36 responses to energy-matched continuous exercise and submaximal high-intensity exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 1, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.A.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Batacan, R.B.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017, 51, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Stensvold, D.; Finlayson, G.; Holst, J.; Wisloff, U.; Kulseng, B.; Morgan, L.; King, N.A. Effect of Moderate- and High-Intensity Acute Exercise on Appetite in Obese Individuals. Med. Sci. Sports Exerc. 2014, 47, 40–48. [Google Scholar] [CrossRef] [PubMed]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. Evidence-Based Guideline for the Management of High Blood Pressure in Adults. JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Fayh, A.P.T.; Matos, V.A.F.; Souza, D.C.; Santos, V.O.A.; Marinho, C.S.R.; Serquiz, A.C.; Friedman, R.; Costa, E.C. Effects of a single session of high-intensity interval exercise and moderate-intensity continuous exercise on biochemical cardiovascular risk factors in obese males. Sport Sci. Health 2018, 1–8. [Google Scholar] [CrossRef]
- Frazão, D.T.; De Farias, L.F.; Dantas, T.C.B.; Krinski, K.; Elsangedy, H.M.; Prestes, J.; Hardcastle, S.J.; Costa, E.C. Feeling of pleasure to high-intensity interval exercise is dependent of the number of work bouts and physical activity status. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Souza, D.C.; Matos, V.A.F.; Santos, V.O.A.; Medeiros, I.F.; Marinho, C.S.R.; Nascimento, P.R.P.; Dorneles, G.P.; Peres, A.; Müller, C.H.; Krause, M.; et al. Effects of High-Intensity Interval and Moderate-Intensity Continuous Exercise on Inflammatory, Leptin, IgA, and Lipid Peroxidation Responses in Obese Males. Front. Physiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Howley, E.T.; Basset, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, L.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Guia Alimentar Para a População Brasileira/Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica.–2.ed.–Brasília: Ministério da saúde. 2014. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/guia_alimentar_populacao_brasileira_2ed.pdf (accessed on 10 December 2017).
- Fisberg, R.M.; Marchioni, D.M.L.; Colucci, A.C.A. Avaliação do consumo alimentar e da ingestão de nutrientes na prática clínica. Arq. Bras. Endocrinol. Metabol. 2009, 53, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.Y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Nakao, H.; Fujimoto, S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J. Endocrinol. 2009, 201, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.A.; King, J.A.; Clayton, D.J.; Jackson, A.P.; Sargeant, J.A.; Thackray, A.E.; Davies, M.J.; Stensel, D.J. Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women. Int. J. Obes. 2017, 41, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Holliday, A.; Blannin, A.K. Very Low Volume Sprint Interval Exercise Suppresses Subjective Appetite, Lowers Acylated Ghrelin, and Elevates GLP-1 in Overweight Individuals: A Pilot Study. Nutrients 2017, 9, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.L.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic highintensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 7, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Evan, M.F.; Meghan, L.B. A new look at the science of weight control: How acceptance and commitment strategies can address the challenge of self-regulation. Appetite 2015, 84, 171–180. [Google Scholar] [CrossRef]
- Alkahtani, S.A.; Byrne, N.M.; Hills, A.P.; King, N.A. Acute interval exercise intensity does not affect appetite and nutrient preferences in overweight and obese males. Asia Pac. J. Clin. Nutr. 2014, 23, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Matos, V.A.F.; Souza, D.C.; Browne, R.A.V.; Santos, V.O.A.; Costa, E.C.; Fayh, A.P.T. Acute effect of high-intensity interval exercise and moderate-intensity continuous exercise on appetite in overweight/obese males: A pilot study. Sport Sci. Health 2017, 13, 403–410. [Google Scholar] [CrossRef]
- Schubert, M.A.; Desbrow, B.; Sabapathy, S.; Leveritt, M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013, 63, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Brashear, M.M.; Johnson, W.D.; Katzmarzyk, P.T. Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese US men and women. Int. J. Behav. Nutr. Phys. Act. 2010, 7. [Google Scholar] [CrossRef]

| Title | Mean ± SD |
|---|---|
| Age (years) | 28.4 ± 2.6 |
| Height (cm) | 177 ± 7 |
| Body weight (kg) | 109.0 ± 17.1 |
| Body mass index (kg/m2) | 35.5 ± 4.5 |
| Fat free mass (kg) | 65.7 ± 10.7 |
| Body fat (%) | 39.8 ± 2.2 |
| Fasting glucose (mg/dL) | 98.6 ± 25.2 |
| Total cholesterol (mg/dL) | 198.1 ± 25.9 |
| HDL-cholesterol (mg/dL) | 42.7 ± 2.3 |
| LDL-cholesterol (mg/dL) | 131.5 ± 27.1 |
| VLDL-cholesterol (mg/dL) | 24 ± 7.4 |
| Tryglicerides (mg/dL) | 119.9 ± 37.1 |
| Physical Activity Level | |
| Walking (min/week) | 16.9 ± 21.3 |
| Moderate activity (min/week) | 10.8 ± 15 |
| Vigorous activity (min/week) | 0 |
| Sitting time (h/day) | 9.7 ± 3.3 |
| Title | CON | MICE | HIIE |
|---|---|---|---|
| Energy Intake (kcal) | |||
| Ad libitum | 766 ± 189 | 713 ± 196 | 761 ± 243 |
| 24 h | 2813 ± 462 | 2737 ± 535 | 2665 ± 435 |
| Carbohydrate (g) | |||
| Ad libitum | 91.3 ± 32.3 | 85.4 ± 27.9 | 91.7 ± 31.1 |
| 24 h | 399.9 (371–465) | 380.2 (327–427) | 362.6 (311–455) |
| Protein (g) | |||
| Ad libitum | 26.9 ± 7.9 | 24.9 ± 6.9 | 25.3 ± 8.9 |
| 24 h | 99.2 (88–143) | 108.2 (90–134) | 112.9 (102–117) |
| Lipids (g) | |||
| Ad libitum | 32.5 ± 8.0 | 30.3 ± 11.1 | 32.5 ± 11.5 |
| 24 h | 86.9 (70–111) | 78.5 (59–93) | 81.1 (68–92) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, V.A.F.; Souza, D.C.; Santos, V.O.A.; Medeiros, Í.F.; Browne, R.A.V.; Nascimento, P.R.P.; Marinho, C.S.R.; Serquiz, A.C.; Costa, E.C.; Fayh, A.P.T. Acute Effects of High-Intensity Interval and Moderate-Intensity Continuous Exercise on GLP-1, Appetite and Energy Intake in Obese Men: A Crossover Trial. Nutrients 2018, 10, 889. https://doi.org/10.3390/nu10070889
Matos VAF, Souza DC, Santos VOA, Medeiros ÍF, Browne RAV, Nascimento PRP, Marinho CSR, Serquiz AC, Costa EC, Fayh APT. Acute Effects of High-Intensity Interval and Moderate-Intensity Continuous Exercise on GLP-1, Appetite and Energy Intake in Obese Men: A Crossover Trial. Nutrients. 2018; 10(7):889. https://doi.org/10.3390/nu10070889
Chicago/Turabian StyleMatos, Victor A. F., Daniel C. Souza, Victor O. A. Santos, Ítalo F. Medeiros, Rodrigo A. V. Browne, Paulo R. P. Nascimento, Cristiane S. R. Marinho, Alexandre C. Serquiz, Eduardo C. Costa, and Ana Paula Trussardi Fayh. 2018. "Acute Effects of High-Intensity Interval and Moderate-Intensity Continuous Exercise on GLP-1, Appetite and Energy Intake in Obese Men: A Crossover Trial" Nutrients 10, no. 7: 889. https://doi.org/10.3390/nu10070889
APA StyleMatos, V. A. F., Souza, D. C., Santos, V. O. A., Medeiros, Í. F., Browne, R. A. V., Nascimento, P. R. P., Marinho, C. S. R., Serquiz, A. C., Costa, E. C., & Fayh, A. P. T. (2018). Acute Effects of High-Intensity Interval and Moderate-Intensity Continuous Exercise on GLP-1, Appetite and Energy Intake in Obese Men: A Crossover Trial. Nutrients, 10(7), 889. https://doi.org/10.3390/nu10070889






