Effectiveness of Essence of Chicken on Cognitive Function Improvement: A Randomized Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Investigational Product
2.4. Cognitive Function Assessment
2.4.1. WAIS Digit Span
2.4.2. Stroop Color–Word Test
2.4.3. Sustained Attention to Response Task (SART)
2.5. Sample Size Calculations
2.6. Statistical Analysis
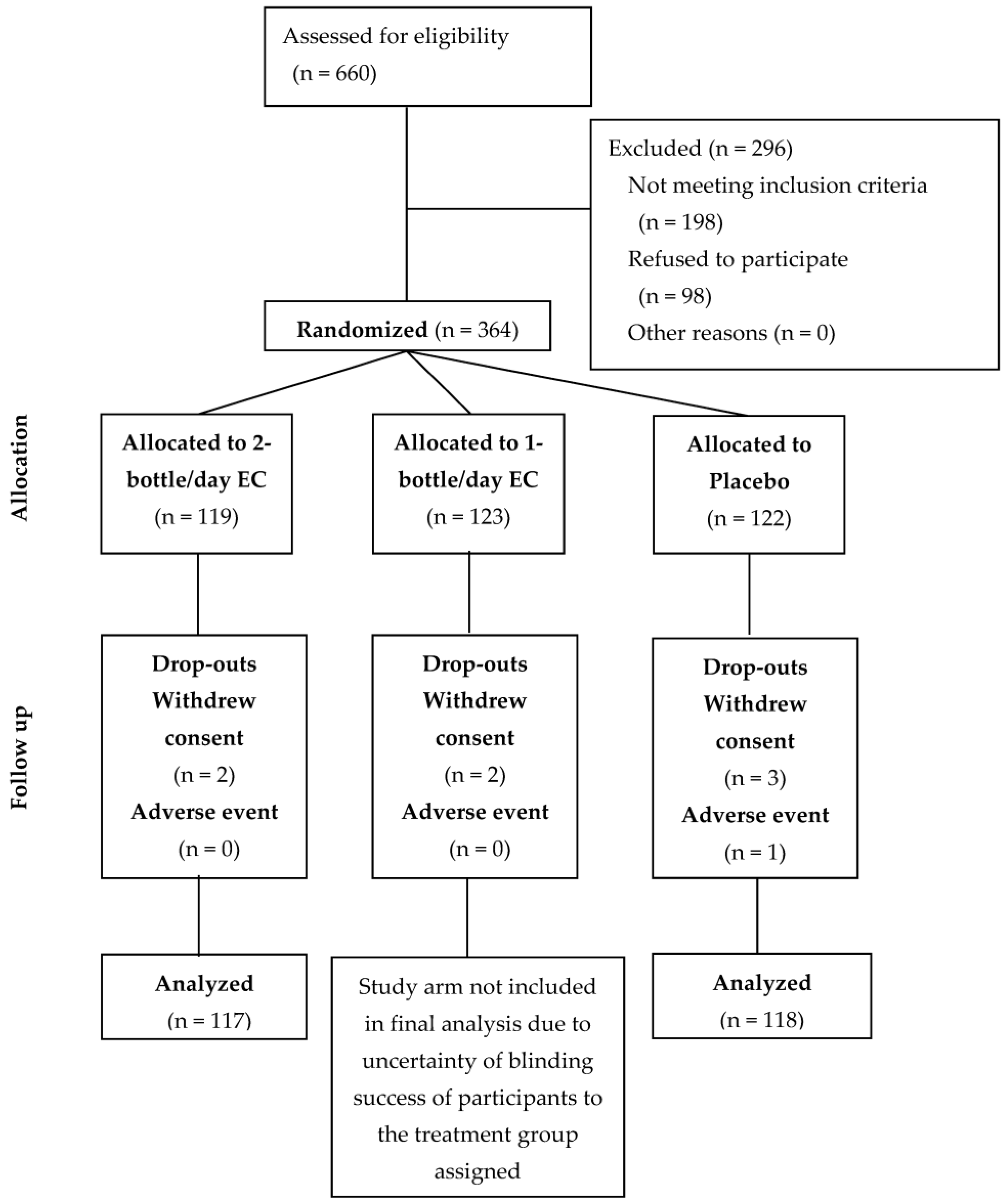
2.7. Protocol Deviations
3. Results
3.1. Participant Demographics
3.2. Cognitive Enhancing Effect of EC
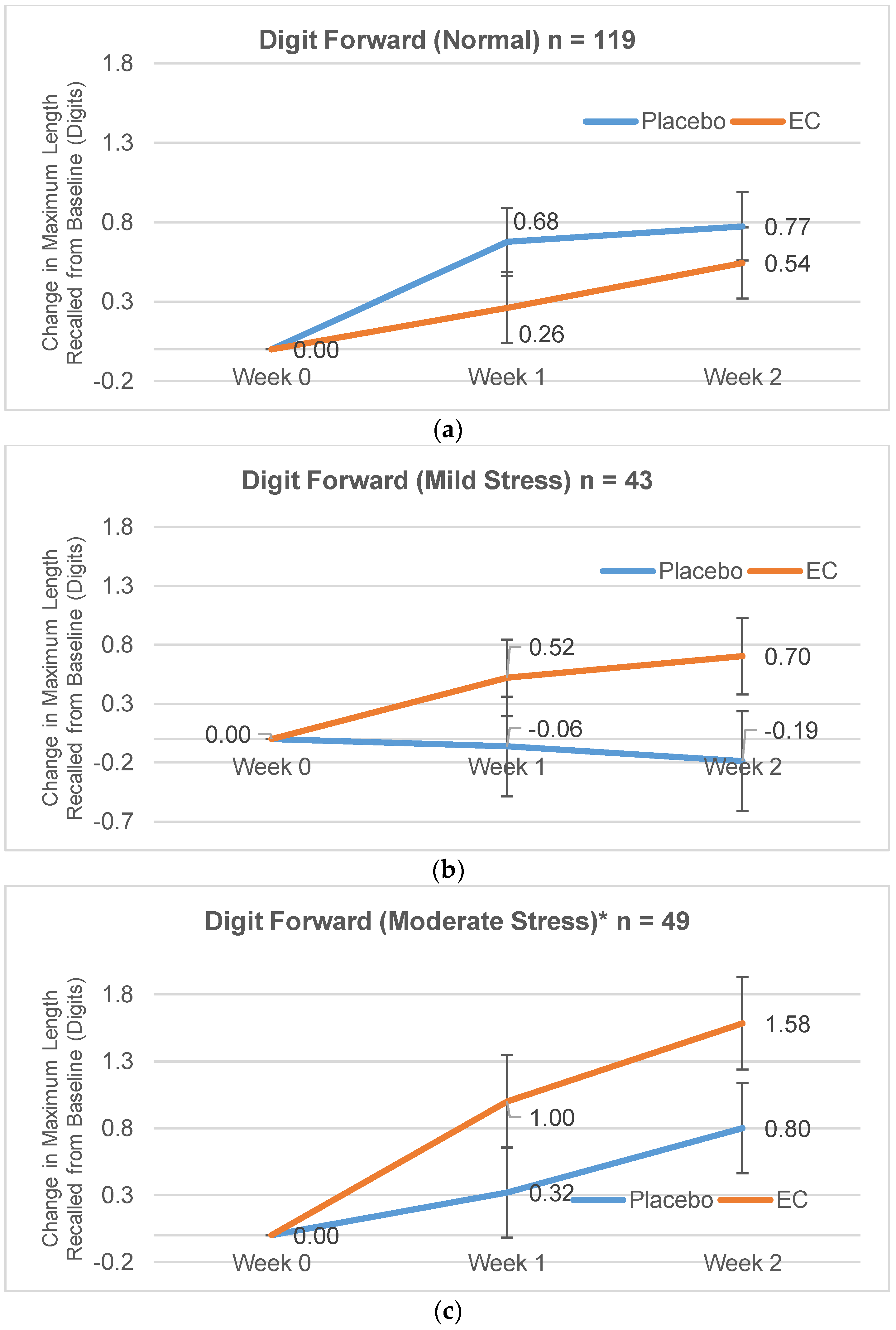
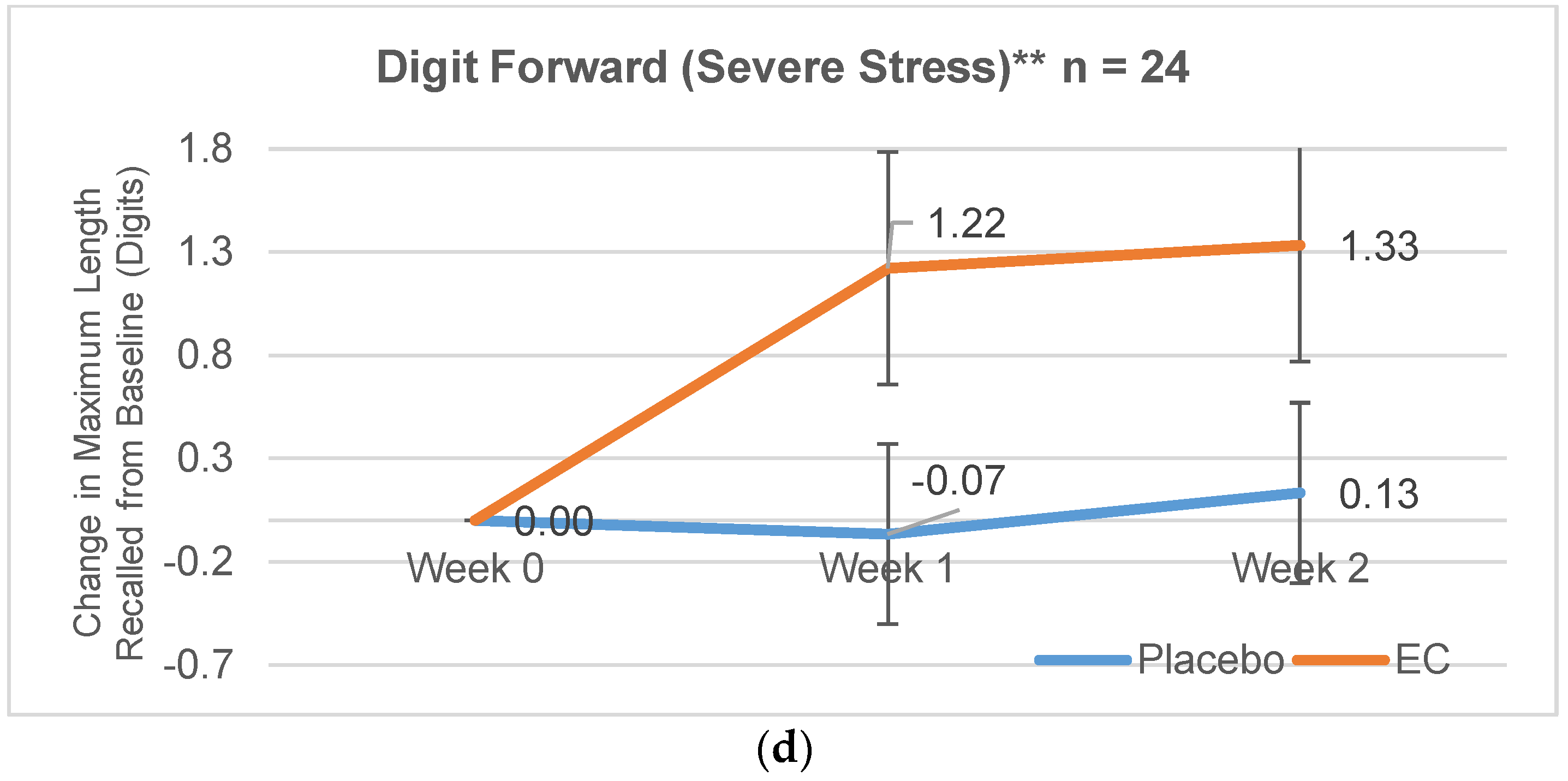
3.2.1. Digit Span Forward
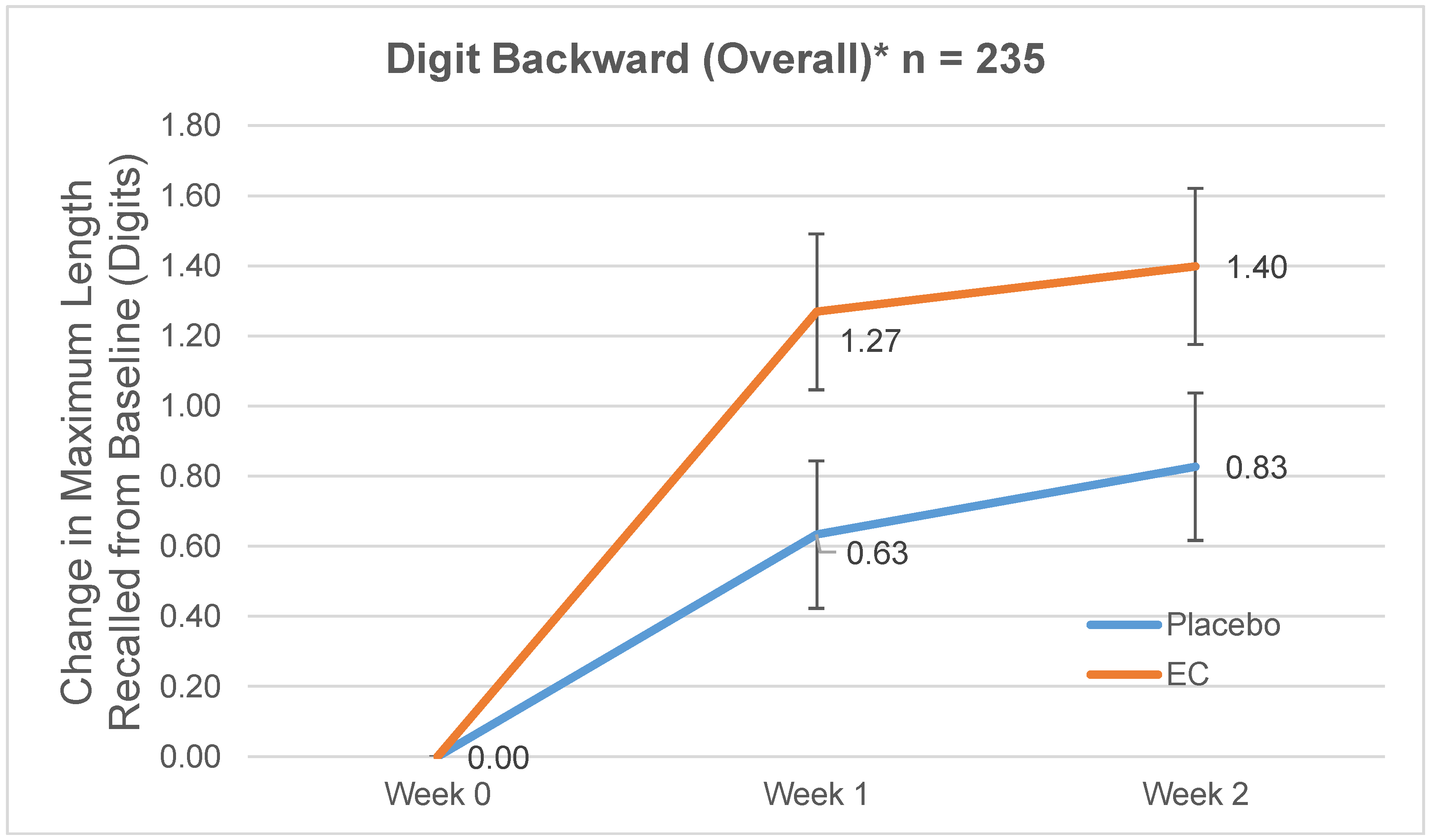
3.2.2. Digit Span Backward
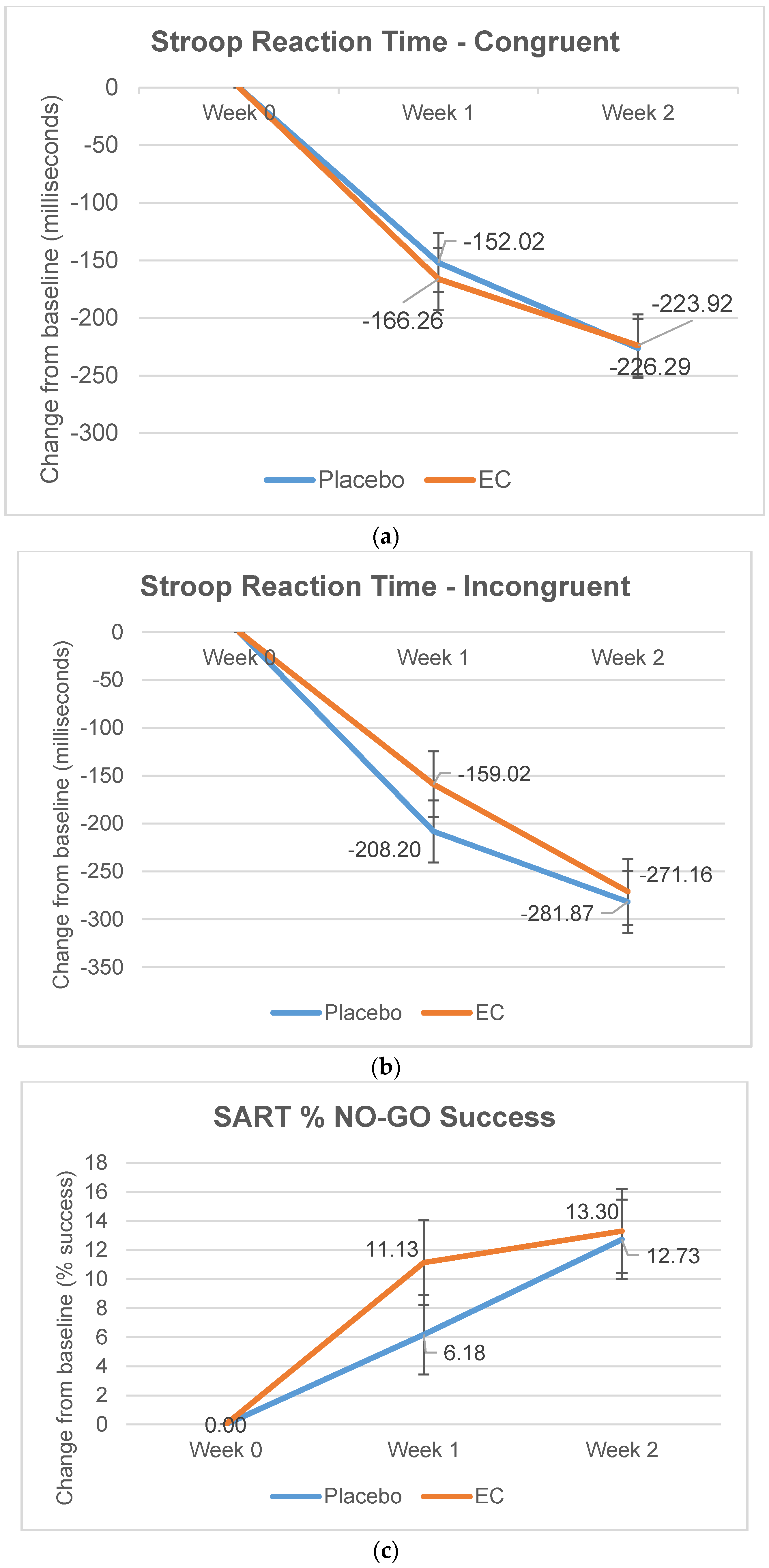
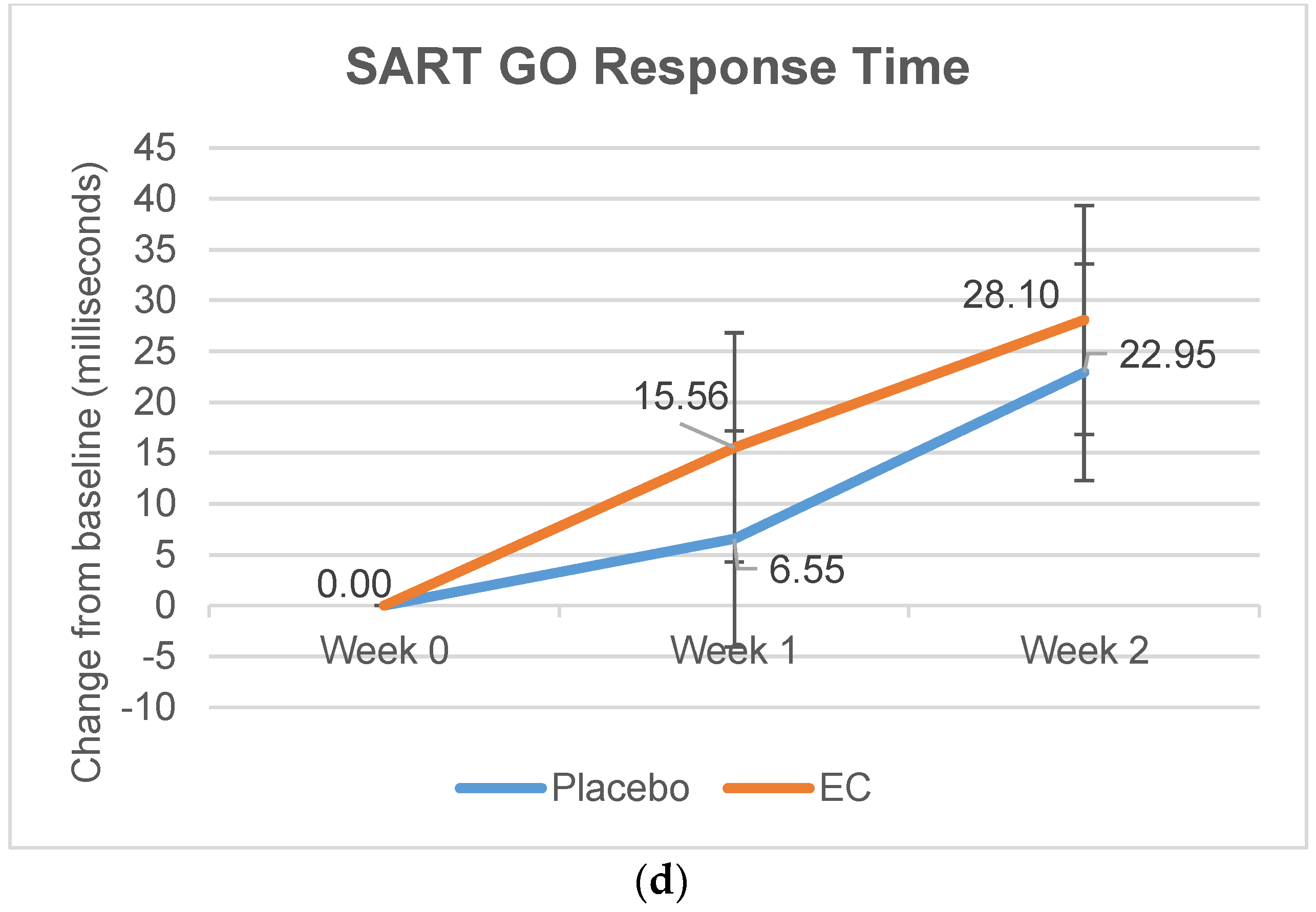
3.2.3. Stroop and SART Tests
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.F.; He, R.R.; Tsoi, B.; Kurihara, H. Bioactivities of chicken essence. J. Food Sci. 2012, 77, R105–R110. [Google Scholar] [CrossRef] [PubMed]
- Geissler, C.; Boroumand-Naini, M.; Tomassen, C. Large acute thermic response to chicken essence in humans. Nutr. Rep. Int. USA 1989, 39, 547–556. [Google Scholar]
- Ikeda, T.; Nishijima, Y.; Kiso, Y.; Shibata, H.; Ono, H.; Moritani, T. Effects of Chicken Essence Tablets on Resting Metabolic Rate. Biosci. Biotechnol. Biochem. 2001, 65, 2083–2086. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.Y.; Lim, J.; Sun, L.; Henry, C.J. Effect of co-ingestion of amino acids with rice on glycaemic and insulinaemic response. Br. J. Nutr. 2015, 114, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tan, K.W.J.; Henry, C.J. Co-ingestion of essence of chicken to moderate glycaemic response of bread. Int. J. Food Sci. Nutr. 2015, 66, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.C.-J.; Tseng, H.-P.; Chang, C.W.; Chien, Y.-Y.; Au, H.K.; Chen, J.-R.; Chen, C.-F. Chicken extract affects colostrum protein compositions in lactating women. J. Nutr. Biochem. 2004, 15, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.; Tsi, D.; Tan, A.C.; Wang, S.; Hsu, M. Effects of postexercise supplementation of chicken essence on the elimination of exercise-induced plasma lactate and ammonia. Chin. J. Physiol. 2005, 48, 187–192. [Google Scholar] [PubMed]
- Young, H.; Benton, D.; Carter, N. The Effect of Chicken Extract on Mood, Cognition and Heart Rate Variability. Nutrients 2015, 7, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.Z.; Razak, A.; Mohsin, S.S.J. The use of chicken essence as an adjunct to psychotherapy in anxious subject—A clinical assessment. Malays. J. Psychiatry 2001, 9, 13–22. [Google Scholar]
- Migita, T.; Mitsuzono, R.; Komiya, S. Effect of BEC (Brands Essence of Chicken) Ingestion on Physical and Mental Fatigue during Intensive Long Distance Running Training. Kurume J. Health Phys. Educ. 1998, 6, 9–14. [Google Scholar]
- Zain, A.M.; Syedsahiljamalulail, S. Effect of taking chicken essence on stress and cognition of human volunteers. Malays. J. Nutr. 2003, 9, 19–29. [Google Scholar] [PubMed]
- Yamano, E.; Tanaka, M.; Ishii, A.; Tsuruoka, N.; Abe, K.; Watanabe, Y. Effects of chicken essence on recovery from mental fatigue in healthy males. Med. Sci. Monit. 2013, 19, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Harada, M.; Nakagawa, M.; Tanaka, T.; Gunadi, B.; Setiabudi, M.L.; Uktolseja, J.L.; Miyata, Y. Effects of chicken extract on the recovery from fatigue caused by mental workload. Appl. Hum. Sci. J. Physiol. Anthropol. 1996, 15, 281–286. [Google Scholar] [CrossRef]
- Azhar, M.Z.; Zubaidah, J.O.; Norjan, K.O.N. Effect of taking chicken essence on cognitive functioning of normal stressed human volunteers. Malays. J. Med. Health Sci. 2008, 4, 57–68. [Google Scholar]
- Benton, D.; Young, H.A. The effect of chicken essence on cognition and mood: A randomized controlled trial. Curr. Top. Nutraceuticals Res. 2015, 13, 61–70. [Google Scholar]
- Teoh, S.L.; Sudfangsai, S.; Lumbiganon, P.; Laopaiboon, M.; Lai, N.M.; Chaiyakunapruk, N. Chicken essence for cognitive function improvement: A systematic review and meta-analysis. Nutrients 2016, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Wang, H.-M.; Chen, K.-Y.; Lin, Y.-C.; Wu, P.-J.; Hsieh, W.-L.; Chen, Y.-R.; Liu, C.-P.; Tsai, H.-Y.; Chen, Y.-R.; et al. Effectiveness of Essence of Chicken in Improving Cognitive Function in Young People Under Work-Related Stress. Medicine 2016, 95, e3640. [Google Scholar] [CrossRef] [PubMed]
- Konagai, C.; Watanabe, H.; Abe, K.; Tsuruoka, N.; Koga, Y. Effects of essence of chicken on cognitive brain function: A near-infrared spectroscopy study. Biosci. Biotechnol. Biochem. 2013, 77, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.-F.; Lord, C.; Andrews, J.; Juster, R.-P.; Sindi, S.; Arsenault-Lapierre, G.; Fiocco, A.J.; Lupien, S.J. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn. Mem. 2011, 96, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Yao, X.-S.; Nagai, H.; Tsuruoka, N.; Shibata, H.; Kiso, Y.; Fukami, H. Anti-Stress Effect of BRAND’S Essence of Chicken (BEC) on Plasma Glucose Levels in Mice Loaded with Restraint Stress. J. Health Sci. 2006, 52, 252–258. [Google Scholar] [CrossRef]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/Carnosine Supplementation Suppresses the Expression of the Inflammatory Chemokine CCL24 in Peripheral Blood Mononuclear Cells from Elderly People. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J. Alzheimers Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily Carnosine and Anserine Supplementation Alters Verbal Episodic Memory and Resting State Network Connectivity in Healthy Elderly Adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Szcześniak, D.; Budzeń, S.; Kopeć, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- Brown, T.A.; Chorpita, B.F.; Korotitsch, W.; Barlow, D.H. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav. Res. Ther. 1997, 35, 79–89. [Google Scholar] [CrossRef]
- Lovibond, S.H. Manual for the Depression Anxiety Stress Scales /S.H. Lovibond, P.F. Lovibond. Available online: http://trove.nla.gov.au/work/30421447?q&sort=holdings+desc&_=1501741463002&versionId=46688692 (accessed on 3 August 2017).
- MacLeod, C.M. Half a century of research on the Stroop effect: An integrative review. Psychol. Bull. 1991, 109, 163–203. [Google Scholar] [CrossRef] [PubMed]
- Homack, S.; Riccio, C.A. A meta-analysis of the sensitivity and specificity of the Stroop Color and Word Test with children. Arch. Clin. Neuropsychol. 2004, 19, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Smilek, D.; Carriere, J.S.A.; Cheyne, J.A. Failures of sustained attention in life, lab, and brain: Ecological validity of the SART. Neuropsychologia 2010, 48, 2564–2570. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.L.; Kishiyama, M.M.; Yund, E.W.; Herron, T.J.; Edwards, B.; Poliva, O.; Hink, R.F.; Reed, B. Improving digit span assessment of short-term verbal memory. J. Clin. Exp. Neuropsychol. 2011, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.H.; Manly, T.; Andrade, J.; Baddeley, B.T.; Yiend, J. ‘Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia 1997, 35, 747–758. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SAS® 9.4 Procedures Guide; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Davidson, D.J.; Zacks, R.T.; Williams, C.C. Stroop Interference, Practice, and Aging. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2003, 10, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Spreen, O.; Strauss, E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary; Oxford University Press: Oxford, UK, 1998; ISBN 978-0-19-510019-8. [Google Scholar]
- Crawford, J.R.; Henry, J.D. The Depression Anxiety Stress Scales (DASS): Normative data and latent structure in a large non-clinical sample. Br. J. Clin. Psychol. 2003, 42, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Conway, A.R.A.; Kane, M.J.; Bunting, M.F.; Hambrick, D.Z.; Wilhelm, O.; Engle, R.W. Working memory span tasks: A methodological review and user’s guide. Psychon. Bull. Rev. 2005, 12, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.H.; Ward, T.; Ridgeway, V.; Nimmo-Smith, I. The structure of normal human attention: The Test of Everyday Attention. J. Int. Neuropsychol. Soc. 1996, 2, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.C.K.; Hoosain, R.; Lee, T.M.C. Reliability and validity of the Cantonese version of the Test of Everyday Attention among normal Hong Kong Chinese: A preliminary report. Clin. Rehabil. 2002, 16, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.C.K.; Lai, M.K.; Robertson, I.H. Latent structure of the Test of Everyday Attention in a non-clinical Chinese sample. Arch. Clin. Neuropsychol. 2006, 21, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Yang, R.; Zhang, Z.; Gao, L.; Wang, J.; Xu, Y.; Zhao, M.; Han, X.; Liu, Z.; Li, Y. Marine collagen peptide isolated from Chum Salmon (Oncorhynchus keta) skin facilitates learning and memory in aged C57BL/6J mice. Food Chem. 2010, 118, 333–340. [Google Scholar] [CrossRef]
- Xu, L.; Dong, W.; Zhao, J.; Xu, Y. Effect of Marine Collagen Peptides on Physiological and Neurobehavioral Development of Male Rats with Perinatal Asphyxia. Mar. Drugs 2015, 13, 3653–3671. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; Vecchio, G.; Cuzzocrea, S.; Calabrese, V.; Rizzarelli, E. Neuroprotective features of carnosine in oxidative driven diseases. Mol. Asp. Med. 2011, 32, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Stvolinsky, S.L.; Fedorova, T.N.; Suslina, Z.A. Carnosine as a Natural Antioxidant and Geroprotector: From Molecular Mechanisms to Clinical Trials. Rejuvenation Res. 2009, 13, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Hase, A.; Jung, S.E.; aan het Rot, M. Behavioral and cognitive effects of tyrosine intake in healthy human adults. Pharmacol. Biochem. Behav. 2015, 133, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jongkees, B.J.; Hommel, B.; Kühn, S.; Colzato, L.S. Effect of tyrosine supplementation on clinical and healthy populations under stress or cognitive demands—A review. J. Psychiatr. Res. 2015, 70, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, I.; Fujimura, N.; Nozawa, Y.; Furuhata, Y.; Sato, H. The effect of histidine on mental fatigue and cognitive performance in subjects with high fatigue and sleep disruption scores. Physiol. Behav. 2015, 147, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Ahmad, S.; Madiha, S.; Khaliq, S.; Shahzad, S.; Batool, Z.; Haider, S. Impact of oral supplementation of Glutamate and GABA on memory performance and neurochemical profile in hippocampus of rats. Pak. J. Pharm. Sci. 2017, 30, 1013–1021. [Google Scholar] [PubMed]
- Ohtsuka, Y.; Nakaya, J. Effect of oral administration of L-arginine on senile dementia. Am. J. Med. 2000, 108, 439. [Google Scholar] [CrossRef]
- Hosseini, M.; Anaeigoudari, A.; Beheshti, F.; Soukhtanloo, M.; Nosratabadi, R. Protective effect against brain tissues oxidative damage as a possible mechanism for beneficial effects of L-arginine on lipopolysaccharide induced memory impairment in rats. Drug Chem. Toxicol. 2018, 41, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Reddy, L.; Ekambaram, P. Prevention of picrotoxin convulsions-induced learning and memory impairment by nitric oxide increasing dose of L-arginine in rats. Pharmacol. Biochem. Behav. 2003, 75, 329–334. [Google Scholar] [CrossRef]
- Dos Santos, F.S.; da Silva, L.A.; Pochapski, J.A.; Raczenski, A.; da Silva, W.C.; Grassiolli, S.; Malfatti, C.R.M. Effects of L-arginine and creatine administration on spatial memory in rats subjected to a chronic variable stress model. Pharm. Biol. 2014, 52, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E.; Hassmén, P.; Ekblom, B.; Newsholme, E.A. Administration of branched-chain amino acids during sustained exercise—Effects on performance and on plasma concentration of some amino acids. Eur. J. Appl. Physiol. 1991, 63, 83–88. [Google Scholar] [CrossRef]
- Blomstrand, E.; Hassmén, P.; Newsholme, E.A. Effect of branched-chain amino acid supplementation on mental performance. Acta Physiol. Scand. 1991, 143, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Hassmén, P.; Blomstrand, E.; Ekblom, B.; Newsholme, E.A. Branched-chain amino acid supplementation during 30-km competitive run: Mood and cognitive performance. Nutrition 1994, 10, 405–410. [Google Scholar] [PubMed]
- Blomstrand, E.; Hassmén, P.; Ek, S.; Ekblom, B.; Newsholme, E.A. Influence of ingesting a solution of branched-chain amino acids on perceived exertion during exercise. Acta Physiol. Scand. 1997, 159, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids 2013, 45, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Branched-chain amino acids and brain function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef] [PubMed]
- Gignac, G.E.; Weiss, L.G. Digit Span is (mostly) related linearly to general intelligence: Every extra bit of span counts. Psychol. Assess. 2015, 27, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV); NCS Pearson: San Antonio, TX, USA, 2008; Volume 22, p. 498. [Google Scholar]
- Gignac, G.E. The magical numbers 7 and 4 are resistant to the Flynn effect: No evidence for increases in forward or backward recall across 85 years of data. Intelligence 2015, 48, 85–95. [Google Scholar] [CrossRef]
- Gignac, G.E.; Kovacs, K.; Reynolds, M.R. Backward and forward serial recall across modalities: An individual differences perspective. Personal. Individ. Differ. 2018, 121, 147–151. [Google Scholar] [CrossRef]
| Content | Essence of Chicken (EC) | Placebo |
|---|---|---|
| Solid (g/100 g) | 8.02 | 9.32 |
| Protein (g/100 g) | 7.51 | 8.10 |
| Carbohydrate (g/100 g) | 0.00 | 0.76 |
| Fat (g/100 g) * | 0.06 | 0.06 |
| Ash (g/100 g) | 0.58 | 0.40 |
| Energy (Kcal/100 g) | 30.58 | 35.98 |
| Number (%) Unless Otherwise Stated | ||
|---|---|---|
| Demographic and Baseline Characteristics | EC n = 117 | Placebo n = 118 |
| Age (years), mean (SD) | 22.9 (4.4) | 22.7(4.8) |
| Male | 36 (30.8) | 38 (31.2) |
| Female | 81 (69.2) | 80 (67.8) |
| Height (m), mean (SD) | 1.63 (0.09) | 1.65 (0.07) |
| Weight (kg), mean (SD) | 54.8 (7.5) | 55.9 (6.4) |
| BMI (kg/m2), Mean (SD) | 20.3 (1.5) | 20.5 (1.3) |
| Highest Education Completed | ||
| Less than high school | 0 (0.00) | 1 (0.85) |
| High school | 80 (68.4) | 82 (69.5) |
| Diploma | 4 (3.42) | 0 (0.00) |
| University Degree | 29 (24.8) | 31 (26.3) |
| Masters’ Degree | 4 (3.42) | 4 (3.39) |
| Alcohol consumption | ||
| None | 94 (80.3) | 91 (77.1) |
| 1 drink per week | 15 (12.8) | 18 (15.3) |
| >1 drink per week | 8 (6.8) | 9 (7.63) |
| Currently smoking | ||
| Yes | 4 (3.42) | 8 (6.78) |
| No | 107 (96.6) | 110 (93.2) |
| Past Medical Conditions * | ||
| Diabetes Mellitus Type II | 1 (0.85) | 2 (1.69) |
| Elevated markers of renal insufficiency | 1 (0.85) | 0 (0.0) |
| Hypertension | 0 (0.0) | 1 (0.85) |
| Pelvic floor dysfunction | 1 (0.85) | 1 (0.85) |
| Pelvic or Abdominal surgery | 2 (1.71) | 1 (0.85) |
| Gastrointestinal disorder | 2 (1.71) | 1 (0.85) |
| Stress Category # | ||
| Normal | 57 (48.7) | 62 (52.5) |
| Mild | 27 (23.1) | 16 (13.6) |
| Moderate | 24 (20.5) | 25 (21.2) |
| Severe/Extremely Severe | 9 (7.69) | 15 (12.7) |
| Cognitive Function Test α | Mean (SD) Score at Baseline | Mean (SD) Score Change from Baseline | ||||
|---|---|---|---|---|---|---|
| EC n = 117 | P n = 118 | Day 7 | Day 14 | |||
| EC | P | EC | P | |||
| WAIS Digit Span | ||||||
| Forward span | 7.88 (1.66) | 7.98 (1.51) | 0.547 (1.53) | 0.407 (1.58) | 0.855 (1.84) | 0.568 (1.86) |
| Backward span | 7.06 (2.00) | 7.16 (2.06) | 1.12 (1.95) | 0.78 (1.81) | 1.34 (2.13) | 1.08 (2.01) |
| Stroop | ||||||
| Proportion Correct, Congruent | 0.98 (0.03) | 0.99 (0.03) | −0.005 (0.033) | −0.005 (0.039) | −0.007 (0.035) | −0.011 (0.041) |
| Proportion Correct, Incongruent | 0.94 (0.08) | 0.94 (0.07) | 0.024 (0.077) | 0.020 (0.072) | 0.028 (0.079) | 0.012 (0.081) |
| Reaction Time, Congruent | 972.15 (278.02) | 996.04 (350.55) | −134.85 (235.57) | −143.82 (237.90) | −204.96 (209.18) | −225.14 (265.94) |
| Reaction Time, Incongruent | 1116.45 (326.09) | 1173.97 (412.54) | −128.83 (316.55) | −202.99 (304.21) | −248.68 (262.49) | −272.52 (330.90) |
| Stoop Interference Index (ms) | 144.30 (192.97) | 177.93 (214.92) | 6.02 (269.59) | −59.174 (257.97) | −43.71 (227.18) | −47.37 (218.06) |
| SART | ||||||
| % No-Go Success | 36.75 (26.92) | 38.88 (27.22) | 11.27 (21.28) | 7.50 (26.48) | 15.56 (24.84) | 13.15 (28.36) |
| % No-Go Omissions | 2.11 (2.94) | 2.08 (4.38) | 0.24 (3.83) | 0.10 (4.43) | 0.03 (4.64) | −0.13 (5.06) |
| Reaction Time, Mean (ms) | 341.02 (92.62) | 348.04 (92.44) | 21.79 (94.10) | 10.60 (98.88) | 38.78 (108.43) | 20.28 (96.11) |
| Cognitive Function Test α | Mean (SD) Score at Baseline | Mean (SD) Score Change from Baseline | ||||
|---|---|---|---|---|---|---|
| EC n = 117 # | P n = 118 * | Day 7 | Day 14 | |||
| EC | P | EC | P | |||
| Digit Span Forward | ||||||
| Normal | 8.26 (1.75) | 7.81 (1.56) | 0.26 (1.62) | 0.68 (1.50) | 0.54 (1.65) | 0.77 (1.93) |
| Mild Stress | 7.89 (1.40) | 8.19 (1.64) | 0.52 (1.40) | −0.06 (1.77) | 0.70 (2.03) | −0.19 (2.37) |
| Moderate Stress | 7.33 (1.49) | 8.04 (1.51) | 1.00 (1.25) | 0.32 (1.46) | 1.58 (1.59) | 0.80 (1.35) |
| Severe Stress | 6.89 (1.62) | 8.4 (1.18) | 1.22 (1.79) | −0.07 (1.79) | 1.33 (2.60) | 0.13 (1.51) |
| Digit Span Backward | ||||||
| Normal | 7.05 (2.16) | 6.94 (2.02) | 1.16 (2.08) | 1.02 (1.86) | 1.49 (2.19) | 1.39 (2.03) |
| Mild Stress | 7.26 (2.11) | 7.56 (2.22) | 0.33 (1.90) | 0.25 (1.57) | 0.63 (1.80) | 0.00 (2.10) |
| Moderate Stress | 7.08 (1.64) | 7.28 (2.20) | 1.58 (1.41) | 0.60 (1.91) | 1.58 (2.30) | 1.32 (2.04) |
| Severe Stress | 6.44 (1.67) | 7.47 (1.85) | 2.0 (1.94) | 0.67 (1.68) | 1.89 (1.96) | 0.60 (1.35) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suttiwan, P.; Yuktanandana, P.; Ngamake, S. Effectiveness of Essence of Chicken on Cognitive Function Improvement: A Randomized Controlled Clinical Trial. Nutrients 2018, 10, 845. https://doi.org/10.3390/nu10070845
Suttiwan P, Yuktanandana P, Ngamake S. Effectiveness of Essence of Chicken on Cognitive Function Improvement: A Randomized Controlled Clinical Trial. Nutrients. 2018; 10(7):845. https://doi.org/10.3390/nu10070845
Chicago/Turabian StyleSuttiwan, Panrapee, Pongsak Yuktanandana, and Sakkaphat Ngamake. 2018. "Effectiveness of Essence of Chicken on Cognitive Function Improvement: A Randomized Controlled Clinical Trial" Nutrients 10, no. 7: 845. https://doi.org/10.3390/nu10070845
APA StyleSuttiwan, P., Yuktanandana, P., & Ngamake, S. (2018). Effectiveness of Essence of Chicken on Cognitive Function Improvement: A Randomized Controlled Clinical Trial. Nutrients, 10(7), 845. https://doi.org/10.3390/nu10070845





