Association between Dietary Inflammatory Index, C-Reactive Protein and Metabolic Syndrome: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Data Collection
2.3. Calculation of DII Scores
2.4. Identification of the Metabolic Syndrome
2.5. Ethics
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Incidence of MetS and DII/CRP
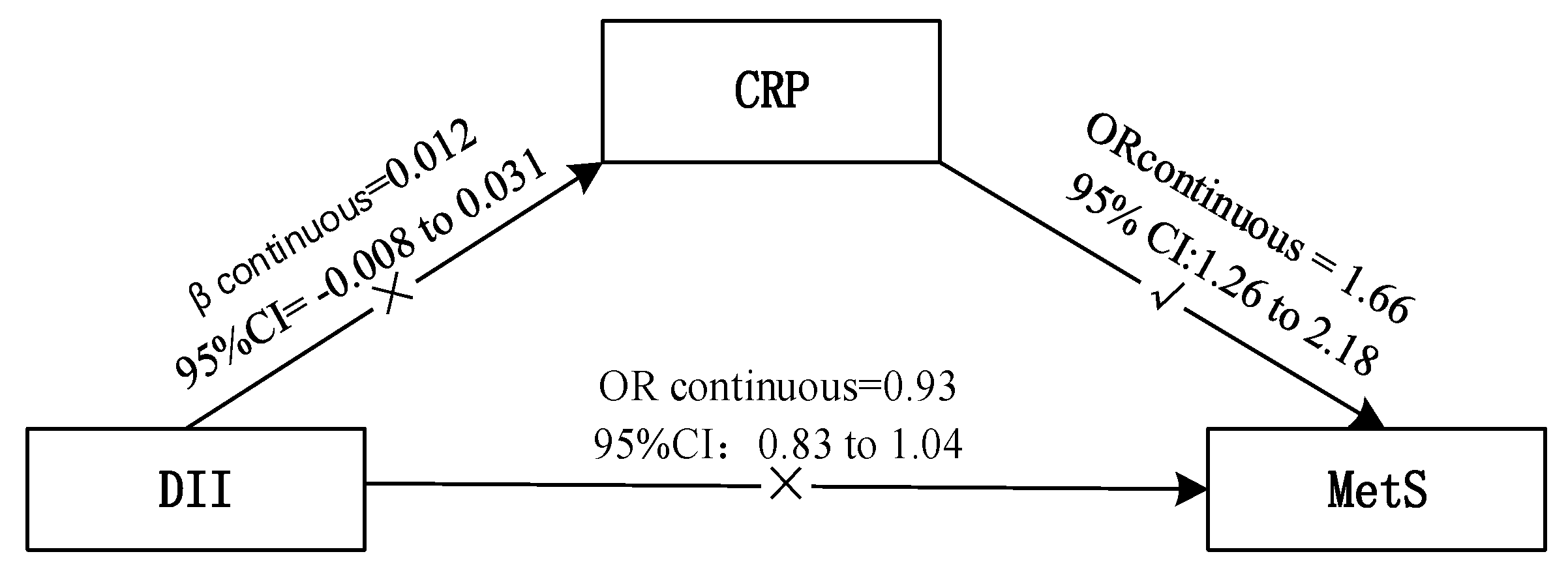
3.3. DII and CRP
3.4. DII and the Frequency of “Shanghuo”
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, P.T.; Loria, P.Z.; Smith, S.J. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, L.; Li, M.; Xu, Y.; Jiang, Y.; Wang, W.; Li, J.; Mi, S.; Zhang, M.; Li, Y.; et al. Metabolic syndrome among adults in China—The 2010 China Noncommunicable Disease Surveillance. J. Clin. Endocrinol. Metab. 2017, 102, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Hernandez, B.P.; Hernandez-Suarez, D.F. Linking chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.J. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Jin, X.; Liang, X.; Zhao, Q.; Zhang, X. Inflammatory mediators involved in the progression of the metabolic syndrome. Diabetes Metab. Res. Rev. 2012, 28, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Hirabara, S.M.; Gorjao, R.; Vinolo, M.A.; Rodrigues, A.C.; Nachbar, R.T.; Curi, R. Molecular targets related to inflammation and insulin resistance and potential interventions. J. Biomed. Biotechnol. 2012, 2012, 379024. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Pennathur, S.; Afshinnia, F. Link of dietary patterns with metabolic syndrome: Analysis of the National Health and Nutrition Examination Survey. Nutr. Diabetes 2017, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Wang, H.; Wang, Z.; Du, W.; Ouyang, Y.; Zhang, B. Relationship between dietary factors and the number of altered metabolic syndrome components in Chinese adults: A cross-sectional study using data from the China Health and Nutrition Survey. BMJ Open 2017, 7, e014911. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Syauqy, A.; Hsu, C.Y.; Rau, H.H.; Chao, J.C. Association of dietary patterns with components of metabolic syndrome and inflammation among middle-aged and older adults with metabolic syndrome in Taiwan. Nutrients 2018, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, M.; Hermansen, K.; Savolainen, M.J.; Schwab, U.; Kolehmainen, M.; Brader, L.; Mortensen, L.S.; Cloetens, L.; Johansson-Persson, A.; Onning, G.; et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome—A randomized study (SYSDIET). J. Intern Med. 2013, 274, 52–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new Dietary Inflammatory Index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Santulli, G. Integrating diet and inflammation to calculate cardiovascular risk. Atherosclerosis 2016, 253, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Bes-Rastrollo, M.; Martínez-González, M.A. The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int. J. Mol. Sci. 2016, 17, 1265. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Huang, Z.S.; Wu, Q.G.; Huang, Z.J.; Wu, L.R.; Yan, W.L.; Chang, D.L.; Yang, Z.; Wang, Z.W. Quantization and diagnosis of Shanghuo (Heatiness) in Chinese medicine using a diagnostic scoring scheme and salivary biochemical parameters. Chin. Med. 2014, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, N.; Gong, J.; Ni, S.F.; Luo, R.F.; Zhao, T.; Lu, F.; Wu, Y.B.; Cao, M.Y. “Shanghuo” “Inflammatory” and the Relationship between Free Radicals (in Chinese). J. Liaoning Univ. TCM 2011, 13, 87–89. [Google Scholar]
- Zhao, A.; Szeto, I.M.; Wang, Y.; Li, C.; Pan, M.; Li, T.; Wang, P.; Zhang, Y. Knowledge, Attitude, and Practice (KAP) of Dairy Products in Chinese Urban Population and the Effects on Dairy Intake Quality. Nutrients 2017, 9, 668. [Google Scholar] [CrossRef]
- Yang, Y. China Food Composition, 1st ed.; Peking University Medical Press: Beijing, China, 2004. [Google Scholar]
- Yang, Y. China Food Composition, 2nd ed.; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J Clin. Nutr. 1997, 65, S1220–S1228. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Burch, J.; Shivappa, N.; Violanti, J.M.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E.; Hartley, T.A.; Miller, D.B.; Mnatsakanova, A.; et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J. Occup. Environ. Med. 2014, 56, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Sokol, A.; Wirth, M.D.; Manczuk, M.; Shivappa, N.; Zatonska, K.; Hurley, T.G.; Hébert, J.R. Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr. Res. 2016, 36, 1298–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkerwi, A.A.; Shivappa, N.; Crichton, G.; Hébert, J.R. No significant independent relationships with cardiometabolic biomarkers were detected in the Observation of Cardiovascular Risk Factors in Luxembourg study population. Nutr. Res. 2014, 34, 1058–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufcourt, L.; Assmann, K.E.; Fezeu, L.K.; Touvier, M.; Graffouillère, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Prospective association between the dietary inflammatory index and metabolic syndrome: Findings from the SU.VI.MAX study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, A.M.; Toledo, E.; Rodriguez-Diez, M.C.; Gea, A.; Lopez-Iracheta, R.; Shivappa, N.; Hébert, J.R.; Martinez-Gonzalez, M.A. Dietary indexes, food patterns and incidence of metabolic syndrome in a Mediterranean cohort: The SUN project. Clin. Nutr. 2015, 34, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naja, F.; Shivappa, N.; Nasreddine, L.; Kharroubi, S.; Itani, L.; Hwalla, N.; Mehio Sibai, A.; Hebert, J.R. Role of inflammation in the association between the western dietary pattern and metabolic syndrome among Lebanese adults. Int. J. Food Sci. Nutr. 2017, 68, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arellano, A.; Ramallal, R.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Shivappa, N.; Schröder, H.; Hébert, J.R.; Ros, E.; Gómez-Garcia, E.; et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the PREDIMED Study. Nutrients 2015, 7, 4124–4138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfutzner, A.; Schöndorf, T.; Hanefeld, M.; Forst, T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: Effects of insulin-sensitizing treatment with pioglitazone. J. Diabetes Sci. Technol. 2010, 4, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.K.; Devaraj, S.; Yuhanna, I.; Shaul, P.; Jialal, I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 2002, 106, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, S.; Wen, J.; Yang, Y.; Yang, Z.; Zhang, Z.; Wang, X.; Hu, R. The New Unified International Diabetes Federation/American Heart Association/National Heart, Lung, and Blood Institute Metabolic Syndrome definition: Does it correlate better with C-reactive protein in Chinese patients diagnosed with type 2 diabetes? J. Int. Med. Res. 2010, 38, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Z.; Ma, R.L.; Ding, Y.S.; Guo, H.; Zhang, J.Y.; Mu, L.T.; Zhang, M.; Liu, J.M.; Rui, D.A.; He, J.; et al. Association of inflammation with metabolic syndrome among low-income rural Kazakh and Uyghur Adults in Far Western China. Mediators Inflamm. 2015, 2015, 706768. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; Kintscher, U.; et al. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. Eur. Heart J. 2016, 37, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Shivappa, N.; Hurley, T.G.; Hébert, J.R. Association between previously diagnosed circulatory conditions and a dietary inflammatory index. Nutr. Res. 2016, 36, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, M.M.; Li, C.I.; Kardia, S.L.; Liu, C.S.; Lin, W.Y.; Lee, Y.D.; Chang, P.C.; Lin, C.C.; Li, T.C. Sex difference in the association of metabolic syndrome with high sensitivity C-reactive protein in a Taiwanese population. BMC Public Health 2010, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Najafi, M. Dietary inflammatory index: A potent association with cardiovascular risk factors among patients candidate for coronary artery bypass grafting (CABG) surgery. Nutr. J. 2018, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Kim, M.; Sohn, C. Dietary inflammatory index and its relationship with high-sensitivity C-reactive protein in Korean: Data from the health examinee cohort. J. Clin. Biochem. Nutr. 2018, 62, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Shivappa, N.; Davis, L.; Hurley, T.G.; Ortaglia, A.; Drayton, R.; Blair, S.N.; Hébert, J.R. Construct validation of the Dietary Inflammatory Index among African Americans. J. Nutr. Health Aging 2017, 21, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Rietzschel, E.R.; Marcos, A.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; González-Gil, E.; Frederic, G.; et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Ghayour-Mobarhan, M.; Yaghootkar, H.; Lanham-New, S.A.; Lamb, D.J.; Ferns, G.A. Association between serum CRP concentrations with dietary intake in healthy and dyslipidaemic patients. Asia Pac. J. Clin. Nutr. 2007, 16, 262–268. [Google Scholar] [PubMed]
- Ohsawa, M.; Itai, K.; Onoda, T.; Tanno, K.; Sasaki, S.; Nakamura, M.; Ogawa, A.; Sakata, K.; Kawamura, K.; Kuribayashi, T.; et al. Dietary intake of n-3 polyunsaturated fatty acids is inversely associated with CRP levels, especially among male smokers. Atherosclerosis 2008, 201, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, Q.I.; Li, S.M.; Zhu, Y.F.; Chen, N.N.; Zheng, W.J.; Xie, Z.J.; Fan, Y.S. Case-control study of inducing factors in ‘Shanghuo’. Chin. J. Tradit. Chin. Med. 2015, 30, 1013–1016. (In Chinese) [Google Scholar]
| Variables | Dietary Inflammatory Index | |||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | pa | |
| N | 566 | 567 | 579 | |
| DII scores | −0.91(0.74) | 0.62(0.30) | 1.63(0.38) | |
| Age | 53.8(15.7) | 51.5(17.6) | 46.2(18.0) | <0.001 * |
| BMI (kg/m2) | 24.0(3.6) | 23.8(3.4) | 23.6(3.6) | 0.157 |
| Gender | 0.047 * | |||
| Male | 173(30.6) | 192(33.9) | 217(37.5) | |
| Female | 393(69.4) | 375(66.1) | 362(62.5) | |
| Education | 0.218 | |||
| Never | 28(4.9) | 16(2.8) | 30(5.2) | |
| Secondary or under | 155(27.4) | 176(31.2) | 160(27.8) | |
| high or equal | 242(42.8) | 238(42.1) | 222(38.6) | |
| Bachelor | 118(20.8) | 108(19.1) | 136(23.7) | |
| Master or above | 23(4.1) | 27(4.8) | 27(4.7) | |
| Family monthly income (RMB: yuan) | 0.105 | |||
| ≤3000 | 113(20.1) | 114(20.2) | 134(23.3) | |
| 3000–4999 | 144(25.6) | 169(30.0) | 176(30.6) | |
| 5000–7999 | 138(24.5) | 106(18.8) | 122(21.2) | |
| 8000–9999 | 61(10.8) | 54(9.6) | 57 (9.9) | |
| 10,000–14,999 | 58 (10.3) | 66(11.7) | 48(8.3) | |
| ≥15,000 | 49(8.7) | 55(9.8) | 39(6.8) | |
| Family monthly expenditure on food (RMB: yuan) | <0.001 * | |||
| ≤500 | 30(5.3) | 36(6.4) | 52(9.0) | |
| 500–999 | 112(19.8) | 138(24.4) | 143(24.8) | |
| 1000–2999 | 288(50.9) | 288(50.9) | 302(52.3) | |
| 3000–4999 | 104(18.4) | 68(12.0) | 65(11.3) | |
| ≥5000 | 32(5.7) | 36(6.4) | 15(2.6) | |
| City | 0.002 * | |||
| Beijing | 120(21.2) | 105(18.5) | 101(17.4) | |
| Chengdu | 53(9.4) | 51(9.0) | 64(11.1) | |
| Chenzhou | 57(10.1) | 45(7.9) | 71(12.3) | |
| Jilin | 33(5.8) | 69(12.2) | 63(10.9) | |
| Guangzhou | 122(21.6) | 107(18.9) | 97(16.8) | |
| Lanzhou | 64(11.3) | 60(10.6) | 51(8.8) | |
| Wuhu | 59(10.4) | 58(10.2) | 79(13.6) | |
| Xuchang | 58(10.3) | 72(12.7) | 53(9.2) | |
| Geographic location | 0.029 * | |||
| South | 291(51.4) | 261(46.0) | 311(53.7) | |
| North | 275(48.6) | 306(54.0) | 268(46.3) | |
| Physical activity | 0.499 | |||
| Low | 109(20.3) | 100(19.3) | 119(21.5) | |
| Medium | 301(56.2) | 291(56.3) | 286(51.6) | |
| High | 126(23.5) | 126(24.4) | 149(26.9) | |
| Smoking status | 0.102 | |||
| Non-smoker | 436(77.7) | 431(76.6) | 431(74.7) | |
| Former smoker | 68(12.1) | 52(9.2) | 62(10.8) | |
| Smoker | 57(10.2) | 80(14.2) | 84(14.6) | |
| Nutrients | Dietary Inflammatory Index | |||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | pb | |
| Carbohydrate (g) | 280.8(239.0,323.5) | 288.2(241.9,326.8) | 272.6(221.7,318.6) | 0.003 * |
| Protein (g) | 73.2(62.4,84.8) | 65.2(56.3,76.4) | 57.8(49.6,67.5) | <0.001 * |
| Total fat (g) | 72.7(54.8,89.2) | 69(53.7,86.3) | 76(57.8,96.7) | <0.001 * |
| Saturated fat (g) | 10.4(6.7,14.5) | 10.2(6.9,14.5) | 10.1(6.7,16.2) | 0.674 |
| MUFA (g) | 19.8(13,27.5) | 18.9(11.8,28.6) | 21(13.6,32.2) | 0.010 * |
| PUFA (g) | 15.4(10.2,22.2) | 13.9(9.9,18.7) | 11.6(8.2,16.2) | <0.001 * |
| Fiber (g) | 17.7(13.3,24.4) | 11(8.6,13.8) | 7.3(5.7,9.5) | <0.001 * |
| Cholesterol (mg) | 341.1(107.5,600.0) | 304.5(111.2,625.6) | 349.3(122.3,564.5) | 0.911 |
| Niacin (mg) | 15.4(12.3,19.5) | 13.3(10.9,16.6) | 11.4(9.3,14.0) | <0.001 * |
| Thiamine (mg) | 1.1(0.9,1.3) | 1.0(0.8,1.2) | 0.9(0.7,1.0) | <0.001 * |
| Riboflavin (mg) | 1.1(0.9,1.5) | 0.9(0.7,1.2) | 0.7(0.6,1.0) | <0.001 * |
| Folic acid (μg) | 349.9(243.5,469.6) | 230.9(165.9,300.2) | 154.7(116.7,196.0) | <0.001 * |
| Vitamins A (RE) | 635.2(388.1,1054.7) | 379.9(222.6,574.2) | 278.8(169.4,408.6) | <0.001 * |
| Vitamins B6 (mg) | 1.3(1.1,1.6) | 1.0(0.8,1.2) | 0.8(0.7,1.0) | <0.001 * |
| Vitamins B12 (μg) | 2.3(0.8,5.1) | 2.2(0.8,4.7) | 1.9(0.9,3.5) | 0.011 * |
| Vitamins C (mg) | 141.8(85.6,198.2) | 74.8(41.3,109.9) | 39.4(20.9,60.9) | <0.001 * |
| Vitamins E (mg) | 33.1(23.7,43.8) | 25.1(17.6,37.0) | 17.8(12.3,30.5) | <0.001 * |
| Iron (mg) | 24.9(21.7,30.3) | 20.5(18.2,23.4) | 17.4(15.4,19.5) | <0.001 * |
| Magnesium (mg) | 368.2(322.8,440.2) | 288.5(258.5,327.7) | 226.4(194.3,262.8) | <0.001 * |
| Selenium (μg) | 44.6(33.7,59.2) | 41.9(31.3,55.4) | 38.8(29.9,48.8) | <0.001 * |
| Zinc (mg) | 13.1(11.7,14.9) | 11.4(10.3,12.8) | 10.0(9.1,11.3) | <0.001 * |
| MetS/Components | Dietary Inflammatory Index OR (95% CI) | CRP (Continuous) OR (95% CI) | |||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Continuous | ||
| Metabolic Syndrome | |||||
| Model 1 b | 1.00(Ref.) | 0.74(0.58–0.95) | 0.72(0.56–0.92) | 0.85(0.78–0.92) | 3.83(3.05–4.80) |
| Model 2 c | 1.00(Ref.) | 0.76(0.56–1.03) | 1.02(0.75–1.40) | 0.93(0.83–1.04) | 1.66(1.26–2.18) * |
| Waist circumference | |||||
| Model 1 b | 1.00(Ref.) | 0.86(0.68–1.09) | 0.69(0.54–0.86) | 0.85(0.79–0.93) | 4.61(3.67–5.79) |
| Model 2 c | 1.00(Ref.) | 0.92(0.64–1.32) | 0.86(0.59–1.24) | 0.93(0.81–1.06) | 1.91(1.36–2.66) * |
| Blood pressure | |||||
| Model 1 b | 1.00(Ref.) | 0.96(0.76–1.21) | 0.86(0.68–1.08) | 0.91(0.84–0.99) | 2.80(2.28–3.45) |
| Model 2 c | 1.00(Ref.) | 1.04(0.77–1.39) | 1.40(1.03–1.89) * | 1.06(0.96–1.18) | 1.17(0.89–1.53) |
| HDL-cholesterol | |||||
| Model 1 b | 1.00(Ref.) | 0.90(0.69–1.17) | 1.01(0.78–1.31) | 0.96(0.88–1.06) | 2.13(1.72–2.64) |
| Model 2 c | 1.00(Ref.) | 0.95(0.71–1.26) | 1.17(0.88–1.56) | 1.02(0.92–1.12) | 1.64(1.27–2.11) * |
| Triglycerides | |||||
| Model 1 b | 1.00(Ref.) | 0.69(0.54–0.88) | 0.73(0.58–0.93) | 0.88(0.81–0.96) | 3.01(2.43–3.72) |
| Model 2 c | 1.00(Ref.) | 0.73(0.55–0.96) | 1.03(0.78–1.37) | 0.99(0.90–1.09) | 1.44(1.12–1.85) * |
| Fasting glucose | |||||
| Model 1 b | 1.00(Ref.) | 0.78(0.61–1.00) | 0.71(0.56–0.92) | 0.86(0.79–0.94) | 2.47(2.00–3.05) |
| Model 2 c | 1.00(Ref.) | 0.78(0.59–1.04) | 0.85(0.64–1.14) | 0.91(0.82–1.00) | 1.73(1.34–2.24) * |
| MetS/Components | Dietary Inflammatory Index OR (95% CI) | CRP (Continuous) OR (95% CI) | |||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Continuous | ||
| Males | |||||
| Metabolic Syndrome | |||||
| Model 1 b | 1.00(Ref.) | 0.86(0.56–1.32) | 0.86(0.57–1.30) | 0.92(0.80–1.07) | 1.67(1.15–2.41) |
| Model 2 c | 1.00(Ref.) | 0.91(0.55–1.50) | 1.06(0.64–1.75) | 0.97(0.82–1.16) | 1.09(0.70–1.70) |
| Waist circumference | |||||
| Model 1 b | 1.00(Ref.) | 1.03(0.68–1.57) | 0.97(0.65–1.46) | 0.95(0.82–1.09) | 2.63(1.79–3.87) |
| Model 2 c | 1.00(Ref.) | 1.07(0.59–1.96) | 1.21(0.66–2.23) | 0.99(0.80–1.23) | 1.73(0.97–3.08) |
| Blood pressure | |||||
| Model 1 b | 1.00(Ref.) | 1.08(0.70–1.66) | 0.72(0.48–1.08) | 0.87(0.75–1.01) | 1.45(1.00–2.09) |
| Model 2 c | 1.00(Ref.) | 1.25(0.76–2.07) | 1.04(0.63–1.70) | 0.99(0.83–1.17) | 0.97(0.62–1.53) |
| HDL-Cholesterol | |||||
| Model 1 b | 1.00(Ref.) | 1.09(0.64–1.86) | 1.40(0.84–2.31) | 1.08(0.91–1.30) | 1.65(1.07–2.53) |
| Model 2 c | 1.00(Ref.) | 1.16(0.65–2.07) | 1.47(0.83–2.58) | 1.10(0.90–1.35) | 1.31(0.80–2.17) |
| Triglycerides | |||||
| Model 1 b | 1.00(Ref.) | 0.77(0.50–1.17) | 0.76(0.50–1.13) | 0.93(0.81–1.08) | 1.52(1.06–2.18) |
| Model 2 c | 1.00(Ref.) | 0.75(0.47–1.19) | 0.78(0.49–1.20) | 0.94(0.80–1.11) | 0.92(0.61–1.39) |
| Fasting glucose | |||||
| Model 1 b | 1.00(Ref.) | 0.88(0.58–1.33) | 0.67(0.45–1.01) | 0.85(0.75–0.94) | 1.81(1.26–2.61) |
| Model 2 c | 1.00(Ref.) | 0.96(0.60–1.54) | 0.90(0.56–1.44) | 0.91(0.77–1.08) | 1.79(1.17–2.73) * |
| Females | |||||
| Metabolic Syndrome | |||||
| Model 1 b | 1.00(Ref.) | 0.69(0.51–0.93) | 0.65(0.48–0.87) | 0.80(0.72–0.89) | 5.96(4.43–8.02) |
| Model 2 c | 1.00(Ref.) | 0.67(0.45–1.00) | 1.01(0.66–1.53) | 0.90(0.78–1.04) | 2.17(1.49–3.15) * |
| Waist circumference | |||||
| Model 1 b | 1.00(Ref.) | 0.81(0.61–1.07) | 0.59(0.44–0.79) | 0.82(0.74–0.91) | 6.70(4.99–9.00) |
| Model 2 c | 1.00(Ref.) | 0.91(0.56–1.46) | 0.74(0.45–1.20) | 0.92(0.77–1.08) | 1.85(1.20–2.88) * |
| Blood pressure | |||||
| Model 1 b | 1.00(Ref.) | 0.87(0.65–1.16) | 0.86(0.64–1.15) | 0.90(0.81–0.99) | 3.53(2.72–4.58) |
| Model 2 c | 1.00(Ref.) | 0.90(0.62–1.32) | 1.72(1.15–2.56) * | 1.12(0.98–1.28) | 1.25(0.88–1.77) |
| HDL-Cholesterol | |||||
| Model 1 b | 1.00(Ref.) | 0.86(0.63–1.17) | 0.94(0.69–1.28) | 0.94(0.84–1.05) | 2.50(1.94–3.23) |
| Model 2 c | 1.00(Ref.) | 0.90(0.65–1.25) | 1.06(0.76–1.49) | 0.99(0.88–1.11) | 1.77(1.31–2.38) * |
| Triglycerides | |||||
| Model 1 b | 1.00(Ref.) | 0.64(0.47–0.86) | 0.70(0.52–0.94) | 0.84(0.76–0.94) | 4.15(3.16–5.45) |
| Model 2 c | 1.00(Ref.) | 0.66(0.46–0.94) * | 1.17(0.80–1.70) | 1.01(0.89–1.15) | 1.86(1.33–2.60) * |
| Fasting glucose | |||||
| Model 1 b | 1.00(Ref.) | 0.69(0.51–0.95) | 0.69(0.50–0.94) | 0.84(0.75–0.94) | 2.77(2.13–3.62) |
| Model 2 c | 1.00(Ref.) | 0.68(0.47–0.98) * | 0.83(0.57–1.21) | 0.89(0.78–1.02) | 1.69(1.21–2.37) * |
| Subjects | Dietary Inflammatory Beta Estimates (95% CI) | |||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Continuous | |
| Whole sample | ||||
| Model 1 b | 1.00(Ref.) | −0.004(−0.064,0.055) | −0.032(−0.101,0.036) | −0.012(−0.033,0.009) |
| Model 2 c | 1.00(Ref.) | 0.035(−0.018,0.089) | 0.040(−0.024,0.103) | 0.012(−0.008,0.031) |
| Subjects with MetS | ||||
| Model 1 b | 1.00(Ref.) | 0.072(−0.010,0.154) | 0.110(0.009,0.211) | 0.033(0.003,0.062) |
| Model 2 c | 1.00(Ref.) | 0.086(0.004,0.167) * | 0.145(0.045,0.245) * | 0.040(0.010,0.069) * |
| Subjects without MetS | ||||
| Model 1 b | 1.00(Ref.) | −0.021(−0.097,0.056) | −0.048(−0.134,0.038) | −0.018(−0.045,0.009) |
| Model 2 c | 1.00(Ref.) | 0.021(−0.050,0.091) | 0.001(−0.080,0.082) | 0.002(−0.024,0.027) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Zhao, A.; Wang, Y.; Meng, L.; Szeto, I.M.-Y.; Li, T.; Gong, H.; Tian, Z.; Zhang, Y.; Wang, P. Association between Dietary Inflammatory Index, C-Reactive Protein and Metabolic Syndrome: A Cross-Sectional Study. Nutrients 2018, 10, 831. https://doi.org/10.3390/nu10070831
Ren Z, Zhao A, Wang Y, Meng L, Szeto IM-Y, Li T, Gong H, Tian Z, Zhang Y, Wang P. Association between Dietary Inflammatory Index, C-Reactive Protein and Metabolic Syndrome: A Cross-Sectional Study. Nutrients. 2018; 10(7):831. https://doi.org/10.3390/nu10070831
Chicago/Turabian StyleRen, Zhongxia, Ai Zhao, Yan Wang, Liping Meng, Ignatius Man-Yau Szeto, Ting Li, Huiting Gong, Zixing Tian, Yumei Zhang, and Peiyu Wang. 2018. "Association between Dietary Inflammatory Index, C-Reactive Protein and Metabolic Syndrome: A Cross-Sectional Study" Nutrients 10, no. 7: 831. https://doi.org/10.3390/nu10070831
APA StyleRen, Z., Zhao, A., Wang, Y., Meng, L., Szeto, I. M.-Y., Li, T., Gong, H., Tian, Z., Zhang, Y., & Wang, P. (2018). Association between Dietary Inflammatory Index, C-Reactive Protein and Metabolic Syndrome: A Cross-Sectional Study. Nutrients, 10(7), 831. https://doi.org/10.3390/nu10070831





