Association between Dietary Vitamin E Intake and Esophageal Cancer Risk: An Updated Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Literature Search, Study Characteristics, and Quality Assessment
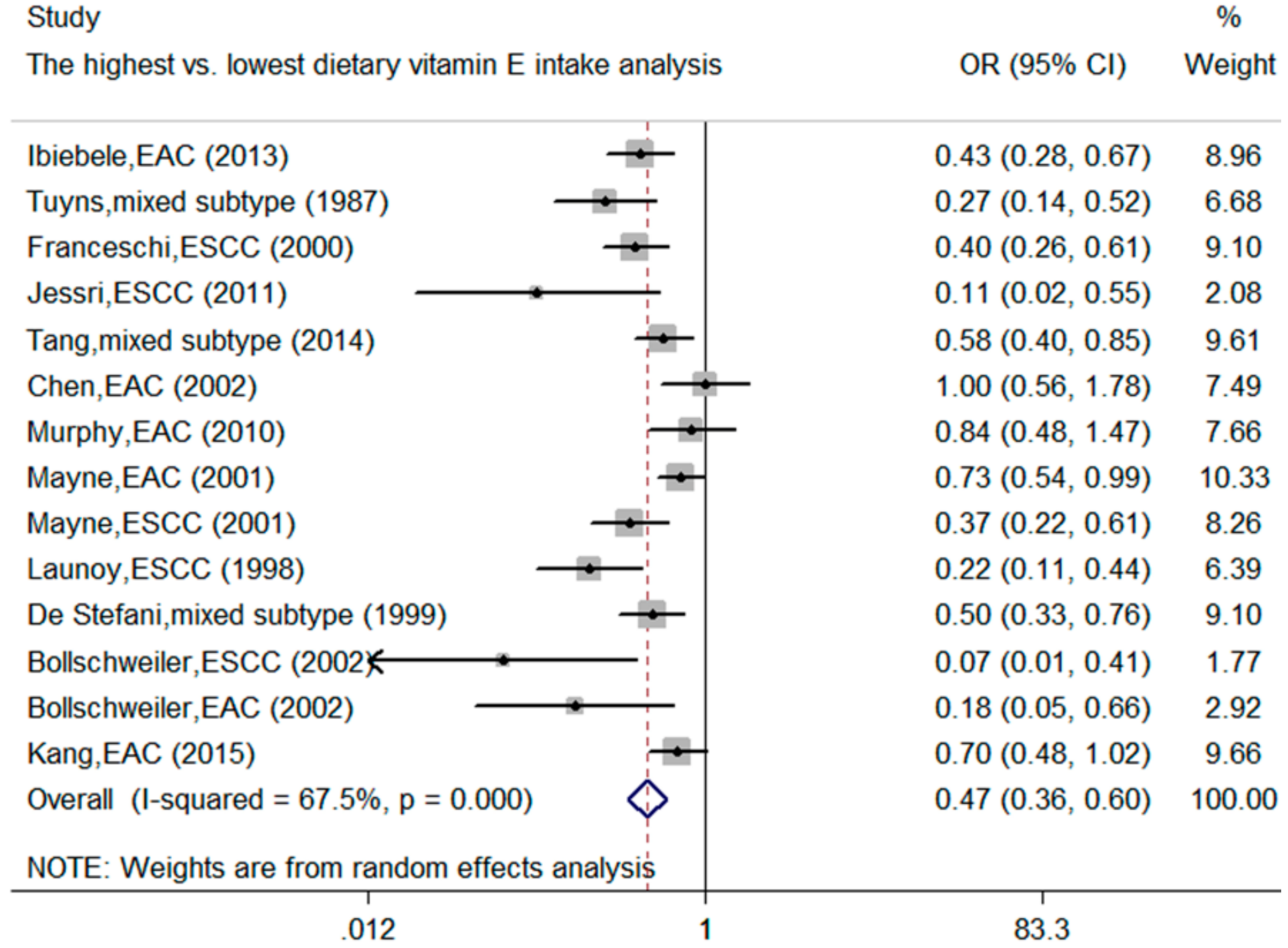
3.2. Meta-Analysis of Dietary Vitamin E Intake and the Esophageal Cancer Risk
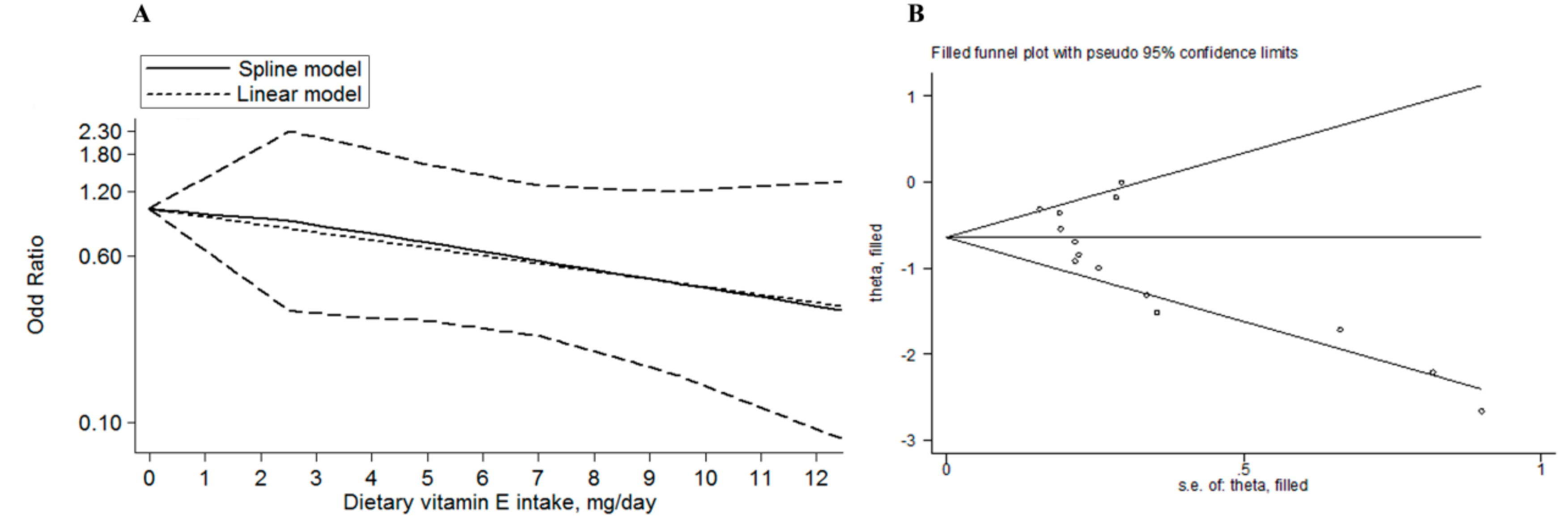
3.3. Meta-Regression and Subgroup Analysis
3.4. Sensitivity Analysis, Publication Bias Analysis, and Trim and Fill Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Joannie Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistic, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Schmassmann, A.; Oldendorf, M.G.; Gebbers, J.O. Changing incidence of gastric and oesophageal cancer subtypes in central Switzerland between 1982 and 2007. Eur. J. Epidemiol. 2009, 24, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.; Cheong, E.; Luben, R.; Igali, L.; Fitzgerald, R.; Khaw, K.T.; Hart, A. Body mass index, smoking, and alcohol and risks of barrett’s esophagus and esophageal adenocarcinoma: A UK prospective cohort study. Dig. Dis. Sci. 2014, 59, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Khor, T.O.; Nair, S.; Reuhl, K.; Suh, N.; Reddy, B.; Newmark, H.; Kong, A.N. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int. J. Cancer 2009, 124, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, S.; Suh, N. Tocopherols in cancer: An update. Mol. Nutr. Food Res. 2016, 60, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, Y.; Cui, X.X.; Goodin, S.; Wang, H.; Du, Z.Y.; Li, D.; Zhang, K.; Tony Kong, A.N.; DiPaola, R.S.; et al. Potent inhibitory effect of δ-tocopherol on prostate cancer cells cultured in vitro and grown as xenograft tumors in vivo. J. Agric. Food Chem. 2014, 62, 10752–10758. [Google Scholar]
- Ibiebele, T.I.; Hughes, M.C.; Nagle, C.M.; Bain, C.J.; Whiteman, D.C.; Webb, P.M. Study of Digestive Health and Australian Cancer Study. Dietary antioxidants and risk of Barrett’s esophagus and adenocarcinoma of the esophagus in an Australian population. Int. J. Cancer 2013, 133, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Tuyns, A.J.; Riboli, E.; Doornbos, G.; Péquignot, G. Diet and esophageal cancer in Calvados (France). Nutr. Cancer 1987, 9, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Bidoli, E.; Negri, E.; Zambon, P.; Talamini, R.; Ruol, A.; Parpinel, M.; Levi, F.; Simonato, L.; La Vecchia, C. Role of macronutrients, vitamins and minerals in the aetiology of squamous-cell carcinoma of the oesophagus. Int. J. Cancer 2015, 86, 626–631. [Google Scholar] [CrossRef]
- Jessri, M.; Rashidkhani, B.; Hajizadeh, B.; Jessri, M.; Gotay, C. Macronutrients, vitamins and minerals intake and risk of esophageal squamous cell carcinoma: A case-control study in Iran. Nutr. J. 2011, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Lee, A.H.; Xu, F.; Zhang, T.; Lei, J.; Binns, C.W. Fruit and vegetable consumption and risk of esophageal cancer: A case-control study in north-west China. Dis. Esophagus 2014, 27, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tucker, K.L.; Graubard, B.I.; Heineman, E.F.; Markin, R.S.; Potischman, N.A.; Russell, R.M.; Weisenburger, D.D.; Ward, M.H. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr. Cancer 2002, 42, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.J.; Anderson, L.A.; Ferguson, H.R.; Johnston, B.T.; Watson, P.R.; McGuigan, J.; Comber, H.; Reynolds, J.V.; Murray, L.J.; Cantwell, M.M. Dietary antioxidant and mineral intake in humans is associated with reduced risk of esophageal adenocarcinoma but not reflux esophagitis or Barrett’s esophagus. J. Nutr. 2010, 140, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Gláucia, F.C.; Marcos, R.D.S.; Tatiani, O.F.; Priscla, S.S.; Thais, K.A.; Ana, R. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2013. [Google Scholar]
- Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, X.; Tian, Y.; Xie, C.; Li, Q.; Cui, H.; Sun, C. Flavonoids, flavonoid subclasses, and esophageal cancer risk: A meta-analysis of epidemiologic studies. Nutrients 2016, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [PubMed]
- Studies, M.O.P. Association of fibrinogen, c-reactive protein, albumin, or leukocyte count with coronary heart disease. J. Am. Med. Assoc. 1998, 279, 1477–1482. [Google Scholar]
- Chêne, G.; Thompson, S.G. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am. J. Epidemiol. 1996, 144, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hou, R.; Gong, T.T.; Wu, Q.J. Dietary fat intake and endometrial cancer risk: Dose-response meta-analysis of epidemiological studies. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Zambon, A.; Quatto, P.; Corrao, G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am. J. Epidemiol. 2004, 159, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Royston, P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat. Med. 2000, 19, 1831–1847. [Google Scholar] [CrossRef]
- Hong, Z.; Tian, C.; Zhang, X. Dietary calcium intake, vitamin D levels, and breast cancer risk: A dose-response analysis of observational studies. Breast Cancer Res. Treat. 2012, 136, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Kang, S.; Zhang, D. Association of vitamin B6, vitamin B12 and methionine with risk of breast cancer: A dose–response meta-analysis. Br. J. Cancer 2013, 109, 1926–1944. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Gilbody, S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. Plant Physiol. 1998, 116, 469–477. [Google Scholar]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Risch, H.A.; Dubrow, R.; Chow, W.H.; Gammon, M.D.; Vaughan, T.L.; Farrow, D.C.; Schoenberg, J.B.; Stanford, J.L.; Ahsan, H.; et al. Nutrient Intake and Risk of Subtypes of Esophageal and Gastric Cancer; American Association for Cancer Research: Philadelphia, PA, USA, 2001. [Google Scholar]
- Launoy, G.; Milan, C.; Day, N.; Pienkowski, M.P.; Gignoux, M.; Faivre, J. Diet and squamous-cell cancer of the oesophagus: A French multicentre case-control study. Int. J. Cancer 1998, 76, 7–12. [Google Scholar] [CrossRef]
- De Stefani, E.; Ronco, A.; Mendilaharsu, M.; Deneo-Pellegrini, H. Diet and risk of cancer of the upper aerodigestive tract—II. Nutrients. Oral Oncol. 1999, 35, 22–26. [Google Scholar] [CrossRef]
- Bollschweiler, E.; Wolfgarten, E.; Nowroth, T.; Rosendahl, U.; Mönig, S.P.; Hölscher, A.H. Vitamin intake and risk of subtypes of esophageal cancer in Germany. J. Cancer Res. Clin. Oncol. 2002, 128, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yates, M.; Luben, R.; Cheong, E.; Alexandre, L.; Lgali, L.; Khaw, K.T.; Hart, A. OC-038 The age-dependent relationship of dietary antioxidant intake and the risk of barrett’s oesophagus and oesophageal adenocarcinoma: A UK prospective cohort study using micronutrient data from food diaries and serum biomarkers. Gut 2015, 64. [Google Scholar] [CrossRef]
- Chen, F.; Li, Q.; Yang, Y.; Yang, W.; Shi, F.; Qu, Y. Association of vitamin C, vitamin D, vitamin E and risk of bladder cancer: A dose-response meta-analysis. Sci. Rep. 2015, 5, 9599. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, H.; Chen, J.; Shi, Y.; Cai, J.; Yang, J.; Wu, Y. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int. J. Cancer 2014, 135, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, J.; Hong, X.; Chai, Z.; Li, Q. Dietary vitamin E intake could reduce the risk of lung cancer: Evidence from a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 6631–6637. [Google Scholar] [PubMed]
- Kubo, A.; Corley, D.A. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am. J. Gastroenterol. 2007, 102, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Duthie, G.G.; Aucott, L.S.; Macdonald, H.M. Vitamin E homologues α- and γ-tocopherol are not associated with bone turnover markers or bone mineral density in peri-menopausal and post-menopausal women. Osteoporosis Int. 2016, 27, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Carman, S.; Kamangar, F.; Freedman, N.D.; Wright, M.E.; Dawsey, S.M.; Dixon, L.B.; Subar, A.; Schatzkin, A.; Abnet, C.C. Vitamin E intake and risk of esophageal and gastric cancers in the NIH-AARP diet and health study. Int. J. Cancer 2009, 125, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Terry, P.; Lagergren, J.; Ye, W.; Nyrén, O.; Wolk, A. Antioxidants and cancers of the esophagus and gastric cardia. Int. J. Cancer 2000, 87, 750–754. [Google Scholar] [CrossRef]
- Clark, L.C.; Dalkin, B.; Krongrad, A.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Witherington, R.; Herlong, J.H.; Janosko, E.; Carpenter, D.; et al. Decreased incidence of prostate cancer with selenium supplementation: Results of a double-blind cancer prevention trial. Br. J. Urol. 1998, 81, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A. The Selenium and Vitamin E Cancer Prevention Trial; Humana Press: Clifton County, NJ, USA, 2009. [Google Scholar]
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch. Surg. 2006, 391, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fang, J.; Jia, X.; Han, C.; Chen, X.; Yang, C.S.; Li, N. Chemopreventive effects of early-stage and late-stage supplementation of vitamin E and selenium on esophageal carcinogenesis in rats maintained on a low vitamin E/selenium diet. Carcinogenesis 2011, 32, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Jia, X.; Chen, X.; Yang, C.S.; Li, N. Time-selective chemoprevention of vitamin E and selenium on esophageal carcinogenesis in rats: The possible role of nuclear factor kappaB signaling pathway. Int. J. Cancer 2012, 131, 1517–1527. [Google Scholar]
- Oze, I.; Matsuo, K.; Ito, H.; Wakai, K.; Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Sasazuki, S.; et al. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Cigarette smoking and esophageal cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2012, 42, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Corley, D.A. Body mass index and adenocarcinomas of the esophagus or gastric cardia: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 872–878. [Google Scholar]
- Olsen, C.M.; Pandeya, N.; Green, A.C.; Webb, P.M.; Whiteman, D.C. Australian Cancer Study. Population attributable fractions of adenocarcinoma of the esophagus and gastroesophageal junction. Am. J. Epidemiol. 2011, 174, 582–590. [Google Scholar] [CrossRef] [PubMed]
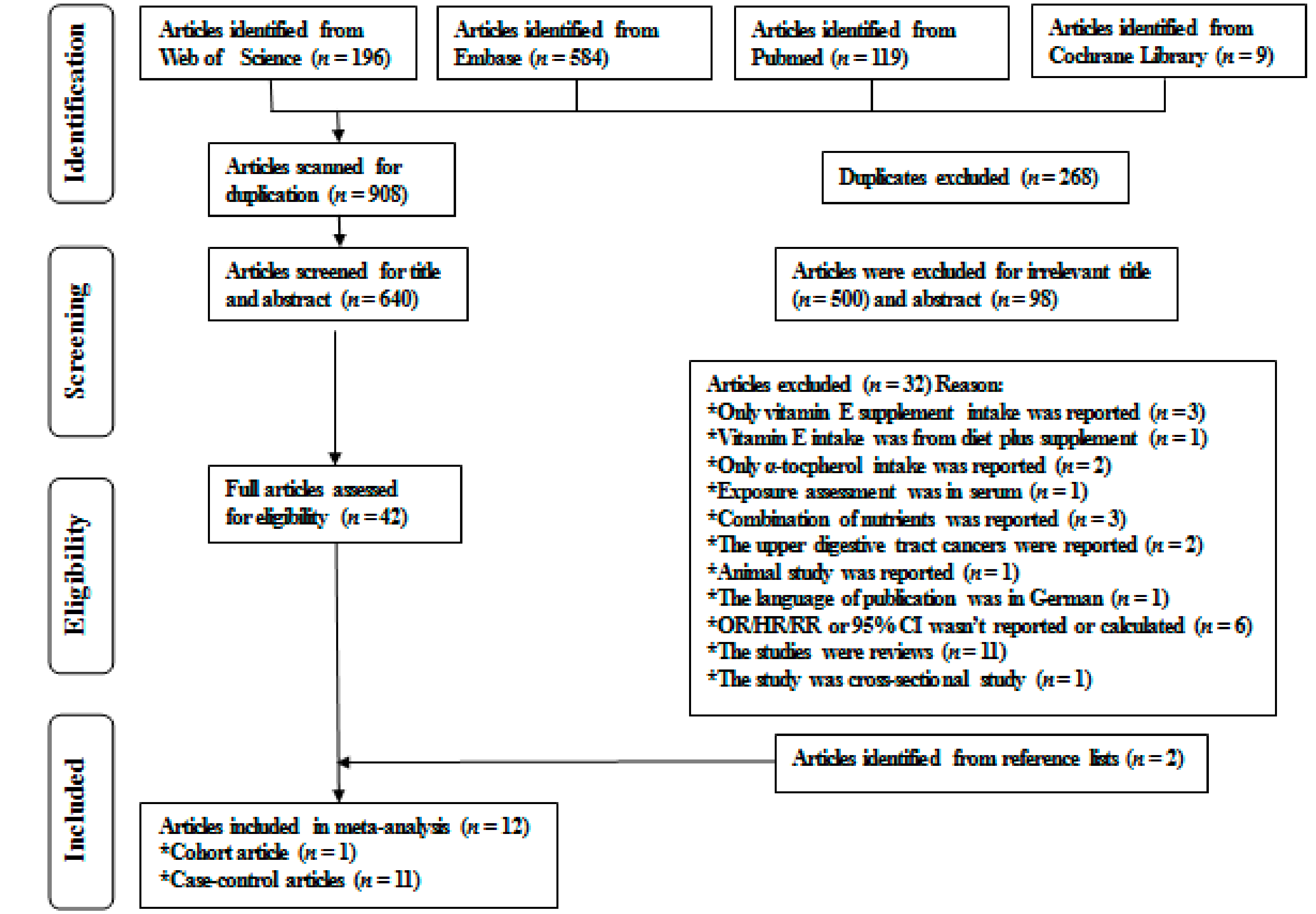
| The First Author, Publication Year | Gender, Age, Study Period | Country, Source of Control, Study Design | Method of Identifying Case, the Number of Case/Non-Case | Methods of Dietary Vitamin E Intake Assessment | OR/HR/RR (95% CI) for Highest vs. Lowest Dietary Vitamin E Intake | Adjustment for Confounders |
|---|---|---|---|---|---|---|
| Ibiebele, 2013 [8] | Both sexes, (18–79) years, 2002–2005 | Australian, population-based case-control | Pathological diagnoses, 299/1507 | Validated-FFQ-135 items | 0.43 (0.28, 0.67) for EAC | Age, gender; education, BMI, frequency of heartburn or acid reflux symptoms, smoking, alcohol, NSAID use, total energy intake |
| Tuyns, 1987 [9] | Both sexes, NA, NA | France, population-based case-control | Cancer registries, 743/1975 | Validated-FFQ-40 items | 0.27 (0.12, 0.45) for mixed subtype a | Age, alcohol, and smoking |
| Franceschi, 2000 [10] | Both sexes, (35–77) years, 1992–1997 | Italian, hospital-based case-control | Pathological diagnoses, 304/743 | Validated-FFQ-78 items | 0.40 (0.30, 0.70) for ESCC | Age, gender, area of residence, education, physical activity, BMI, smoking, alcohol drinking and non-alcohol energy |
| Jessri, 2011 [11] | Both sexes, (40–75) years, NA | Iran, hospital-based case-control | Pathological diagnoses, 47/96 | Validated-FFQ-125 items | 0.11 (0.03, 0.74) for ESCC | Age, sex, gastroesophageal reflux disease symptoms, BMI, smoking, physical activity, education |
| Tang, 2014 [12] | Both sexes, (48.8–72.4) years, 2008–2009 | China, hospital-based case-control | Pathological diagnoses, 350/380 | Validated-FFQ-137 items | 0.58 (0.40, 0.85) for mixed subtype a | Age, gender, education, BMI, energy intake, smoking, alcohol, family history of cancer |
| Chen, 2002 [13] | Both sexes, (42.2–74.7) years, 1992–1994 | America, population-based case-control | Cancer registries, 124/449 | Validated-FFQ-60 items | 1.00 (0.60, 1.90) for EAC | Age, age squared, gender, respondent type, BMI, alcohol, tobacco, education, family history of cancers, vitamin supplement |
| Murphy, 2010 [14] | Both sexes, (53–75) years, 2002–2004 | Ireland, population-based case-control | Pathological diagnoses, 224/256 | Validated-FFQ-188 items | 0.84 (0.48, 1.47) for EAC | Age, sex, BMI, energy intake, smoking, education, occupation, alcohol, NSAID use, gastroesophageal reflux disease, location, H. pylori infection |
| Mayne, 2001 [30] | Both sexes, (30–79) years, 1996–1999 | America, population-based case-control | Cancer registries, 282/687 for EAC; 206/687 for ESCC | Validated-FFQ-104 items | 0.73 (0.54, 1.00) for EAC; 0.37 (0.22, 0.60) for ESCC | Sex, site, age, race, proxy status, income, education, BMI, cigarettes, alcohol, and energy intake |
| Launoy, 1998 [31] | Male, NA, 1991–1994 | France, hospital-based case-control | Pathological diagnoses, 208/399 | Non-validated-FFQ-39 items | 0.22 (0.11, 0.44) for ESCC | Interviewer, age, smoking, beer, aniseed aperitives, hot Calvados, whisky, total alcohol, total energy intake and other significant food groups, PUFA, SFA |
| De Stefani, 1999 [32] | Both sexes, (30–89) years, 1996–1997 | Uruguay, hospital-based case-control | Pathological diagnoses, 66/393 | Not-validated-FFQ-64 items | 0.50 (0.30, 0.70) for mixed subtype a | Age, sex, residence, urban/rural status, education, BMI, smoking, alcohol and energy intake |
| Bollschweiler, 2002 [33] b | Male, (56–62.6) years, 1997–2000 | Germany, population-based case-control | Pathological diagnoses, 52/50 for ESCC; 47/50 for EAC | Not-validated-FFQ-1100 items | 0.07 (0.01, 0.34) for ESCC; 0.18 (0.05, 0.67) for EAC | None |
| Kang, 2015 [34] | Both sexes, (40–65) years, NA-2008 c | UK, cohort | Pathological diagnoses, 61/3712 | Not-validated-FFQ | 0.70 (0.48, 1.01) for EAC | Age, gender, BMI and smoking |
| Outcome of Interest | No. of Studies | No. of Cases/Non-Cases | ORs (95% CIs) | p for Test | Heterogeneity Test | |
|---|---|---|---|---|---|---|
| I2 (%) | phet | |||||
| Dietary Vitamin E | 14 | 3013/11,384 | 0.47 (0.36, 0.6) | <0.001 | 67.5 | <0.001 |
| Pathological type | ||||||
| EAC | 6 | 1037/6661 | 0.66 (0.49, 0.88) | 0.005 | 53.5 | 0.057 |
| ESCC | 5 | 817/1975 | 0.29 (0.18, 0.44) | <0.001 | 43.4 | 0.133 |
| Mixed subtype * | 3 | 1159/2748 | 0.46 (0.32, 0.68) | <0.001 | 48.7 | 0.142 |
| Geographic location | ||||||
| Europe | 7 | 1639/7185 | 0.37 (0.23, 0.60) | <0.001 | 73.9 | 0.001 |
| America | 4 | 678/2216 | 0.60 (0.41, 0.88) | 0.008 | 65.8 | 0.032 |
| Others † | 3 | 696/1983 | 0.44 (0.27, 0.73) | 0.001 | 55 | 0.108 |
| Study design | ||||||
| Case-control | 13 | 2952/7672 | 0.44 (0.37, 0.58) | <0.001 | 68 | <0.001 |
| Cohort | 1 | 61/3712 | 0.7 (0.48, 1.02) | 0.06 | NA | NA |
| Source of control | ||||||
| Population | 9 | 2038/9373 | 0.51 (0.36, 0.71) | <0.001 | 70.3 | 0.001 |
| Hospital | 5 | 975/2011 | 0.40 (0.28, 0.58) | <0.001 | 57.1 | 0.054 |
| Sample size | ||||||
| <500 | 6 | 497/4557 | 0.43 (0.25, 0.72) | 0.002 | 68.5 | 0.007 |
| ≥500 | 8 | 2516/6827 | 0.47 (0.35, 0.63) | <0.001 | 70.3 | 0.001 |
| Method of case identified | ||||||
| Pathological diagnoses | 10 | 1658/7586 | 0.44 (0.32, 0.59) | <0.001 | 63.3 | 0.004 |
| Cancer registries | 4 | 1355/3798 | 0.53 (0.32, 0.9) | 0.02 | 78.2 | 0.003 |
| Dietary vitamin E assessment method | ||||||
| Validated-FFQ | 9 | 2579/6780 | 0.52 (0.38, 0.68) | <0.001 | 66.3 | 0.003 |
| Unvalidated-FFQ | 5 | 434/4604 | 0.34 (0.19, 0.62) | <0.001 | 74.4 | 0.004 |
| Quality score | ||||||
| >5 | 9 | 2579/6780 | 0.51 (0.38, 0.68) | <0.001 | 66.3 | 0.003 |
| ≤5 | 5 | 434/4604 | 0.34 (0.19, 0.62) | <0.001 | 74.4 | 0.004 |
| Adjusted factors | ||||||
| Age | ||||||
| Yes | 12 | 2914/11,284 | 0.50 (0.39, 0.64) | <0.001 | 65.9 | <0.001 |
| No | 2 | 99/100 | 0.13 (0.05, 0.37) | <0.001 | 0 | 0.398 |
| Gender | ||||||
| Yes | 10 | 1963/8910 | 0.56 (0.45, 0.70) | <0.001 | 56.5 | 0.014 |
| No | 4 | 1050/2474 | 0.22 (0.14, 0.34) | <0.001 | 0 | 0.556 |
| Smoking | ||||||
| Yes | 12 | 2914/11,284 | 0.50 (0.39, 0.64) | <0.001 | 65.9 | 0.001 |
| No | 2 | 99/100 | 0.13 (0.05, 0.37) | <0.001 | 0 | 0.398 |
| Drinking | ||||||
| Yes | 10 | 2806/7476 | 0.50 (0.39, 0.64) | <0.001 | 65.9 | 0.002 |
| No | 4 | 207/3908 | 0.21 (0.06, 0.73) | 0.014 | 77.9 | 0.004 |
| BMI | ||||||
| Yes | 10 | 1963/8910 | 0.56 (0.45, 0.70) | <0.001 | 56.5 | 0.014 |
| No | 4 | 1050/2474 | 0.22 (0.14, 0.34) | <0.001 | 0 | 0.556 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Li, L.; Tian, Y.; Xu, F.; Qiao, T. Association between Dietary Vitamin E Intake and Esophageal Cancer Risk: An Updated Meta-Analysis. Nutrients 2018, 10, 801. https://doi.org/10.3390/nu10070801
Cui L, Li L, Tian Y, Xu F, Qiao T. Association between Dietary Vitamin E Intake and Esophageal Cancer Risk: An Updated Meta-Analysis. Nutrients. 2018; 10(7):801. https://doi.org/10.3390/nu10070801
Chicago/Turabian StyleCui, Lingling, Li Li, Yalan Tian, Fan Xu, and Tianyi Qiao. 2018. "Association between Dietary Vitamin E Intake and Esophageal Cancer Risk: An Updated Meta-Analysis" Nutrients 10, no. 7: 801. https://doi.org/10.3390/nu10070801
APA StyleCui, L., Li, L., Tian, Y., Xu, F., & Qiao, T. (2018). Association between Dietary Vitamin E Intake and Esophageal Cancer Risk: An Updated Meta-Analysis. Nutrients, 10(7), 801. https://doi.org/10.3390/nu10070801




