Effects of Glucose with Casein Peptide Supplementation on Post-Exercise Muscle Glycogen Resynthesis in C57BL/6J Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experimental Animals and Materials
2.3. Experimental Procedures
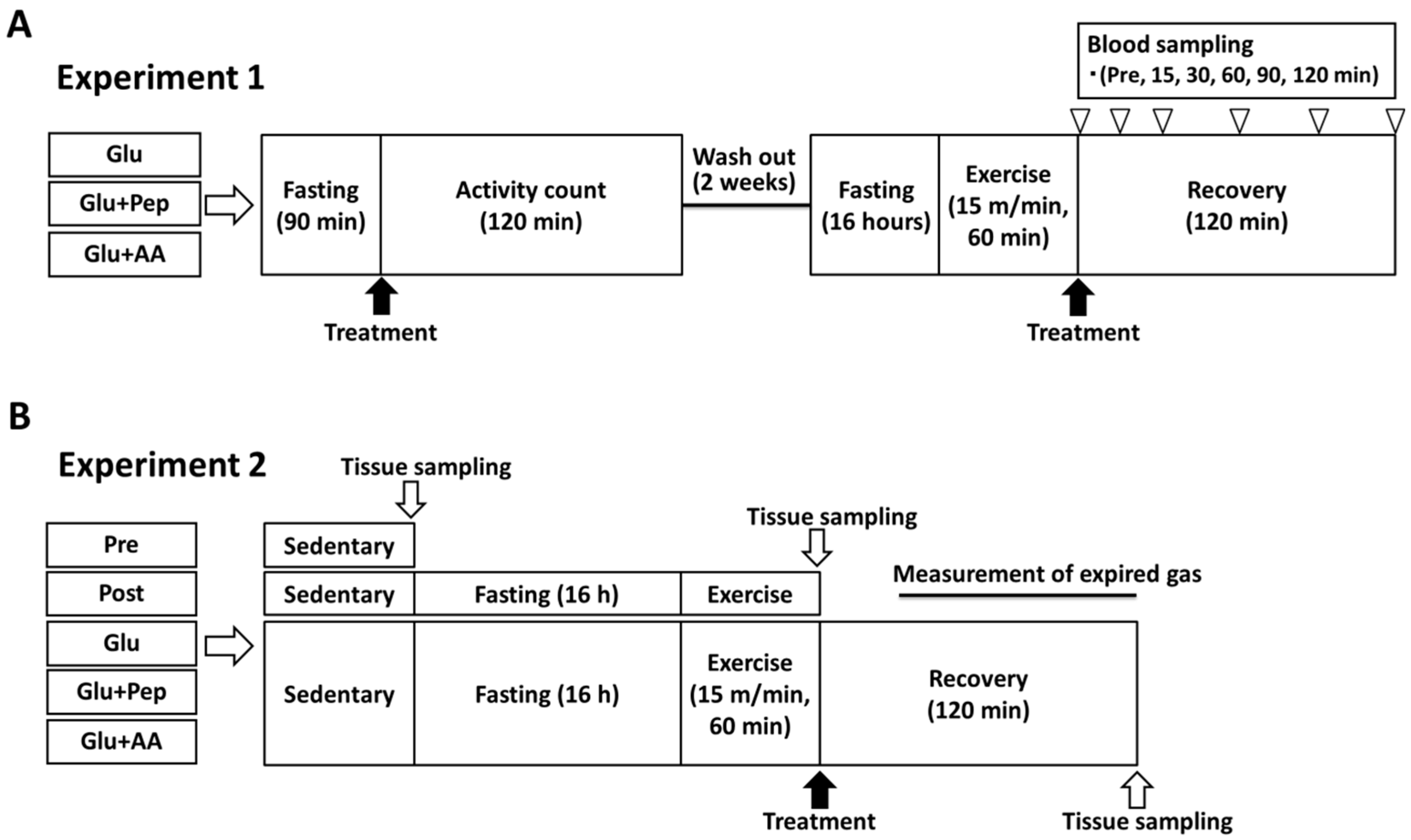
2.3.1. Experiment 1
2.3.2. Experiment 2
2.4. Analysis
2.4.1. Locomotor Activity
2.4.2. Blood and Plasma Substrate Concentrations
2.4.3. Liver and Muscle Glycogen Concentrations
2.4.4. Western Blot Analysis
2.4.5. Expired Gas Analysis
2.4.6. Statistical Analysis
3. Results
3.1. Experiment 1
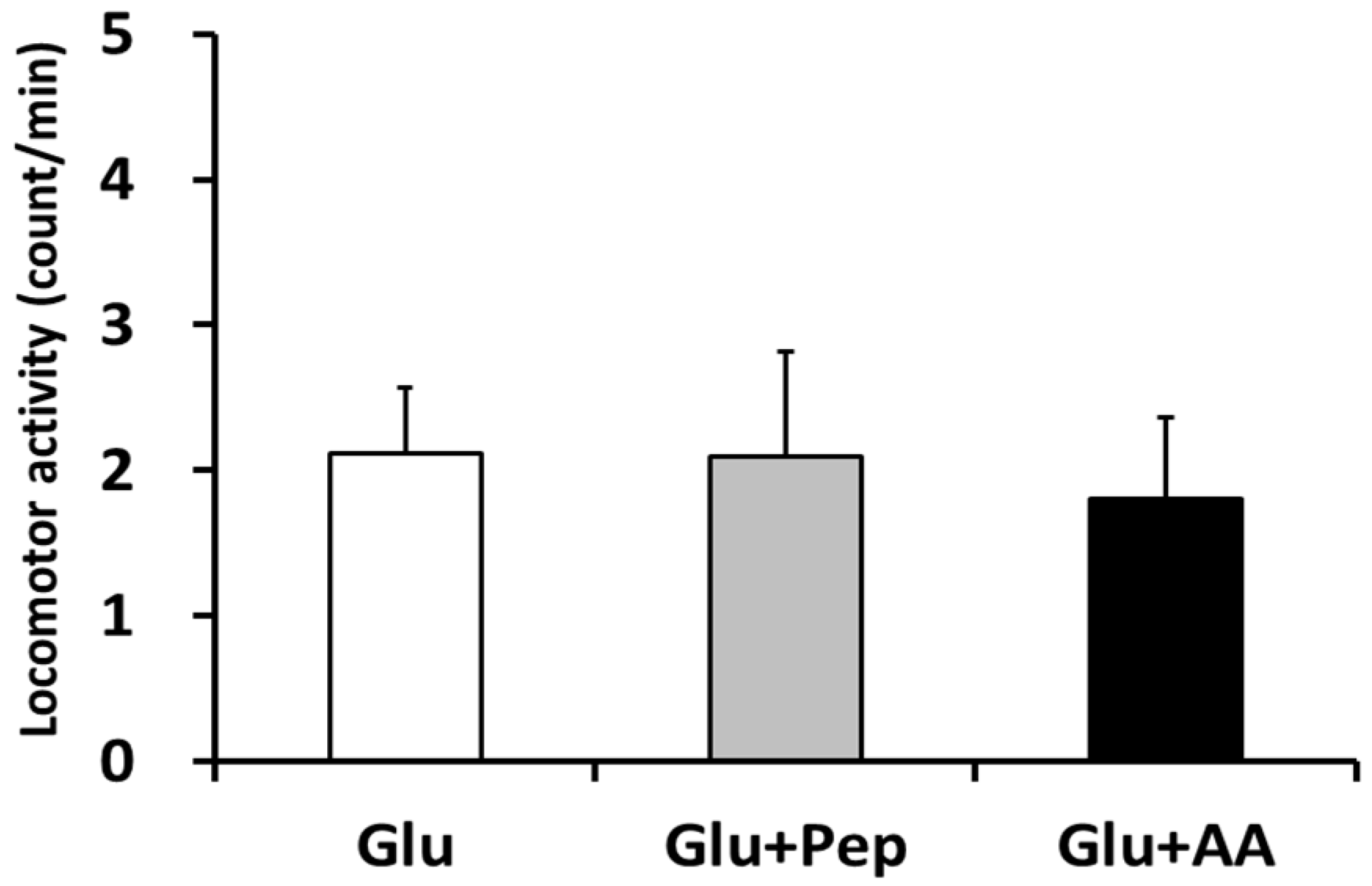
3.1.1. Locomotor Activity
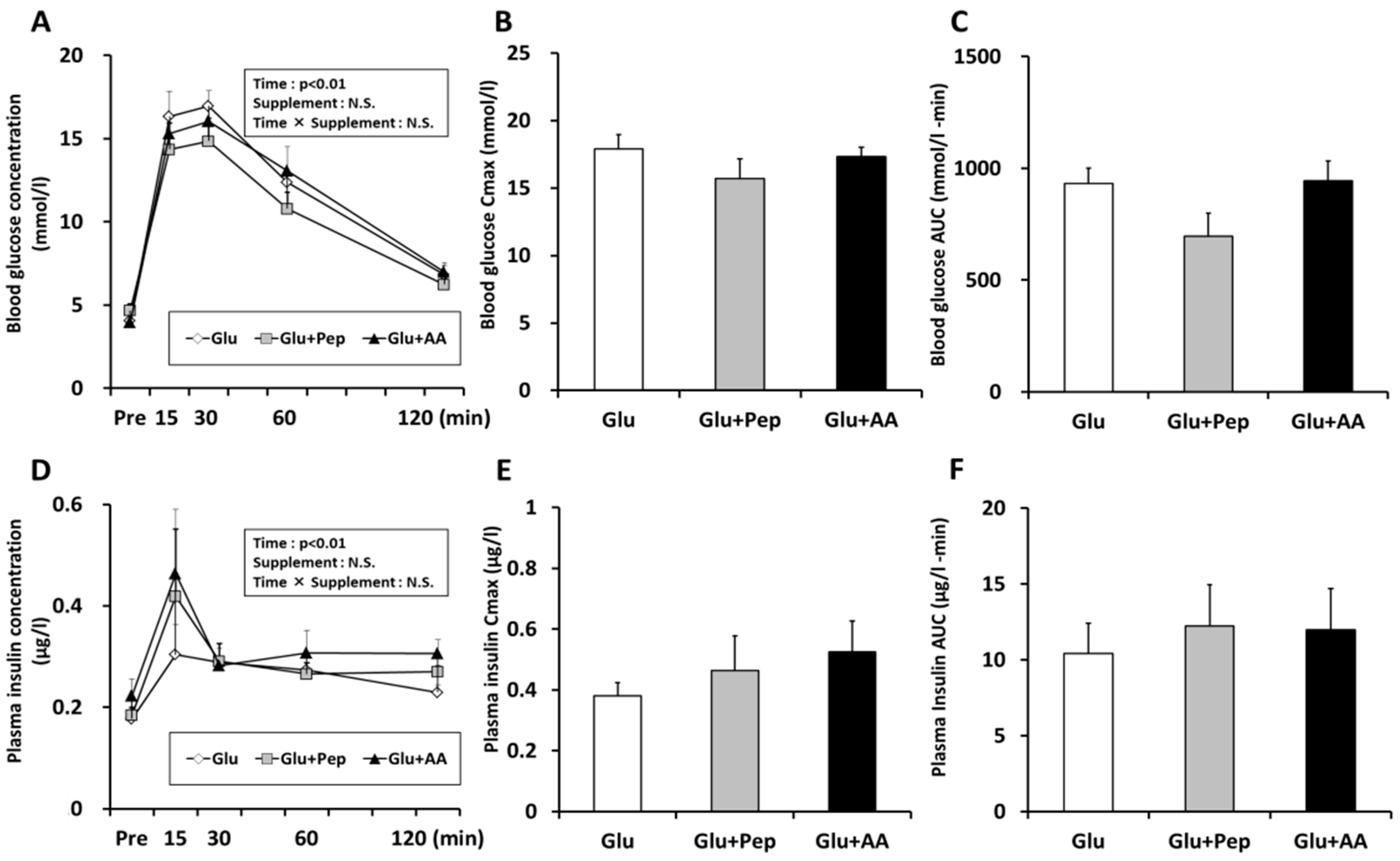
3.1.2. Blood Substrate Concentrations
3.2. Experiment 2
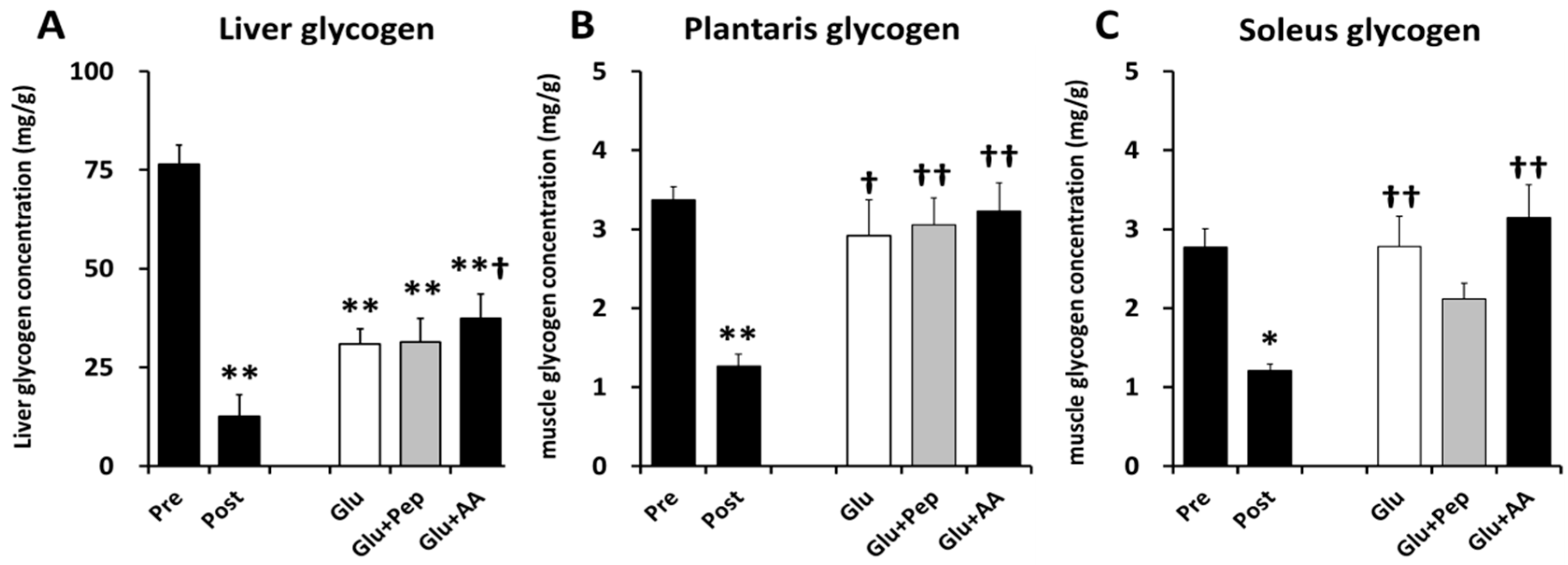
3.2.1. Liver and Muscle Glycogen Concentrations
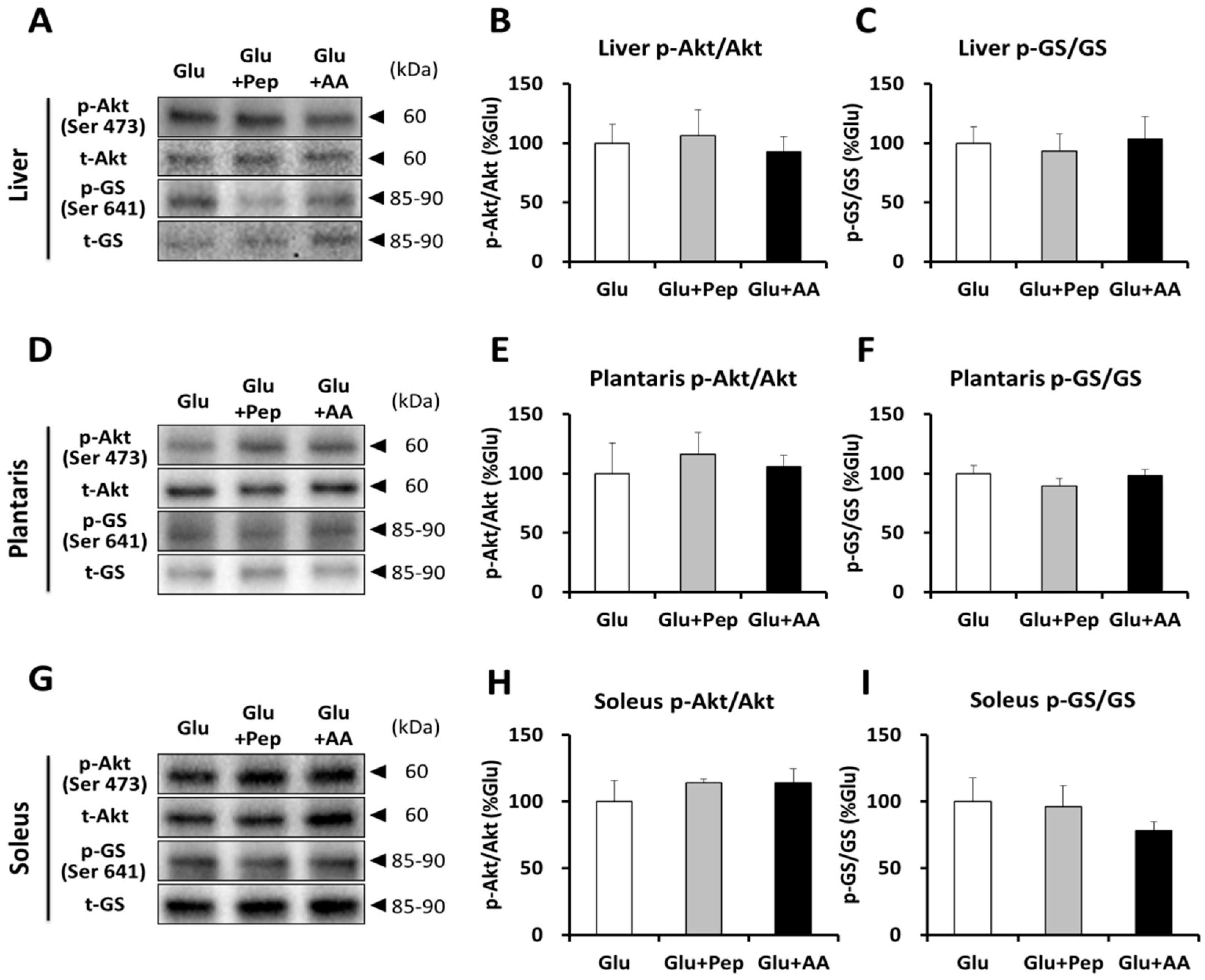
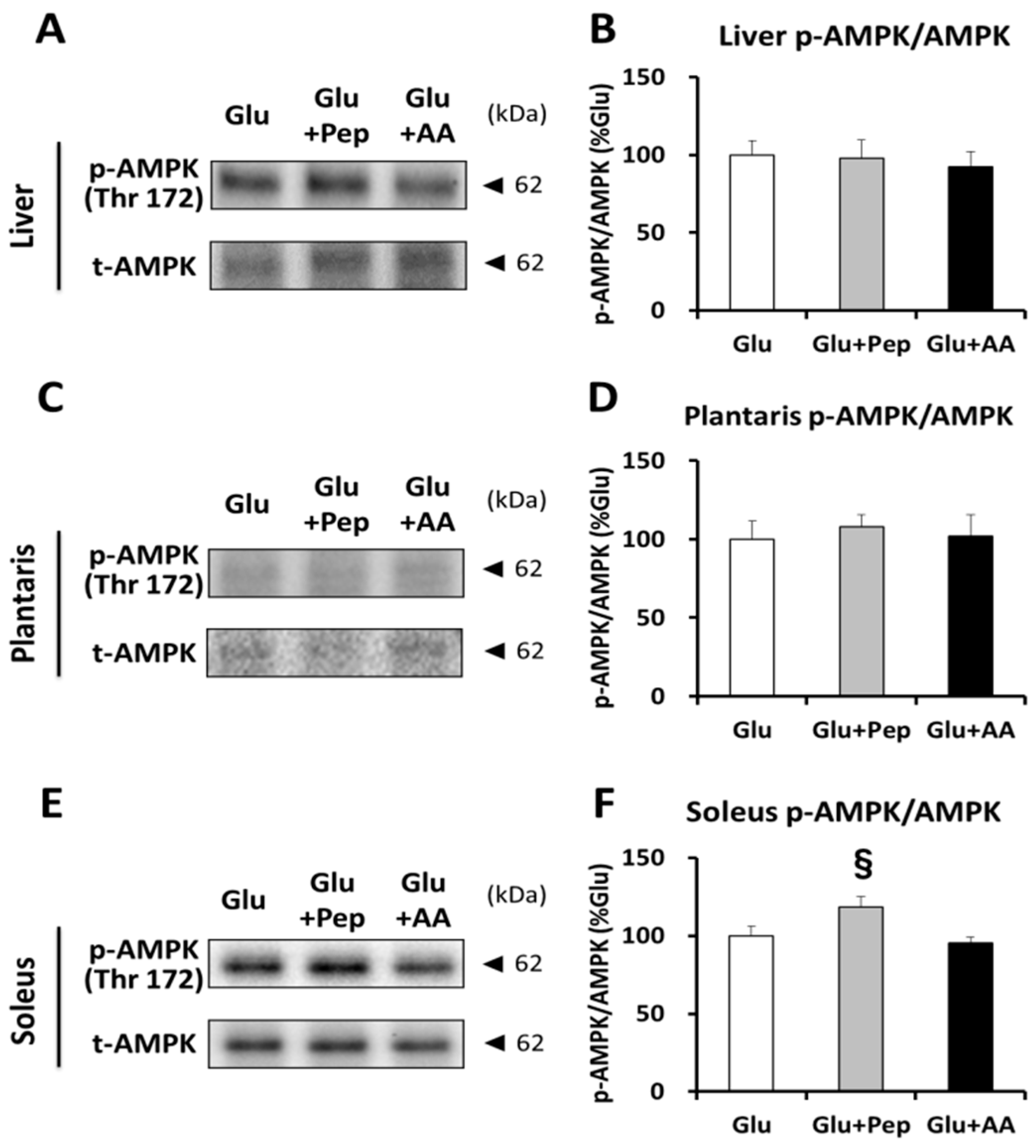
3.2.2. Insulin Signaling and Glycogen Synthesis
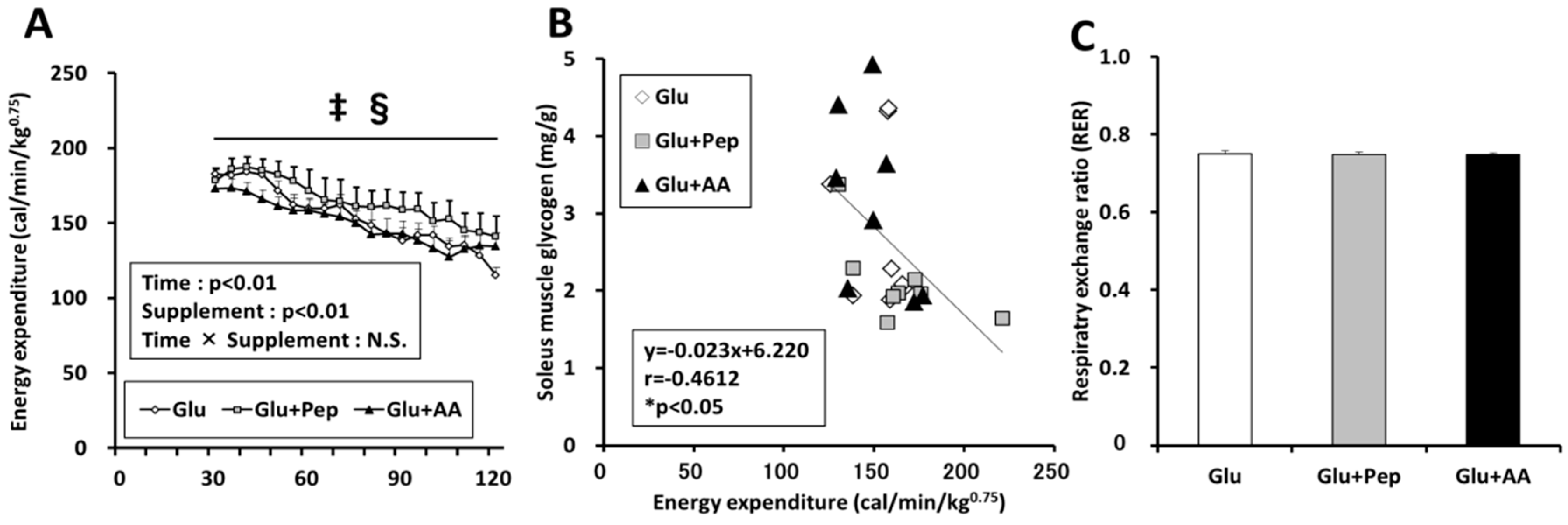
3.2.3. Energy Expenditure in the Recovery Period
3.2.4. Energy Consumption State
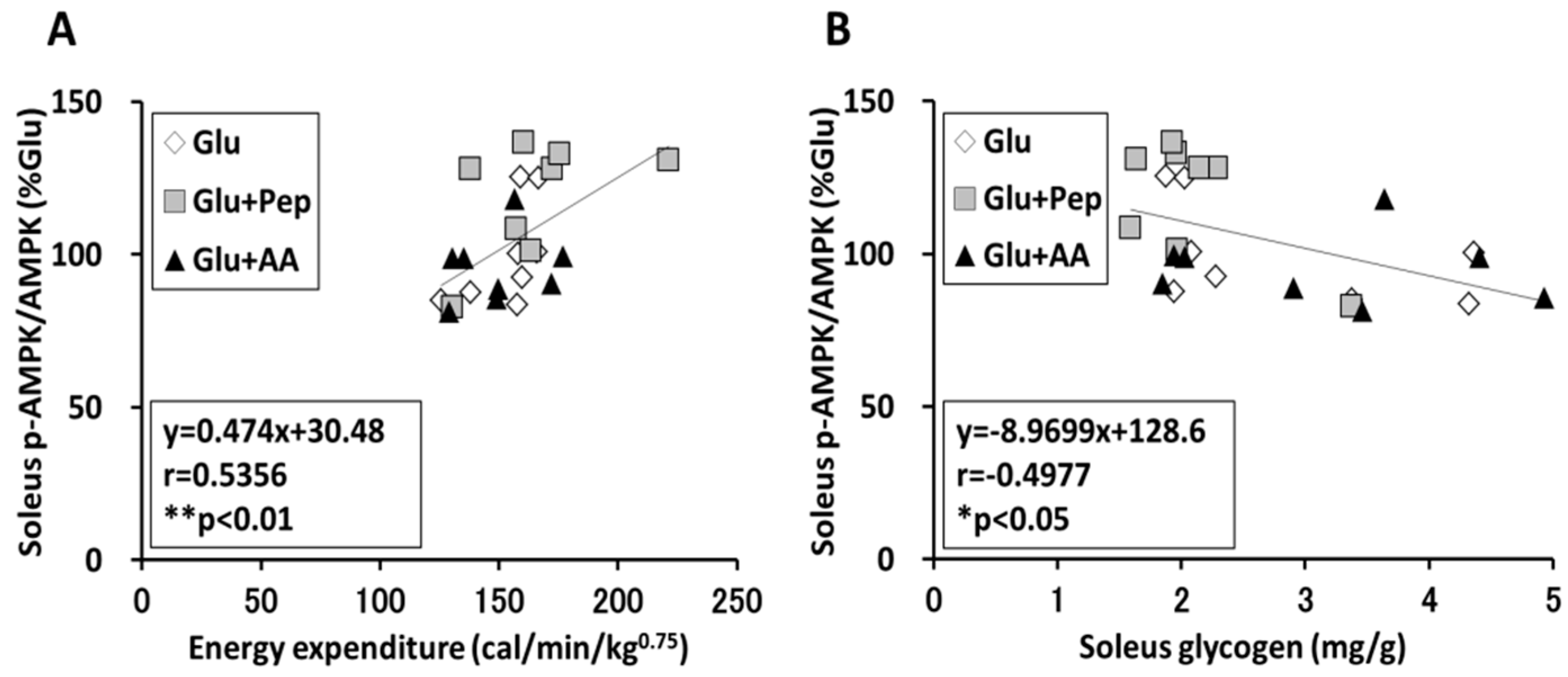
3.2.5. Relation of Energy Expenditure and Muscle Glycogen Concentration with AMPK Phosphorylation in the Soleus Muscle
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bergström, J.; Hultman, E. A study of the glycogen metabolism during exercise in man. Scand. J. Clin. Lab. Investig. 1967, 19, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.L.; Lee, M.C.; Brozinick, J.T., Jr.; Reed, M.J. Muscle glycogen storage after different amounts of carbohydrate ingestion. J. Appl. Physiol. 1988, 65, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, K.M.; Yaspelkis, B.B., 3rd; Ivy, J.L. Carbohydrate-protein complex increases the rate of muscle glycogen storage after exercise. J. Appl. Physiol. 1992, 72, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.L.; Goforth, H.W., Jr.; Damon, B.M.; McCauley, T.R.; Parsons, E.C.; Price, T.B. Early postexercise muscle glycogen recovery is enhanced with a carbohydrate-protein supplement. J. Appl. Physiol. 2002, 93, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Morifuji, M.; Koga, J.; Kawanaka, K.; Higuchi, M. Branched–chain amino acid–containing dipeptides, identified from whey protein hydrolysates, stimulate glucose uptake rate in L6 myotubes and isolated skeletal muscles. J. Nutr. Sci. Vitaminol. 2009, 55, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Morifuji, M.; Kanda, A.; Koga, J.; Kawanaka, K.; Higuchi, M. Post-exercise carbohydrate plus whey protein hydrolysates supplementation increases skeletal muscle glycogen level in rats. Amino Acids 2010, 38, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Deglaire, A.; Fromentin, C.; Fouillet, H.; Airinei, G.; Gaudichon, C.; Boutry, C.; Benamouzig, R.; Moughan, P.J.; Tomé, D.; Bos, C. Hydrolyzed dietary casein as compared with the intact protein reduces postprandial peripheral, but not whole-body, uptake of nitrogen in humans. Am. J. Clin. Nutr. 2009, 90, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Tamura, Y.; Sakata, Y.; Nonaka, Y.; Saito, N.; Nakamura, H.; Shimizu, T.; Takeda, Y.; Terada, S.; Hatta, H. Comparison between pre-exercise casein peptide and intact casein supplementation on glucose tolerance in high-fat diet-fed mice. Appl. Physiol. Nutr. Metab. 2018, 43, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Inai, M.; Nishimura, S.; Urashima, S.; Nonaka, Y.; Kimura, M.; Terada, S. Co-ingestion of milk and glucose promotes post-exercise glycogen resynthesis in mouse skeletal muscle and liver. Jpn. J. Sports Nutr. 2017, 10, 38–47. (In Japanese) [Google Scholar]
- Lo, S.; Russell, J.C.; Taylor, A.W. Determination of glycogen in small tissue samples. J. Appl. Physiol. 1970, 28, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tadaishi, M.; Miura, S.; Kai, Y.; Kano, Y.; Oishi, Y.; Ezaki, O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS ONE. 2011, 6, e28290. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Kruijshoop, M.; Verhagen, H.; Saris, W.H.; Wagenmakers, A.J. Ingestion of protein hydrolysate and amino acid-carbohydrate mixtures increases postexercise plasma insulin responses in men. J. Nutr. 2000, 130, 2508–2513. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Saris, W.H.; Kruijshoop, M.; Wagenmakers, A.J. Maximizing postexercise muscle glycogen synthesis: Carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am. J. Clin. Nutr. 2000, 72, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.; Jeukendrup, A. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med. 2003, 33, 117–144. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G.; Shirreffs, S.M.; Calbet, J.A. Muscle glycogen resynthesis during recovery from cycle exercise: No effect of additional protein ingestion. J. Appl. Physiol. 2000, 88, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.; Gutman, R.A.; Cresto, J.C.; Selawry, H.; Lavine, R.; Recant, L. Glucose-induced insulin release patterns: Effect of starvation. Diabetologia 1974, 10, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Qvigstad, E.; Bjerve, K.S.; Grill, V. Effects of long-term fasting on insulin responses to fatty acids in man. Scand. J. Clin. Lab. Investig. 2002, 62, 271–277. [Google Scholar] [CrossRef]
- Raben, A.; Agerholm-Larsen, L.; Flint, A.; Holst, J.J.; Astrup, A. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am. J. Clin. Nutr. 2003, 77, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Lillefosse, H.H.; Tastesen, H.S.; Du, Z.Y.; Ditlev, D.B.; Thorsen, F.A.; Madsen, L.; Kristiansen, K.; Liaset, B. Hydrolyzed casein reduces diet-induced obesity in male C57BL/6J mice. J. Nutr. 2013, 143, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Tamura, Y.; Takahashi, Y.; Masuda, H.; Hoshino, D.; Kitaoka, Y.; Saito, N.; Nakamura, H.; Takeda, Y.; Hatta, H. Pre-exercise casein peptide supplementation enhances endurance training-induced mitochondrial enzyme activity in slow twitch muscle, but not fast twitch muscle of high fat diet-fed mice. J. Phys. Fit. Sports Med. 2015, 4, 377–384. [Google Scholar] [CrossRef]
- Miyamoto, L.; Toyoda, T.; Hayashi, T.; Yonemitsu, S.; Nakano, M.; Tanaka, S.; Ebihara, K.; Masuzaki, H.; Hosoda, K.; Ogawa, Y.; et al. Effect of acute activation of 5’-AMP-activated protein kinase on glycogen regulation in isolated rat skeletal muscle. J. Appl. Physiol. 2007, 102, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Kawanaka, K.; Tabata, I.; Tanaka, A.; Higuchi, M. Effects of high-intensity intermittent swimming on glucose transport in rat epitrochlearis muscle. J. Appl. Physiol. 1998, 84, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Sonou, T.; Higuchi, M.; Terada, S. An acute bout of high-intensity intermittent swimming induces glycogen supercompensation in rat skeletal muscle. Eur. J. Sport Sci. 2008, 8, 413–420. [Google Scholar] [CrossRef]
- Chen, Z.P.; Stephens, T.J.; Murthy, S.; Canny, B.J.; Hargreaves, M.; Witters, L.A.; Kemp, B.E.; McConell, G.K. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 2003, 52, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H.; Armstrong, R.B. Muscular blood flow distribution patterns as a function of running speed in rats. Am. J. Physiol. 1982, 243, H296–H306. [Google Scholar] [CrossRef] [PubMed]
- Holten, M.K.; Zacho, M.; Gaster, M.; Juel, C.; Wojtaszewski, J.F.; Dela, F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 2004, 53, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, A.; Han, D.H.; Hansen, P.A.; Nolte, L.A.; Host, H.H.; Hickner, R.C.; Holloszy, J.O. Effect of endurance exercise training on muscle glycogen supercompensation in rats. J. Appl. Physiol. 1997, 82, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Sakurai, T.; Ochi, D.; Mitsuyama, E.; Yamauchi, K.; Abe, F. Antihypertensive effect of the bovine casein-derived peptide Met-Lys-Pro. Food Chem. 2015, 172, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Min, L.J.; Kobayashi, Y.; Mogi, M.; Tsukuda, K.; Yamada, A.; Yamauchi, K.; Abe, F.; Iwanami, J.; Xiao, J.Z.; Horiuchi, M. Administration of bovine casein-derived peptide prevents cognitive decline in Alzheimer disease model mice. PLoS ONE 2017, 12, e0171515. [Google Scholar] [CrossRef] [PubMed]
- Soga, M.; Ohashi, A.; Taniguchi, M.; Matsui, T.; Tsuda, T. The di-peptide Trp-His activates AMP-activated protein kinase and enhances glucose uptake independently of insulin in L6 myotubes. FEBS Open Bio 2014, 4, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Sakamoto, T.; Kitano, T.; Inoue, N.; Sugihara, F.; Harada, N.; Yamaji, R. The collagen derived dipeptide hydroxyprolyl-glycine promotes C2C12 myoblast differentiation and myotube hypertrophy. Biochem. Biophys. Res. Commun. 2016, 478, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
| Essential Amino Acids (mg/g) | |||
| Lysine | 68 | Leucine | 72 |
| Threonine | 44 | Phenylalanine | 31 |
| Valine | 55 | Tyrosine | 33 |
| Methionine | 24 | Tryptophan | 2 |
| Cysteine | 3 | Histidine | 22 |
| Isoleucine | 46 | ||
| Non-Essential Amino Acids (mg/g) | |||
| Aspartic acid + Asparagine | 75 | Glycine | 19 |
| Serine | 56 | Alanine | 30 |
| Glutamic acid + Glutamine | 250 | Arginine | 22 |
| Proline | 96 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaga, Y.; Sakata, Y.; Yago, T.; Nakamura, H.; Shimizu, T.; Takeda, Y. Effects of Glucose with Casein Peptide Supplementation on Post-Exercise Muscle Glycogen Resynthesis in C57BL/6J Mice. Nutrients 2018, 10, 753. https://doi.org/10.3390/nu10060753
Matsunaga Y, Sakata Y, Yago T, Nakamura H, Shimizu T, Takeda Y. Effects of Glucose with Casein Peptide Supplementation on Post-Exercise Muscle Glycogen Resynthesis in C57BL/6J Mice. Nutrients. 2018; 10(6):753. https://doi.org/10.3390/nu10060753
Chicago/Turabian StyleMatsunaga, Yutaka, Yasuyuki Sakata, Takumi Yago, Hirohiko Nakamura, Takashi Shimizu, and Yasuhiro Takeda. 2018. "Effects of Glucose with Casein Peptide Supplementation on Post-Exercise Muscle Glycogen Resynthesis in C57BL/6J Mice" Nutrients 10, no. 6: 753. https://doi.org/10.3390/nu10060753
APA StyleMatsunaga, Y., Sakata, Y., Yago, T., Nakamura, H., Shimizu, T., & Takeda, Y. (2018). Effects of Glucose with Casein Peptide Supplementation on Post-Exercise Muscle Glycogen Resynthesis in C57BL/6J Mice. Nutrients, 10(6), 753. https://doi.org/10.3390/nu10060753




