Impact of First Meal Size during Prolonged Sitting on Postprandial Glycaemia in Individuals with Prediabetes: A Randomised, Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
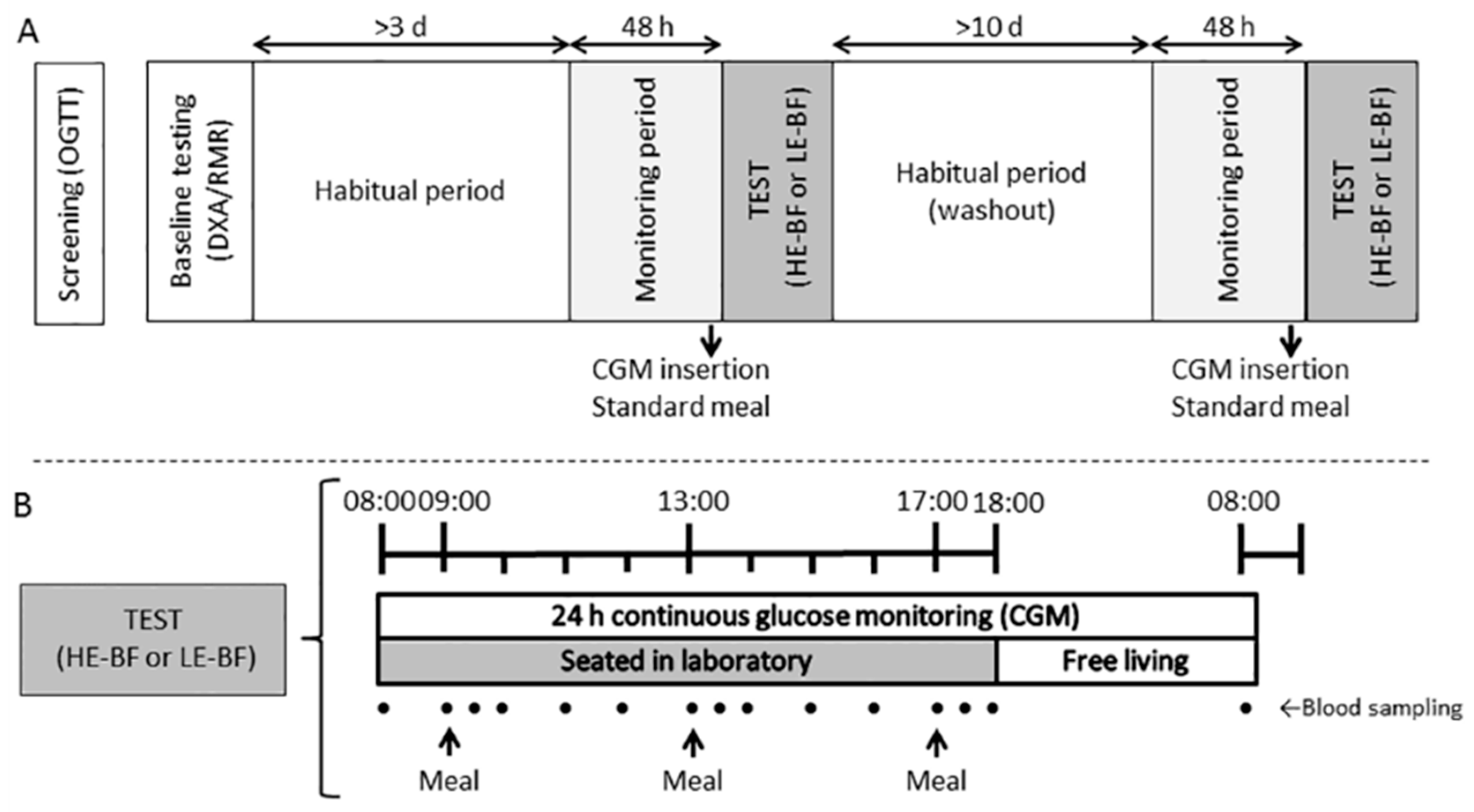
2.2. Study Design
2.3. Preliminary Measures
2.4. Study Protocol and Trial Conditions
Meal Composition
2.5. Biochemical Analysis
2.6. Data Analysis
2.6.1. Sample Size Calculations
2.6.2. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Interventions
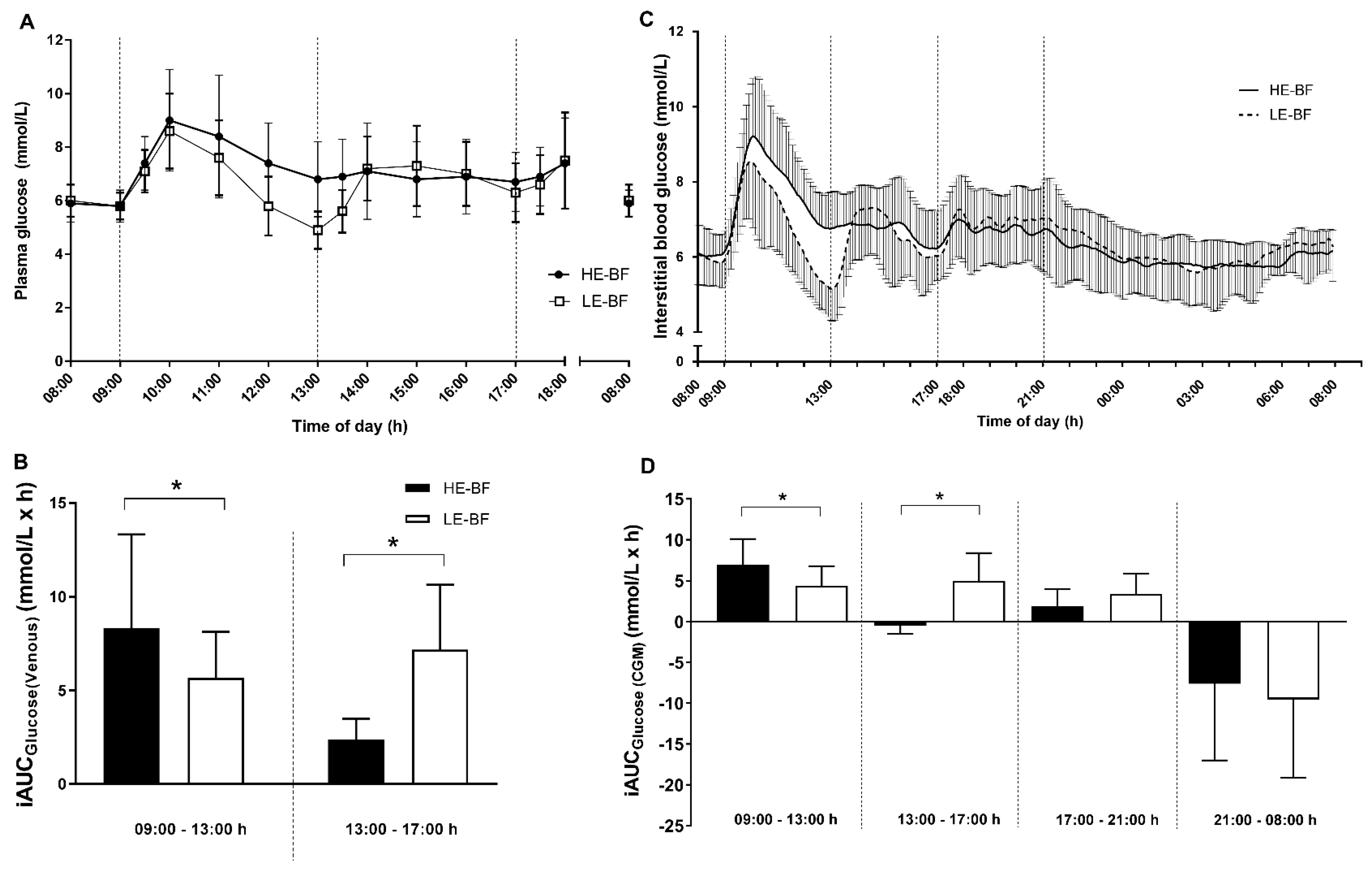
3.3. Glycaemic Control
3.4. Insulin, C-Peptide and tGLP-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrannini, E.; Gastaldelli, A.; Iozzo, P. Pathophysiology of prediabetes. Med. Clin. N. Am. 2011, 95, 327–339. [Google Scholar] [CrossRef] [PubMed]
- De Vegt, F.; Dekker, J.M.; Jager, A.; Hienkens, E.; Kostense, P.J.; Stehouwer, C.D.; Nijpels, G.; Bouter, L.M.; Heine, R.J. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA 2001, 285, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bell, D.S.H. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Vizcaíno, J.; Piñol, J.L.; Cabré, J.J.; Fuentes, C.M. Relevance of casual undetected hyperglycemia among high-risk individuals for developing diabetes. Diabetes Res. Clin. Pract. 2007, 78, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Chen, K.Y.; Freedson, P.S.; Buchowski, M.S.; Beech, B.M.; Pate, R.R.; Troiano, R.P. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol. 2008, 167, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Bellettiere, J.; Winkler, E.A.H.; Chastin, S.F.M.; Kerr, J.; Owen, N.; Dunstan, D.W.; Healy, G.N. Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS ONE 2017, 12, e0180119. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.C.; Blankenship, J.M.; Larsen, R.N.; Sacre, J.W.; Sethi, P.; Straznicky, N.E.; Cohen, N.D.; Cerin, E.; Lambert, G.W.; Owen, N.; et al. Interrupting prolonged sitting in type 2 diabetes: Nocturnal persistence of improved glycaemic control. Diabetologia 2017, 60, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Davies, M.J.; Bodicoat, D.H.; Edwardson, C.L.; Gill, J.M.R.; Stensel, D.J.; Tolfrey, K.; Dunstan, D.W.; Khunti, K.; Yates, T. Breaking Up Prolonged Sitting With Standing or Walking Attenuates the Postprandial Metabolic Response in Postmenopausal Women: A Randomized Acute Study. Diabetes Care 2016, 39, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitz, H.R.; Boaz, M.; Ganz, T.; Jakubowicz, D.; Matas, Z.; Madar, Z.; Wainstein, J. Big breakfast rich in protein and fat improves glycemic control in type 2 diabetics. Obesity 2014, 22, E46–F54. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Wainstein, J.; Ahrén, B.; Bar-Dayan, Y.; Landau, Z.; Rabinovitz, H.R.; Froy, O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: A randomised clinical trial. Diabetologia 2015, 58, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Shapiro, E.T.; Tillil, H.; Polonsky, K.S. Circadian modulation of glucose and insulin responses to meals: Relationship to cortisol rhythm. Am. J. Physiol. 1992, 262, E467–E475. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.C.; Resuehr, H.E.; Goree, L.L.; Locher, J.L.; Bray, M.S.; Soleymani, T.; Gower, B.A. A High-Fat Compared with a High-Carbohydrate Breakfast Enhances 24-Hour Fat Oxidation in Older Adults. J. Nutr. 2018, 148, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Gan, Y.; Yang, C.; Chen, Y.; Tong, X.; Lu, Z. Breakfast skipping and the risk of type 2 diabetes: A meta-analysis of observational studies. Public Health Nutr 2015, 18, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.L.; Aragon, F.F.; Padovani, C.R.; Pimenta, W.P. Daytime variations in glucose tolerance in people with impaired glucose tolerance. Diabetes Res. Clin. Pract. 2006, 74, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Sulk, S.; Helbig, M.; Thomas, A.; Kohler, C. Differences in Glycemic Variability between Normoglycemic and Prediabetic Subjects. J. Diabetes Sci. Technol. 2014, 8, 286–290. [Google Scholar] [CrossRef] [PubMed]
- WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Service, F.J.; Molnar, G.D.; Rosevear, J.W.; Ackerman, E.; Gatewood, L.C.; Taylor, W.F. Mean Amplitude of Glycemic Excursions, a Measure of Diabetic Instability. Diabetes 1970, 19, 644–655. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.M.; Donath, S.M.; Vidmar, S.I.; Werther, G.A.; Cameron, F.J. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol. Ther. 2005, 7, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Oliver, N.S.; Choudhary, P.; Levy, J.C.; Hindmarsh, P.; Matthews, D.R. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol. Ther. 2011, 13, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.; Mignault, D.; Allison, D.B.; Rabasa-Lhoret, R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am. J. Clin. Nutr. 2007, 85, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.N.; Kingwell, B.A.; Robinson, C.; Hammond, L.; Cerin, E.; Shaw, J.E.; Healy, G.N.; Hamilton, M.T.; Owen, N.; Dunstan, D.W. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin. Sci. 2015, 129, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Baldi, J.C.; Manning, P.J.; Lucas, S.J.E.; Hawley, J.A.; Williams, M.J.A.; Cotter, J.D. “Exercise snacks” before meals: A novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia 2014, 57, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating Glucose Is More Deleterious to Endothelial Function and Oxidative Stress Than Mean Glucose in Normal and Type 2 Diabetic Patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.-P.; Colette, C. Activation of Oxidative Stress by Acute Glucose Fluctuations Compared With Sustained Chronic Hyperglycemia in Patients With Type 2 Diabetes. JAMA 2006, 295, 1681. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.F.; Knowler, W.C.; Pettitt, D.J.; Nelson, R.G.; Charles, M.A.; Bennett, P.H. A two-step model for development of non-insulin-dependent diabetes. Am. J. Med. 1991, 90, 229–235. [Google Scholar] [CrossRef]
- Faber, O.K.; Hagen, C.; Binder, C.; Markussen, J.; Naithani, V.K.; Blix, P.M.; Kuzuya, H.; Horwitz, D.L.; Rubenstein, A.H.; Rossing, N. Kinetics of human connecting peptide in normal and diabetic subjects. J. Clin. Investig. 1978, 62, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Morgan, L.M.; Tredger, J.A.; Deacon, S.; Wright, J.; Marks, V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: Acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993, 138, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Tura, A.; Mari, A.; Ko, S.-H.; Kwon, H.-S.; Song, K.-H.; Yoon, K.-H.; Lee, K.-W.; Ahn, Y.-B. Potentiation of the early-phase insulin response by a prior meal contributes to the second-meal phenomenon in type 2 diabetes. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E984–E990. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Stentz, F.B.; Brewer, A.; Wan, J.; Garber, C.; Daniels, B.; Sands, C.; Kitabchi, A.E. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: Randomized control trial. BMJ Open Diabetes Res. Care 2016, 4, e000258. [Google Scholar] [CrossRef] [PubMed]
- Service, F.J.; Hall, L.D.; Westland, R.E.; O’Brien, P.C.; Go, V.L.W.; Haymond, M.W.; Rizza, R.A. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia 1983, 25, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Peter, R.; Dunseath, G.; Luzio, S.D.; Chudleigh, R.; Roy Choudhury, S.; Owens, D.R. Daytime variability of postprandial glucose tolerance and pancreatic B-cell function using 12-h profiles in persons with Type 2 diabetes. Diabet. Med. 2010, 27, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Mann, J.I.; Williams, S.; Venn, B.J. Advice to walk after meals is more effective for lowering postprandial glycaemia in type 2 diabetes mellitus than advice that does not specify timing: A randomised crossover study. Diabetologia 2016, 59, 2572–2578. [Google Scholar] [CrossRef] [PubMed]
- Heden, T.D.; Winn, N.C.; Mari, A.; Booth, F.W.; Rector, R.S.; Thyfault, J.P.; Kanaley, J.A. Postdinner resistance exercise improves postprandial risk factors more effectively than predinner resistance exercise in patients with type 2 diabetes. J. Appl. Physiol. 2015, 118, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]


| Condition | ||
|---|---|---|
| HE-BF | LE-BF | |
| Body mass (trial day; kg) a | 91.6 ± 13.8 | 91.3 ± 13.8 |
| Body composition b | ||
| Body mass (kg) | 91.5 ± 12.4 | |
| Fat mass (kg) | 38.2 ± 7.1 | |
| Lean mass (kg) | 50.7 ± 10.4 | |
| Visceral adipose tissue (kg) | 2.1 ± 0.7 | |
| Fasting concentrations c | ||
| Plasma glucose (mmol/L) | 5.52 ± 0.76 | 5.22 ± 0.63 |
| Plasma insulin (mIU/mL) | 23.6 ± 13.4 | 21.7 ± 10.8 |
| Plasma C-peptide (nmol/L) | 0.69 ± 0.66 | 0.64 ± 0.49 |
| Plasma tGLP-1 (pmol/L) | 40.6 ± 9.6 | 39.7 ± 10.1 |
| Triglycerides (mmol/L) | 1.47 ± 0.51 | 1.69 ± 0.61 |
| Total Cholesterol (mmol/L) | 4.79 ± 1.42 | 5.06 ± 1.40 |
| HDL cholesterol (mmol/L) | 1.02 ± 0.28 | 1.03 ± 0.23 |
| LDL cholesterol (mmol/L) | 3.11 ± 1.27 | 3.24 ± 1.21 |
| HOMA2-%beta | 165 ± 80 | 183 ± 75 |
| HOMA2-%S | 41 ± 15 | 44 ± 19 |
| HOMA2-IR | 2.84 ± 1.21 | 2.74 ± 1.24 |
| Physical activity time (min/day) d | ||
| Light-intensity | 156 ± 52 | 149 ± 42 |
| Moderate-intensity | 49 ± 38 | 48 ± 24 |
| Vigorous-intensity | 0 ± 0 | 0 ± 0 |
| Proportion of waking hours spent sedentary (%) e | 65 ± 12 | 67 ± 12 |
| Estimated daily energy expenditure (kJ/day) d | 10,296 ± 2275 | 9772 ± 1461 |
| Diet | ||
| Total energy intake (kJ/day) | 7665 ± 2651 | 7519 ± 3128 |
| Total carbohydrate (% of energy intake) | 46.0 ± 7.0 | 46.0 ± 8.6 |
| Total fat (% of energy intake) | 31.6 ± 8.0 | 30.1 ± 5.6 |
| Total protein (% of energy intake) | 18.6 ± 5.3 | 19.3 ± 5.2 |
| Resting energy expenditure (kJ/day) f | 7311 ± 1221 | |
| Measure | Time | HE-BF | LE-BF | Difference (95% CI) |
|---|---|---|---|---|
| Sitting (%) a | Pre-trial | 64 ± 11 | 65 ± 11 | −1 (−6, 4) |
| Trial day | 98 ± 2 * | 98 ± 2 * | 1 (−4, 6) | |
| Post-trial | 68 ± 10 | 71 ± 10 | −2 (−6, 2) | |
| Standing (%) a | Pre-trial | 27 ± 7 | 26 ± 8 | 1 (−3, 5) |
| Trial day | 2 ± 1 * | 2 ± 2 * | 0 (−5, 4) | |
| Post-trial | 22 ± 8 | 20 ± 8 † | 2 (−2, 6) | |
| Stepping (%) a | Pre-trial | 9 ± 6 | 9 ± 4 | 0 (−2, 2) |
| Trial day | 1 ± 1 * | 1 ± 1 * | −1 (−3, 1) | |
| Post-trial | 9 ± 5 | 9 ± 4 | 0 (−1, 2) | |
| Sitting 30 min blocks (%) a | Pre-trial | 46 ± 15 | 50 ± 15 | 0 (−2, 2) |
| Trial day | 96 ± 4 * | 98 ± 3 * | −1 (−3, 1) | |
| Post-trial | 57 ± 15 | 56 ± 23 | 0 (−2, 2) | |
| Energy expenditure (kJ/day) b | Trial day | 9053 ± 1715 | 8561 ± 1370 | 613 (−243, 1469) |
| Measure | HE-BF | LE-BF | Difference (95% CI) | p-Value |
|---|---|---|---|---|
| AUCtotal (mmol/h/L) | 155.4 ± 15.8 | 153.5 ± 16.8 | 1.91 (−7.22, 11.03) | 0.66 |
| AUCtotal8-6 (mmol/h/L) | 69.8 ± 8.4 | 65.3 ± 7.4 | 4.43 (0.64, 8.21) | 0.026 |
| Mean glucose (mmol/L) | 6.53 ± 0.65 | 6.45 ± 0.70 | 0.08 (−0.30, 0.46) | 0.66 |
| SDglucose (mmol/L) | 0.99 ± 0.34 | 1.00 ± 0.43 | −0.02 (−0.27, 0.24) | 0.89 |
| MAGE (mmol/L) | 2.48 ± 1.24 | 2.63 ± 1.27 | −0.15 (−1.0, 0.70) | 0.71 |
| CONGA-1 | 6.05 ± 0.62 | 5.80 ± 0.63 | 0.25 (0.63, −0.13) | 0.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parr, E.B.; Devlin, B.L.; Pinto, S.K.; Dunstan, D.W.; Hawley, J.A. Impact of First Meal Size during Prolonged Sitting on Postprandial Glycaemia in Individuals with Prediabetes: A Randomised, Crossover Study. Nutrients 2018, 10, 733. https://doi.org/10.3390/nu10060733
Parr EB, Devlin BL, Pinto SK, Dunstan DW, Hawley JA. Impact of First Meal Size during Prolonged Sitting on Postprandial Glycaemia in Individuals with Prediabetes: A Randomised, Crossover Study. Nutrients. 2018; 10(6):733. https://doi.org/10.3390/nu10060733
Chicago/Turabian StyleParr, Evelyn B., Brooke L. Devlin, Samuel K. Pinto, David W. Dunstan, and John A. Hawley. 2018. "Impact of First Meal Size during Prolonged Sitting on Postprandial Glycaemia in Individuals with Prediabetes: A Randomised, Crossover Study" Nutrients 10, no. 6: 733. https://doi.org/10.3390/nu10060733
APA StyleParr, E. B., Devlin, B. L., Pinto, S. K., Dunstan, D. W., & Hawley, J. A. (2018). Impact of First Meal Size during Prolonged Sitting on Postprandial Glycaemia in Individuals with Prediabetes: A Randomised, Crossover Study. Nutrients, 10(6), 733. https://doi.org/10.3390/nu10060733





