Iron Promotes Intestinal Development in Neonatal Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Piglets and Experimental Design
2.2. Iron Measurement and Hepatic Iron Staining
2.3. Real-Time PCR Analysis
2.4. Histomorphology Analysis
2.5. Western Blot Analysis
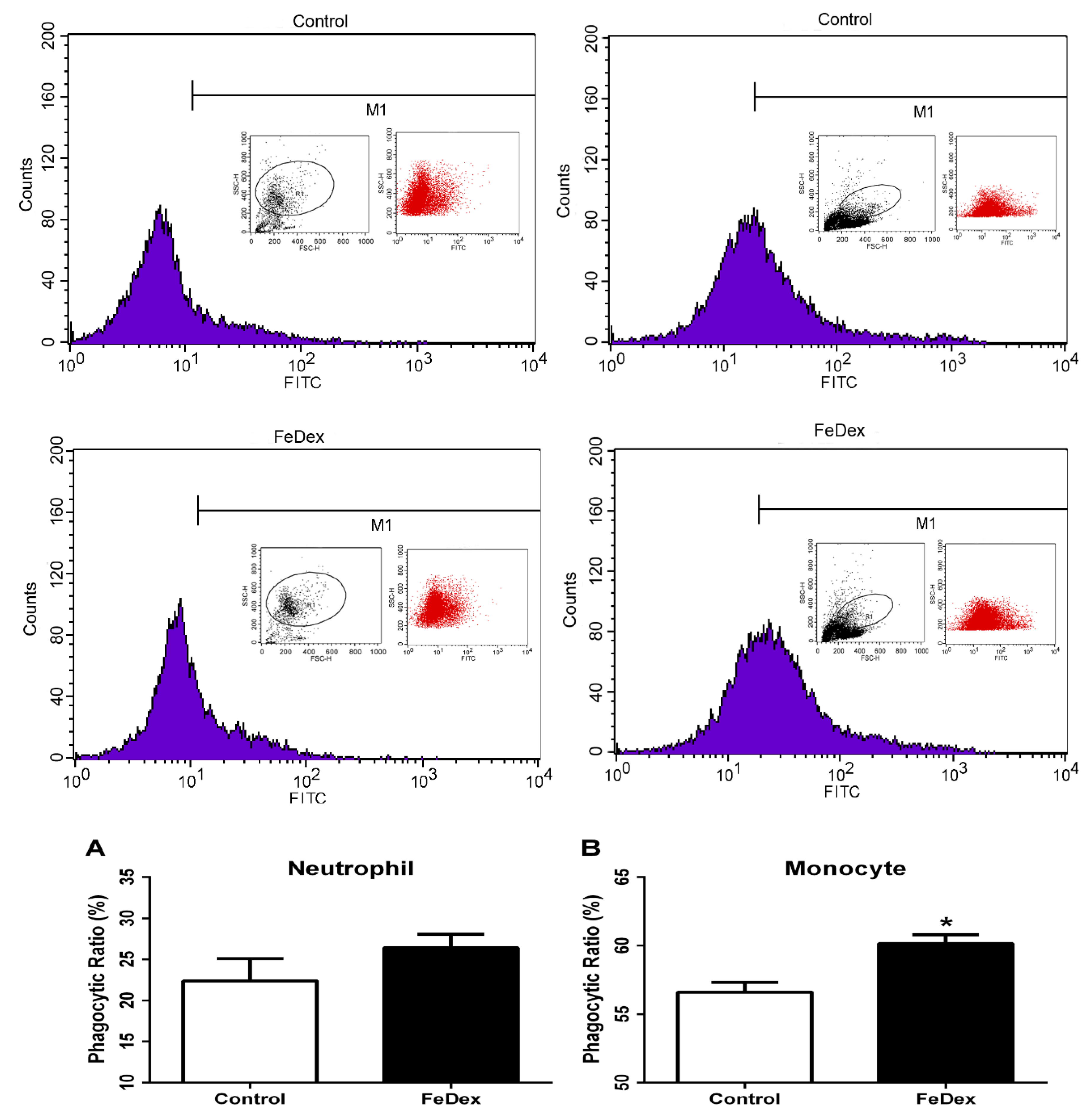
2.6. Phagocytosis Assay
2.7. Statistical Analysis
3. Results
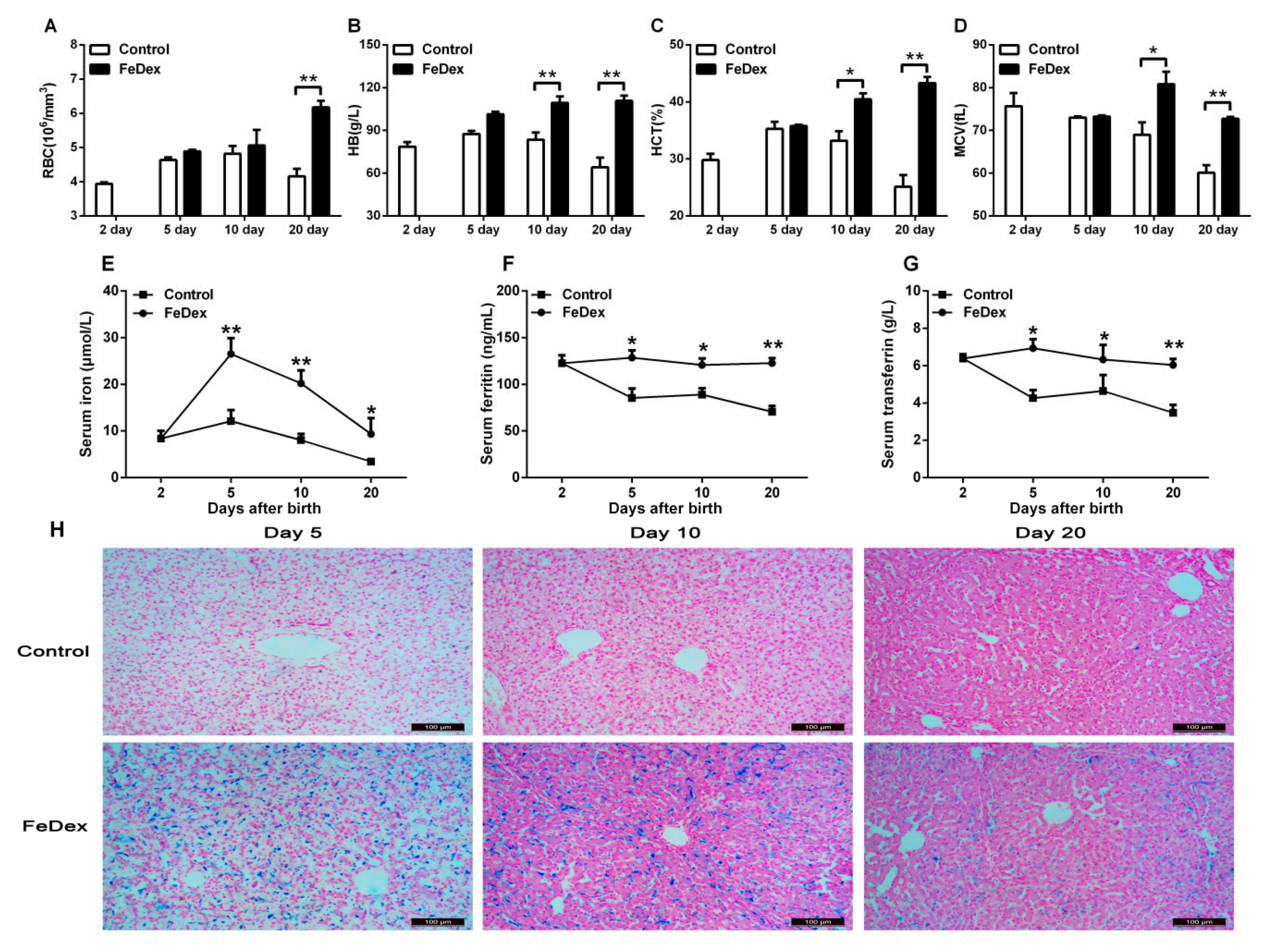
3.1. Iron Supplementation Improved Iron Status of Piglets
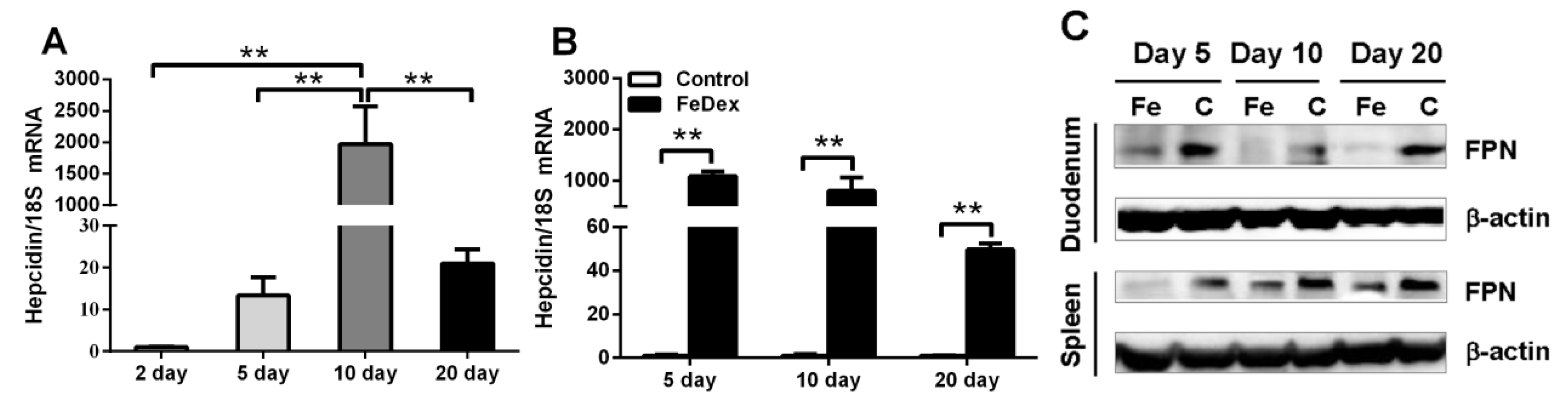
3.2. Relationship between Iron Status of Piglets and Hepcidin mRNA Level
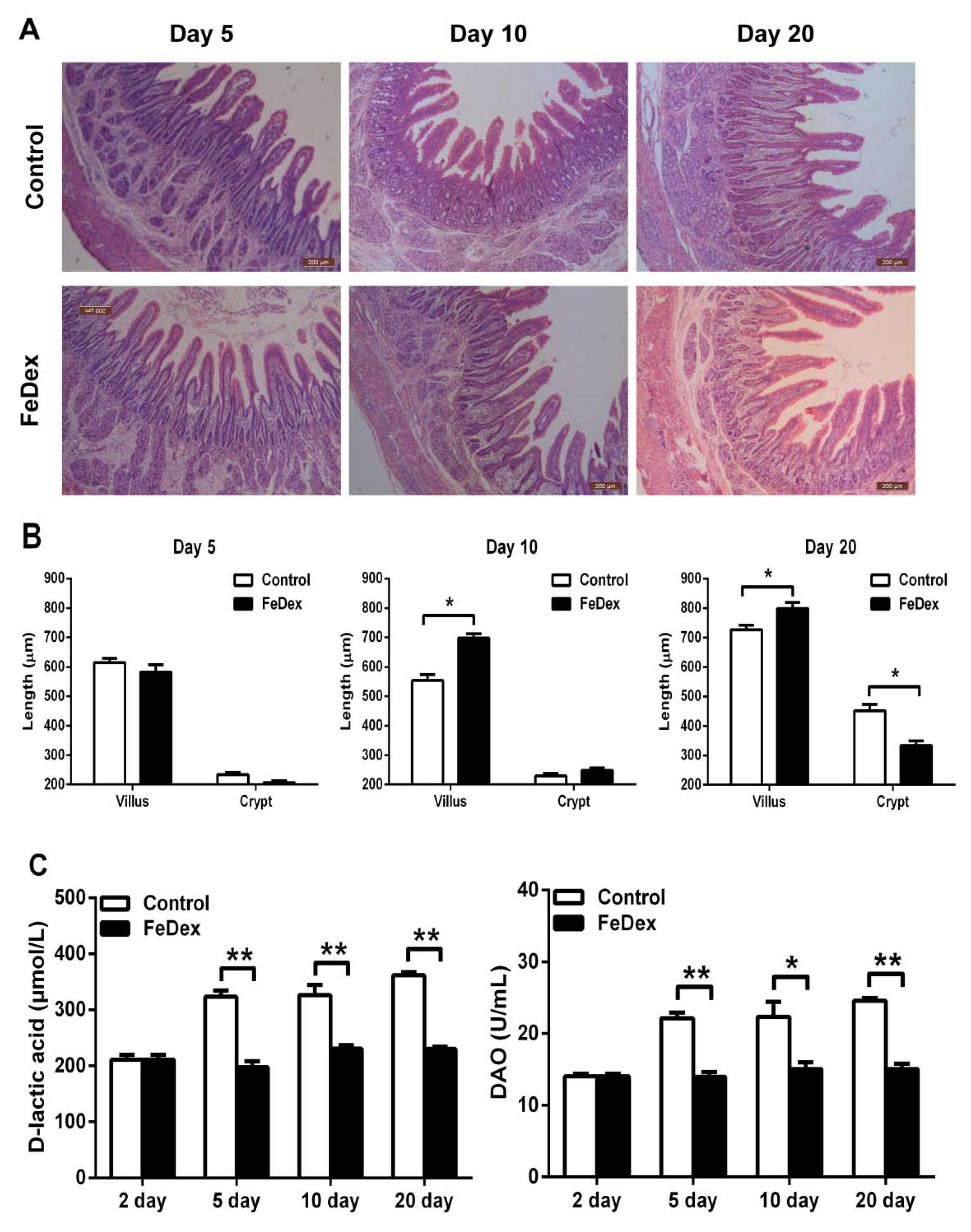
3.3. Iron Supplementation Attenuated the Damage of Duodenal Mucosal
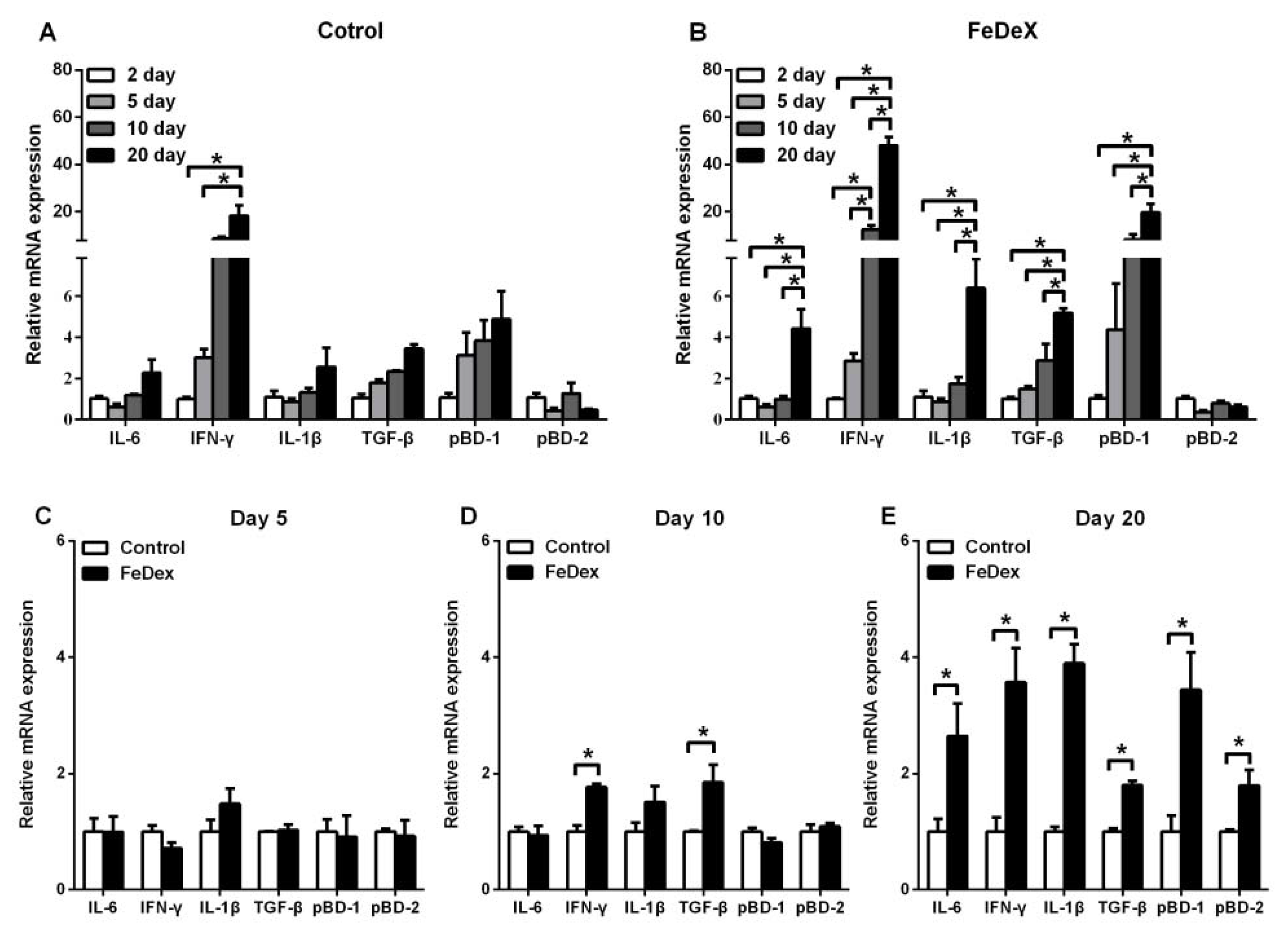
3.4. Iron Supplementation Stimulated the Expression of Cytokines Genes of Intestine
3.5. Iron Supplementation Enhanced Phagocytotic Capacity of Monocytes
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.F. Iron deficiency anemia: Diagnosis and management. Curr. Opin. Gastroenterol. 2009, 25, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, P.; Starzynski, R.R.; Canonne-Hergaux, F.; Tudek, B.; Oliński, R.; Kowalczyk, P.; Dziaman, T.; Thibaudeau, O.; Gralak, M.A.; Smuda, E.; et al. Benefits and risks of iron supplementation in anemic neonatal pigs. Am. J. Pathol. 2010, 177, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Arosio, P. Iron homeostasis in health and disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Georgieff, M.K. Iron in fetal and neonatal nutrition. Semin. Fetal Neonatal Med. 2007, 12, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568S–579S. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.L.; Baltathakis, I.; Alcantara, O.S.; Boldt, D.H. Differentiation of functional dendritic cells and macrophages from human peripheral blood monocyte precursors is dependent on expression of p21 (WAF1/CIP1) and requires iron. Br. J. Haematol. 2002, 117, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Hallquist, N.A.; McNeil, L.K.; Lockwood, J.F.; Sherman, A.R. Maternal-iron-deficiency effects on peritoneal macrophage and peritoneal natural-killer-cell cytotoxicity in rat pups. Am. J. Clin. Nutr. 1992, 55, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi-Riani, E.; Danger, R.; Lozano, J.J.; Martinez-Picola, M.; Kodela, E.; Mas-Malavila, R.; Bruguera, M.; Collins, H.L.; Hider, R.C.; Martinez-Llordella, M.; et al. Iron Deficiency Impairs Intra-Hepatic Lymphocyte Mediated Immune Response. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Gutiérrez, A.G.; González-Rosendo, G.; Sánchez-Muñoz, J.; Polo-Pozo, J.; Rodríguez-Jerez, J.J. Bioavailability of heme iron in biscuit filling using piglets as an animal model for humans. Int. J. Biol. Sci. 2008, 4, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Drabek, J. Iron deficiency in suckling piglets: Etiology, clinical aspects and diagnosis. Folia Vet. 2005, 49, 104–111. [Google Scholar]
- Egeli, A.K.; Framstad, T. An evaluation of iron-dextran supplementation in piglets administered by injection on the first, third or fourth day after birth. Res. Vet. Sci. 1999, 66, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, T.; Ono, M.; Kubota, K.; Hirose, A.; Hayashi, Y.; Saibara, T.; Inanami, O.; Ogawa, Y.; Enzan, H.; Onishi, S.; et al. Super paramagnetic iron oxide MRI shows defective Kupffer cell uptake function in non-alcoholic fatty liver disease. Gut 2010, 59, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Valore, E.V.; Nemeth, E.; Goodnough, J.B.; Gabayan, V.; Ganz, T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood 2007, 110, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Yuan, L.; Azevedo, M.S.; Jeong, K.I.; Gonzalez, A.M.; Saif, L.J. Transfer of maternal cytokines to suckling piglets: In vivo and in vitro models with implications for immunomodulation of neonatal immunity. Vet. Immunol. Immunopathol. 2007, 117, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Comstock, S.S.; Reznikov, E.A.; Contractor, N.; Donovan, S.M. Dietary bovine lactoferrin alters mucosal and systemic immune cell responses in neonatal piglets. J. Nutr. 2014, 144, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Trakooljul, N.; Liu, H.C.; Moeser, A.J.; Spears, J.W. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J. Nutr. 2009, 139, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Bencze, K.Z.; Stemmler, T.L.; Philpott, C.C. A cytosolic iron chaperone that delivers iron to ferritin. Science 2008, 320, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Massupha, W.; Boonrit, T.; Sarinee, K.T. Growth and small intestine histomorphology of low and normal birth weight piglets during the early suckling period. Livest. Sci. 2013, 158, 215–222. [Google Scholar] [CrossRef]
- Watson, A.; Lipina, C.; McArdle, H.J.; Taylor, P.M.; Hundal, H.S. Iron depletion suppresses mTORC1-directed signaling in intestinal Caco-2 cells via induction of REDD1. Cell Signal 2016, 28, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C. Nutritionally mediated programming of the developing immune system. Adv. Nutr. 2011, 2, 377–395. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Y.; Li, S.; Xiong, H.; Zhang, X.; Wang, Y.; Du, H. Iron Promotes Intestinal Development in Neonatal Piglets. Nutrients 2018, 10, 726. https://doi.org/10.3390/nu10060726
Pu Y, Li S, Xiong H, Zhang X, Wang Y, Du H. Iron Promotes Intestinal Development in Neonatal Piglets. Nutrients. 2018; 10(6):726. https://doi.org/10.3390/nu10060726
Chicago/Turabian StylePu, Yutian, Shuhui Li, Haitao Xiong, Xiaofeng Zhang, Yizhen Wang, and Huahua Du. 2018. "Iron Promotes Intestinal Development in Neonatal Piglets" Nutrients 10, no. 6: 726. https://doi.org/10.3390/nu10060726
APA StylePu, Y., Li, S., Xiong, H., Zhang, X., Wang, Y., & Du, H. (2018). Iron Promotes Intestinal Development in Neonatal Piglets. Nutrients, 10(6), 726. https://doi.org/10.3390/nu10060726




