Combining Short-Term Interval Training with Caloric Restriction Improves ß-Cell Function in Obese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Low-Calorie Diet (LCD)
2.3. Exercise Training
2.4. Metabolic Control
2.5. Cardiorespiratory Fitness
2.6. Body Composition
2.7. Pancreatic ß-Cell Function
2.8. Biochemical Analyses
2.9. Statistical Analysis
3. Results
3.1. Diet and Exercise Compliance
3.2. Body Composition and Fitness
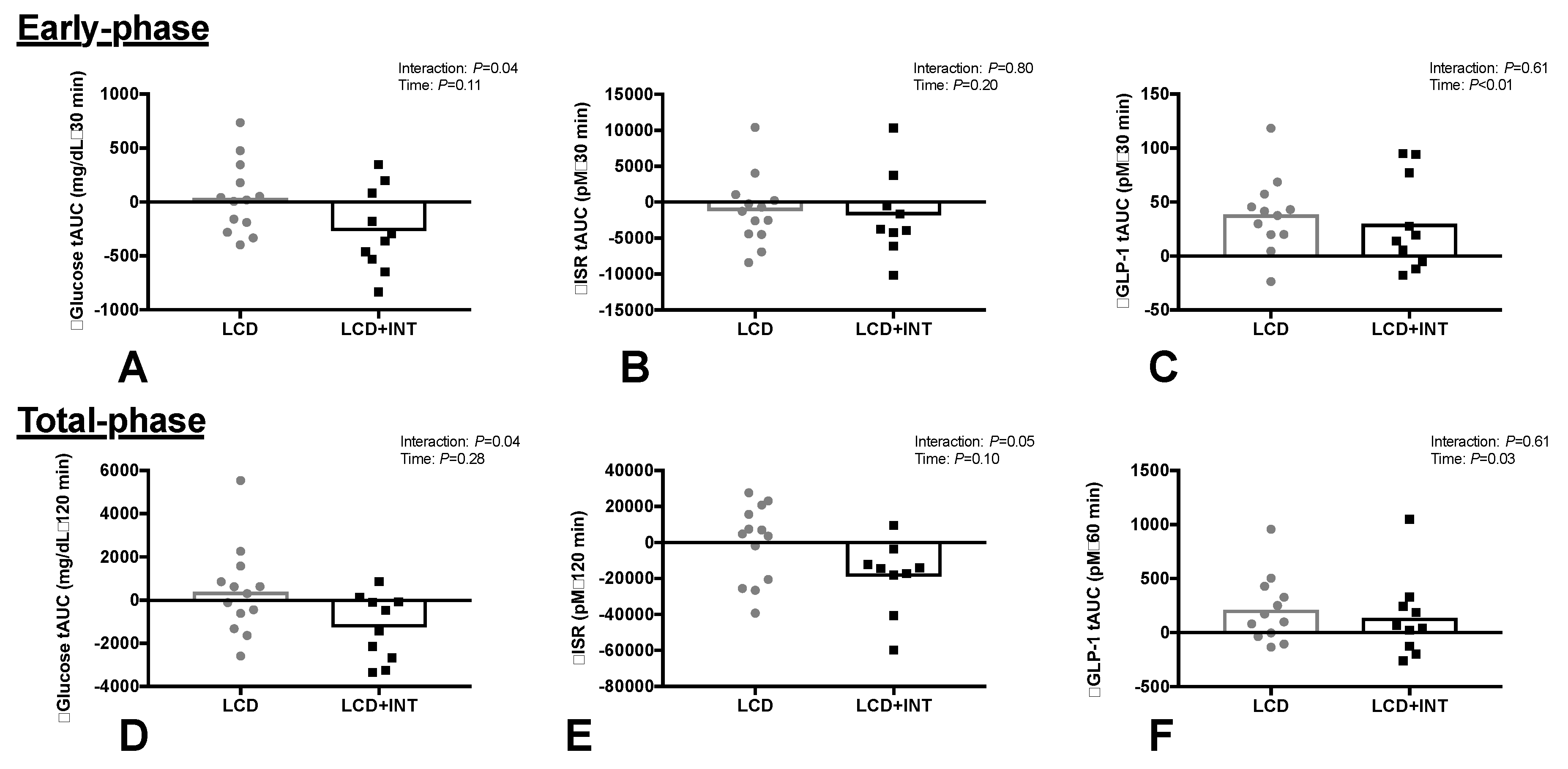
3.3. Glucose and Hormone Responses
3.4. Insulin Resistance
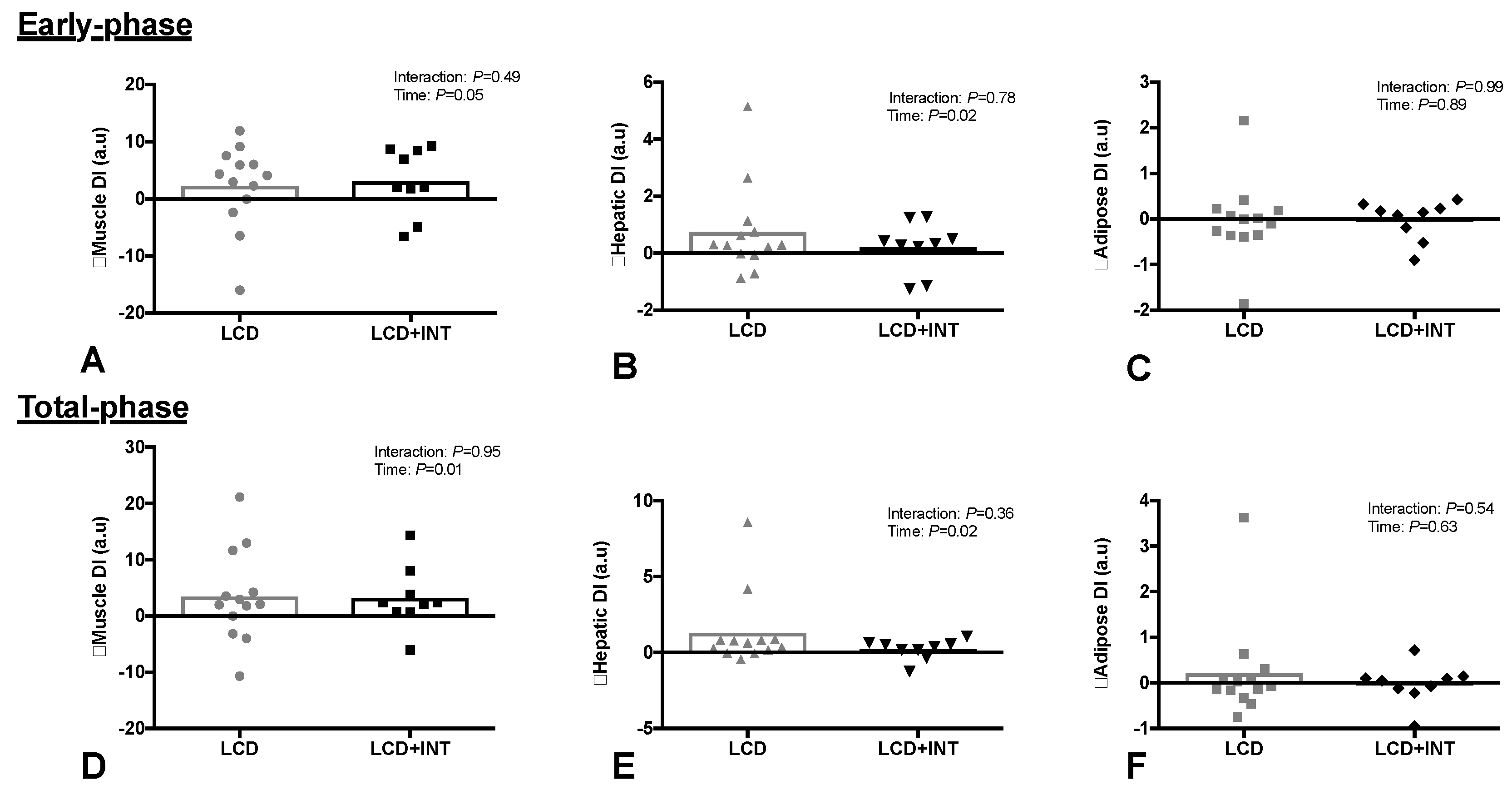
3.5. Pancreatic ß-Cell Function
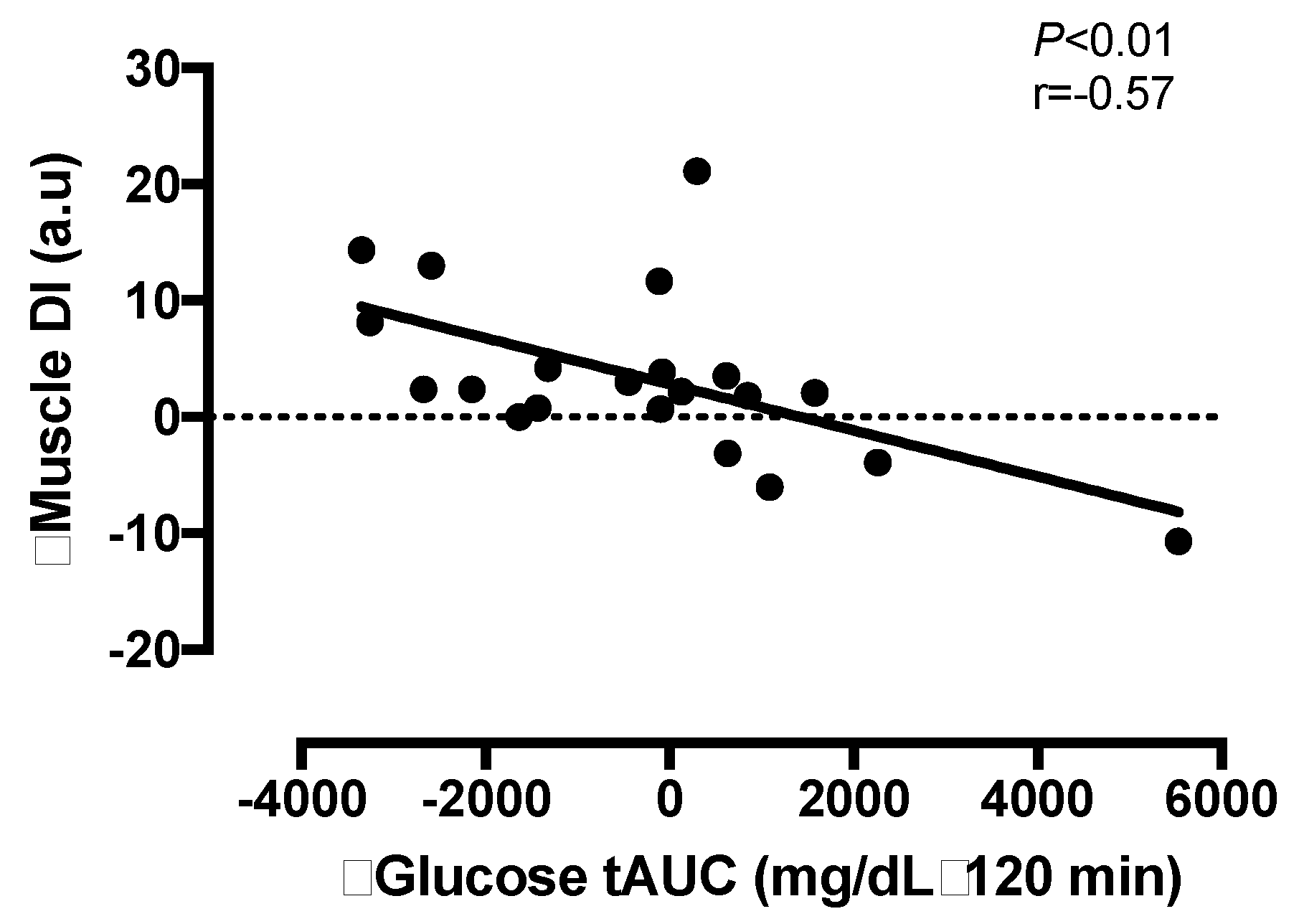
3.6. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight factsheet from the WHO. Health. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/index.html (accessed on 28 April 2018).
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, K.; Given, B.; Van Cauter, E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Investig. 1988, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E. The importance of β-cell failure in the development and progression of type 2 diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 4047–4058. [Google Scholar] [PubMed]
- Kahn, S.E.; Prigeon, R.L.; McCulloch, D.K.; Boyko, E.J.; Bergman, R.N.; Schwartz, M.W.; Neifing, J.L.; Ward, W.K.; Beard, J.C.; Palmer, J.P. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: Evidence for a hyperbolic function. Diabetes 1993, 42, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.P.; Malin, S.K.; Karstoft, K.; Knudsen, S.H.; Haus, J.M.; Laye, M.J.; Pedersen, M.; Pedersen, B.K.; Kirwan, J.P. Determining pancreatic β-cell compensation for changing insulin sensitivity using an oral glucose tolerance test. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E822–E829. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, K.M.; Prigeon, R.L.; Faulenbach, M.V.; Tong, J.; Carr, D.B.; Boyko, E.J.; Leonetti, D.L.; McNeely, M.J.; Fujimoto, W.Y.; Kahn, S.E. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009, 32, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.; Weyer, C. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia 2001, 44, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.P.; Albert, S.G.; Reeds, D.N.; Kress, K.S.; Ezekiel, U.R.; McDaniel, J.L.; Patterson, B.W.; Klein, S.; Villareal, D.T. Calorie restriction and matched weight loss from exercise: Independent and additive effects on glucoregulation and the incretin system in overweight women and men. Diabetes Care 2015, 38, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Weinsier, R.L.; James, L.D.; Darnell, B.E.; Wooldridge, N.H.; Birch, R.; Hunter, G.R.; Bartolucci, A.A. Lipid and insulin concentrations in obese postmenopausal women: Separate effects of energy restriction and weight loss. Am. J. Clin. Nutr. 1992, 56, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, A.P.; Iozzo, P.; Borra, R.; Kankaanpää, M.; Karmi, A.; Lautamäki, R.; Järvisalo, M.; Parkkola, R.; Rönnemaa, T.; Guiducci, L.; et al. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.M.; Thorup, A.C.; Overgaard, K.; Jeppesen, P.B. High intensity interval training improves glycaemic control and pancreatic β cell function of type 2 diabetes patients. PLoS ONE 2015, 10, e0133286. [Google Scholar] [CrossRef] [PubMed]
- Slentz, C.A.; Tanner, C.J.; Bateman, L.A.; Durheim, M.T.; Huffman, K.M.; Houmard, J.A.; Kraus, W.E. Effects of exercise training intensity on pancreatic β-cell function. Diabetes Care 2009, 32, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Solomon, T.P.; Blaszczak, A.; Finnegan, S.; Filion, J.; Kirwan, J.P. Pancreatic β-cell function increases in a linear dose-response manner following exercise training in adults with prediabetes. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1248–E1254. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.L.; Pollock, N.K.; Waller, J.L.; Allison, J.D.; Dennis, B.A.; Bassali, R.; Meléndez, A.; Boyle, C.A.; Gower, B.A. Exercise dose and diabetes risk in overweight and obese children: A randomized controlled trial. JAMA 2012, 308, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Durrer, C.; Pistaweeka, K.J.; Halperin, F.A.; Chang, C.; Little, J.P. Combined interval training and post-exercise nutrition in type 2 diabetes: A randomized control trial. Front. Physiol. 2017, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; James, N.G.; Scheel, M.M.; Olesen, J.; Holst, J.J.; Pedersen, B.K.; Solomon, T.P.J. Mechanisms behind the superior effects of interval vs continuous training on glycaemic control in individuals with type 2 diabetes: A randomised controlled trial. Diabetologia 2014, 57, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Rynders, C.A.; Weltman, J.Y.; Barrett, E.J. Weltman A Exercise intensity modulates glucose-stimulated insulin secretion when adjusted for adipose, liver and skeletal muscle insulin resistance. PLoS ONE 2016, 11, e0154063. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G. Effect of degree of weight loss on health benefits. Obes. Res. 1995, 3, 211s–216s. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Weiss, E.P.; Albert, S.G.; Reeds, D.N.; Kress, K.S.; McDaniel, J.L.; Klein, S.; Villareal, D.T. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: A randomized intervention trial. Am. J. Clin. Nutr. 2016, 10, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, N.M.; Paisley, A.S.; Kranz, S.; Weltman, A.; Kirby, J.L.; Hallowell, P.T.; Malin, S.K. Bariatric Surgery Resistance: Using Preoperative Lifestyle Medicine and/or Pharmacology for Metabolic Responsiveness. Obes. Surg. 2017, 27, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; Nielsen, J.S.; Thomsen, C.; Pedersen, B.K.; Solomon, T.P.J. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients. Diabetes Care 2013, 36, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Mestrez, F.; Sturis, J.; Polonsky, K.S. Estimation of insulin secretion rates from C-peptide levels: Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992, 41, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Kanat, M.; DeFronzo, R.A.; Abdul-Ghani, M.A. Treatment of prediabetes. World J. Diabetes 2015, 6, 1207. [Google Scholar] [CrossRef] [PubMed]
- Assali, A.R.; Ganor, A.; Beigel, Y.; Shafer, Z.; Hershcovici, T. Fainaru Insulin resistance in obesity: Body-weight or energy balance? J. Endocrinol. 2001, 171, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rojo, R.; Alcala-Diaz, J.F.; Wopereis, S.; Perez-Martinez, P.; Quintana-Navarro, G.M.; Marin, C.; Ordovas, J.M.; van Ommen, B.; Perez-Jimenez, F.; Delgado-Lista, J.; et al. The insulin resistance phenotype (muscle or liver) interacts with the type of diet to determine changes in disposition index after 2 years of intervention: The CORDIOPREV-DIAB randomised clinical trial. Diabetologia 2016, 59, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.P.; Haus, J.M.; Kelly, K.R.; Rocco, M.; Kashyap, S.R.; Kirwan, J.P. Improved pancreatic β-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010, 33, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Heilbronn, L.K.; Redman, L.M.; Newcomer, B.R.; Frisard, M.I.; Anton, S.; Smith, S.R.; Alfonso, A.; Ravussin, E.; The Pennington CALERIE Team. Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006, 29, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.P.; Solomon, T.P. Do skeletal muscle-secreted factors influence the function of pancreatic beta β-cells? Am. J. Physiol. Endocrinol. Metab. 2018, 314, E297–E307. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.; Schwartz, T.; Lovgreen, N.; Pedersen, O.; Beck-Nielsen, H. Diurnal profile of pancreatic polypeptide, pancreatic glucagon, gut glucagon and insulin in human morbid obesity. Int. J. Obes. 1983, 7, 529–538. [Google Scholar] [PubMed]
- Stamets, K.; Taylor, D.S.; Kunselman, A.; Demers, L.M.; Pelkman, C.L.; Legro, R.S. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil. Steril. 2004, 81, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.R.; Brooks, L.M.; Solomon, T.P.; Kashyap, S.R.; O'Leary, V.B.; Kirwan, J.P. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1269–E1274. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Vardarli, I.; Deacon, C.; Holst, J.J.; Meier, J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia 2011, 54, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Umeda, L.M.; Silva, E.A.; Carneiro, G.; Arasaki, C.H.; Geloneze, B.; Zanella, M.T. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes. Surg. 2011, 21, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Cani, P.D. Gut microbiota and GLP-1. Rev. Endocr. Metab. Disord. 2014, 15, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Heden, T.D.; Liu, Y.; Kearney, M.L.; Park, Y.; Dellsperger, K.C.; Thomas, T.R.; Kanaley, J.A. Prior exercise and postprandial incretin responses in lean and obese individuals. Med. Sci. Sports Exerc. 2013, 45, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, M.; Abolfathi, F.; Alizadeh, A.A. Serum Glucagon-Like Peptide-1 Changes in Women with Type 2 Diabetes Following a Four Weeks Aerobic Exercise. Iran. J. Diab. Obes. 2016, 8, 61–66. [Google Scholar]
- Kullman, E.L.; Kelly, K.R.; Haus, J.M.; Fealy, C.E.; Scelsi, A.R.; Pagadala, M.R.; Flask, C.A.; McCullough, A.J.; Kirwan, J.P. Short-term aerobic exercise training improves gut peptide regulation in nonalcoholic fatty liver disease. J. Appl. Physiol. 2016, 120, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
| LCD | LCD + INT | Time (p-Value) | Interaction (p-Value) | |||
|---|---|---|---|---|---|---|
| Pre | Change | Pre | Change | |||
| N, (M/F) | 12 (1/11) | 11 (0/11) | ||||
| Age (year) | 45 ± 12 | 47 ± 14 | ||||
| Body Composition | ||||||
| Height (cm) | 166 ± 6 | 168 ± 6 | ||||
| Body mass (kg) | 103.2 ± 15.8 | −2.9 ± 0.9 | 107.3 ± 20.2 | −1.7 ± 1.3 | <0.01 | 0.02 |
| BMI (kg/m2) | 37.4 ± 6.3 | −1.0 ± 0.3 | 38.0 ± 7.7 | −0.3 ± 0.8 | <0.01 | <0.01 |
| Body fat (%) | 46.7 ± 6.4 | −0.3 ± 0.9 | 48.2 ± 5.7 | 0.1 ± 1.1 | 0.46 | 0.20 |
| Body fat (kg) | 49.0 ± 12.7 | −1.4 ± 1.0 | 52.2 ± 14.6 | −0.6 ± 1.0 | <0.01 | 0.07 |
| Fat-free mass (kg) | 51.4 ± 6.3 | −0.7 ± 0.4 | 52.3 ± 9.0 | 1.0 ± 0.9 | 0.05 | 0.02 |
| Body Water (L) | 41.3 ± 5.7 | −0.8 ± 0.5 | 40.0 ± 5.8 | −0.7 ± 1.0 | <0.01 | 0.67 |
| Fitness | ||||||
| VO2 peak (L/min) | 1.9 ± 0.5 | −0.1 ± 0.3 | 1.9 ± 0.4 | 0.1 ± 0.3 | 0.98 | 0.04 |
| VO2 peak (mg/kg/min) | 19.7 ± 4.9 | −0.5 ± 1.6 | 18.6 ± 4.9 | 1.4 ± 2.1 | 0.28 | 0.03 |
| Bloods | ||||||
| FPG (mg/dL) | 96.6 ± 4.8 | −3.6 ± 8.0 | 97.7 ± 7.7 | −2.2 ± 6.7 | 0.01 | 0.93 |
| 2 h PG (mg/dL) | 110.5 ± 21.7 | 7.7 ± 22.7 | 123.2 ± 21.3 | −5.1 ± 24.5 | 0.61 | 0.13 |
| FIns (µU/mL) | 15.4 ± 9.0 | −2.9 ± 6.0 | 21.7 ± 20.1 | −4.1 ± 7.7 | 0.05 | 0.73 |
| 2 h Ins (µU/mL) | 76.1 ± 52.0 | −0.9 ± 22.7 | 148.0 ± 110.4 | −26.3 ± 61.1 | 0.15 | 0.17 |
| FC-pep (ng/mL) | 2.2 ± 0.7 | −0.3 ± 0.5 | 2.6 ± 1.3 | −0.4 ± 0.5 | <0.01 | 0.84 |
| 2 h C-pep (ng/mL) | 8.0 ± 2.9 | 0.5 ± 1.9 | 11.8 ± 4.0 | −2.5 ± 2.5 | 0.04 | <0.01 |
| Diet | ||||||
| Energy intake (kcal) | 2243 ± 759 | −854 ± 768 | 2110 ± 648 | −639 ± 580 | <0.01 | 0.47 |
| CHO (%) | 46 ± 7 | 7 ± 7 | 46 ± 11 | 7 ± 10 | <0.01 | 0.89 |
| Protein (%) | 16 ± 4 | 2 ± 4 | 16 ± 4 | 3 ± 1 | 0.01 | 0.61 |
| Fat (%) | 38 ± 7 | −9 ± 7 | 39 ± 8 | −12 ± 2 | <0.01 | 0.37 |
| LCD | LCD + INT | Time (p-Value) | Interaction (p-Value) | |||
|---|---|---|---|---|---|---|
| Pre | Change | Pre | Change | |||
| Early-Phase Responses | ||||||
| Insulin tAUC30 (µU/mL•30 min) | 2206 ± 1154 | −408 ± 885 | 2544 ± 1238 | −368 ± 670 | 0.07 | 0.66 |
| C-peptide tAUC30 (ng/mL•30 min) | 148 ± 46 | −11 ± 35 | 164 ± 57 | −22 ± 35 | 0.25 | 0.62 |
| ISR (pM •30 min) | 20652 ± 1878 | −1211 ± 4828 | 23647 ± 2299 | −1804 ± 5938 | 0.20 | 0.80 |
| GSIS (pM•min/mg/dL) | 5.31 ± 1.29 | −0.26 ± 1.01 | 6.50 ± 1.41 | −0.05 ± 1.52 | 0.44 | 0.55 |
| HC (µU/mL•mg/dL−1) | 12.5 ± 5.1 | −0.6 ± 3.3 | 13.9 ± 3.7 | −0.4 ± 2.0 | 0.44 | 0.91 |
| Total phase responses | ||||||
| Insulin tAUC120 (µU/mL•120 min) | 11094 ± 5665 | −1685 ± 3882 | 14872 ± 8951 | −2525 ± 3185 | 0.01 | 0.71 |
| C-peptide tAUC120 (ng/mL•120 min) | 895 ± 260 | −10 ± 167 | 1092 ± 340 | −109 ± 122 | 0.14 | 0.22 |
| ISR (pM•120 min) | 96013 ± 8088 | −283 ± 2129 | 130861 ± 14511 | −18956 ± 20239 | 0.10 | 0.05 |
| GSIS (pM•min/mg/dL) | 5.94 ± 1.73 | 0.23 ± 0.91 | 7.95 ± 1.68 | −0.52 ± 1.1 | 0.24 | 0.41 |
| HC (µU/mL•mg/dL−1) | 11.7 ± 3.8 | −1.7 ± 2.7 | 13.4 ± 4.5 | −1.2 ± 2.2 | 0.01 | 0.60 |
| LCD | LCD + INT | Time (p-Value) | Interaction (p-Value) | |||
|---|---|---|---|---|---|---|
| Pre | Change | Pre | Change | |||
| Skeletal muscle IR | 0.38 ± 0.21 | −0.08 ± 0.13 | 0.64 ± 0.53 | −0.16 ± 0.19 | <0.01 | 0.24 |
| Hepatic IR | 3.3 ± 2.1 | −1.0 ± 1.7 | 5.8 ± 5.7 | −1.3 ± 2.5 | 0.01 | 0.59 |
| Adipose IR | 7.6 ± 4.6 | 0.1 ± 2.9 | 13.3 ± 13.3 | −2.4 ± 6.2 | 0.14 | 0.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francois, M.E.; Gilbertson, N.M.; Eichner, N.Z.M.; Heiston, E.M.; Fabris, C.; Breton, M.; Mehaffey, J.H.; Hassinger, T.; Hallowell, P.T.; Malin, S.K. Combining Short-Term Interval Training with Caloric Restriction Improves ß-Cell Function in Obese Adults. Nutrients 2018, 10, 717. https://doi.org/10.3390/nu10060717
Francois ME, Gilbertson NM, Eichner NZM, Heiston EM, Fabris C, Breton M, Mehaffey JH, Hassinger T, Hallowell PT, Malin SK. Combining Short-Term Interval Training with Caloric Restriction Improves ß-Cell Function in Obese Adults. Nutrients. 2018; 10(6):717. https://doi.org/10.3390/nu10060717
Chicago/Turabian StyleFrancois, Monique E., Nicole M. Gilbertson, Natalie Z. M. Eichner, Emily M. Heiston, Chiara Fabris, Marc Breton, J. Hunter Mehaffey, Taryn Hassinger, Peter T. Hallowell, and Steven K. Malin. 2018. "Combining Short-Term Interval Training with Caloric Restriction Improves ß-Cell Function in Obese Adults" Nutrients 10, no. 6: 717. https://doi.org/10.3390/nu10060717
APA StyleFrancois, M. E., Gilbertson, N. M., Eichner, N. Z. M., Heiston, E. M., Fabris, C., Breton, M., Mehaffey, J. H., Hassinger, T., Hallowell, P. T., & Malin, S. K. (2018). Combining Short-Term Interval Training with Caloric Restriction Improves ß-Cell Function in Obese Adults. Nutrients, 10(6), 717. https://doi.org/10.3390/nu10060717






