Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function
Abstract
1. Introduction
2. Materials and Methods
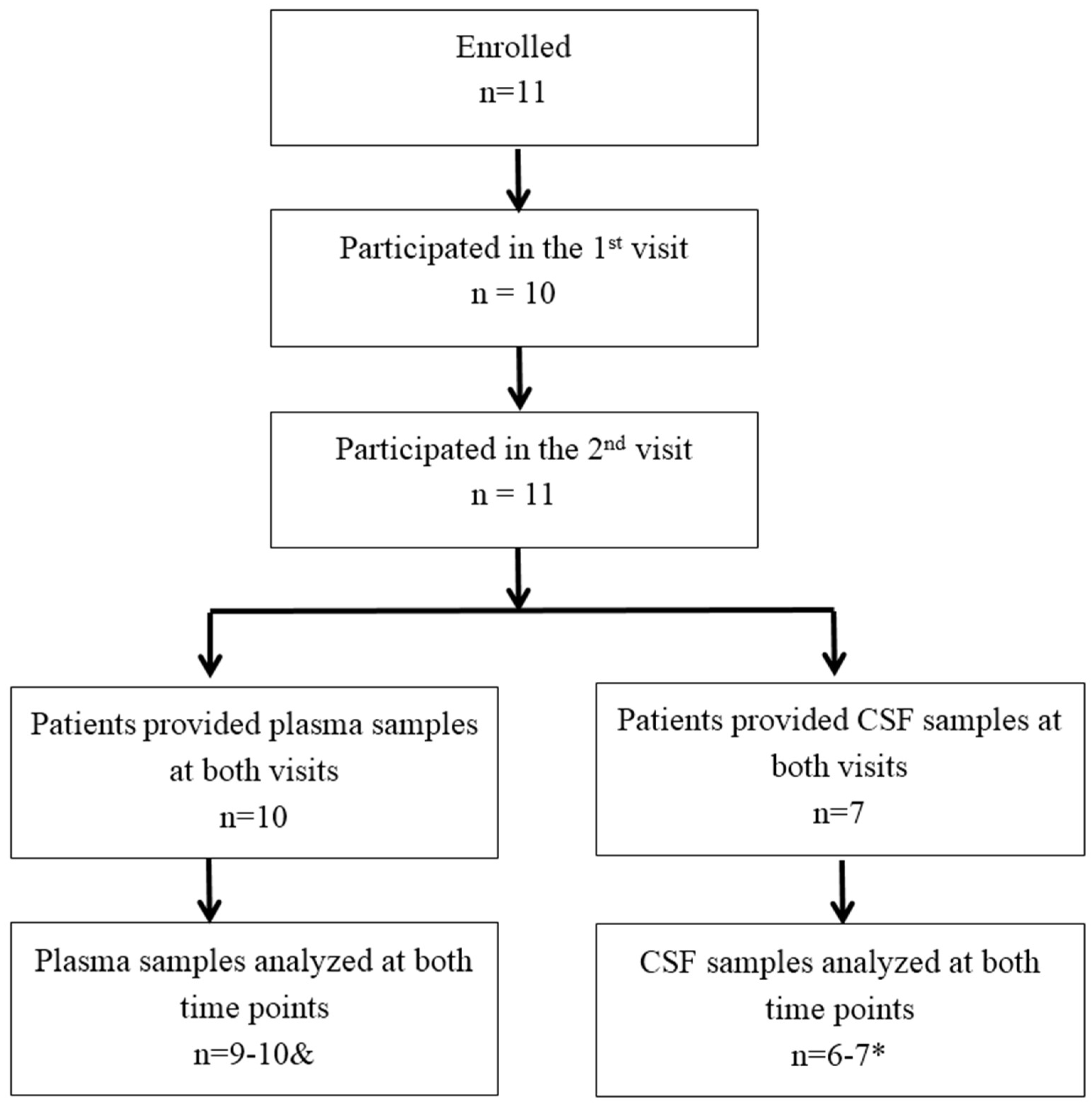
2.1. Recruitment and Clinical Information of Parkinson Patients
2.2. Trial Design
2.3. Samples Collection
2.3.1. CSF and Plasma Samples
2.3.2. In Vitro Samples
2.4. cGP Assays
2.5. High Performance Liquid Chromatography Mass Spectrometry Assay (HPLC-ms)
2.6. ELISA
2.7. Statistical Analysis
3. Results
3.1. Analysis of cGP Concentration in the BCA
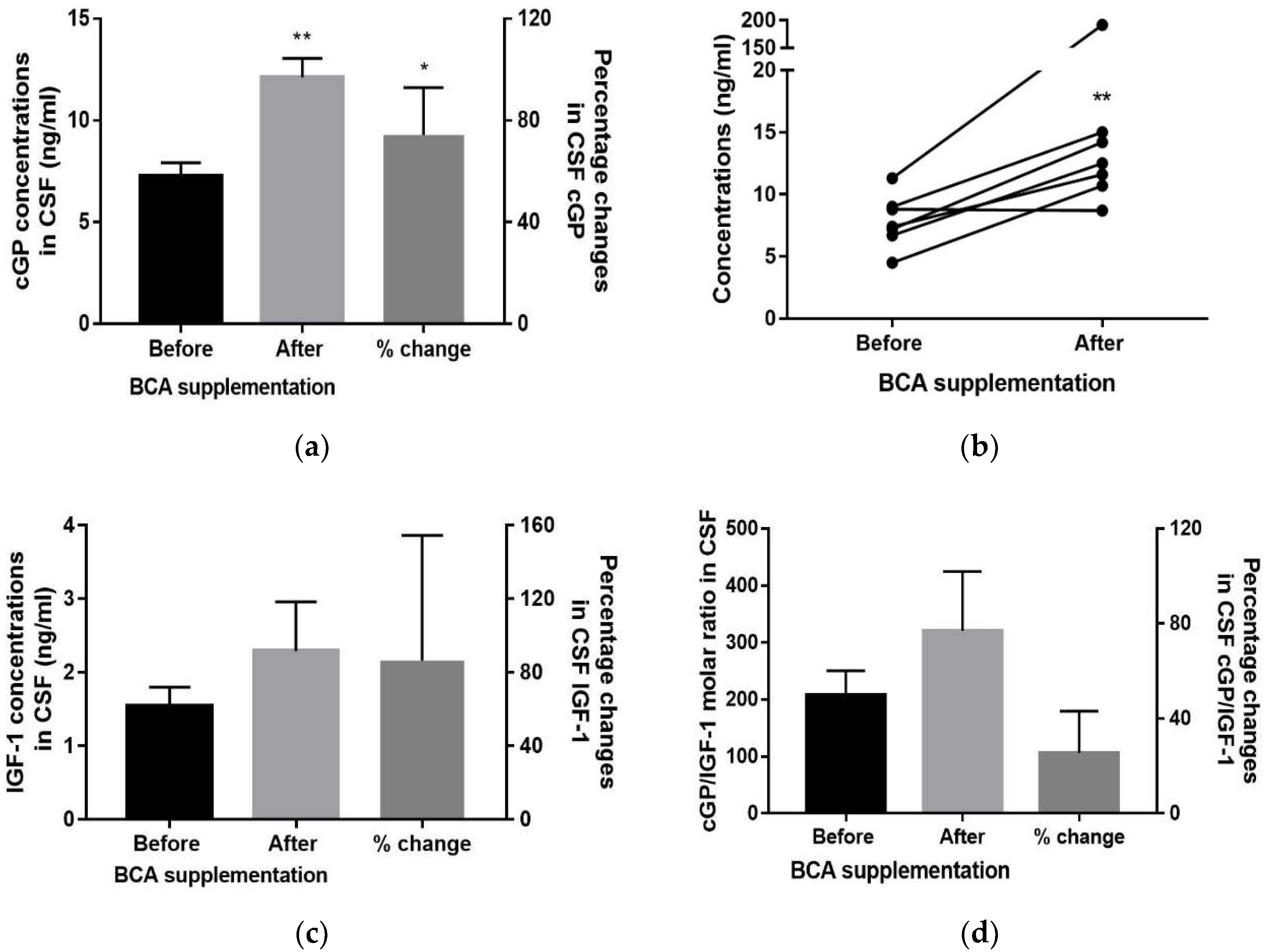
3.2. CSF
3.3. Plasma
3.4. CSF vs. Plasma Concentration
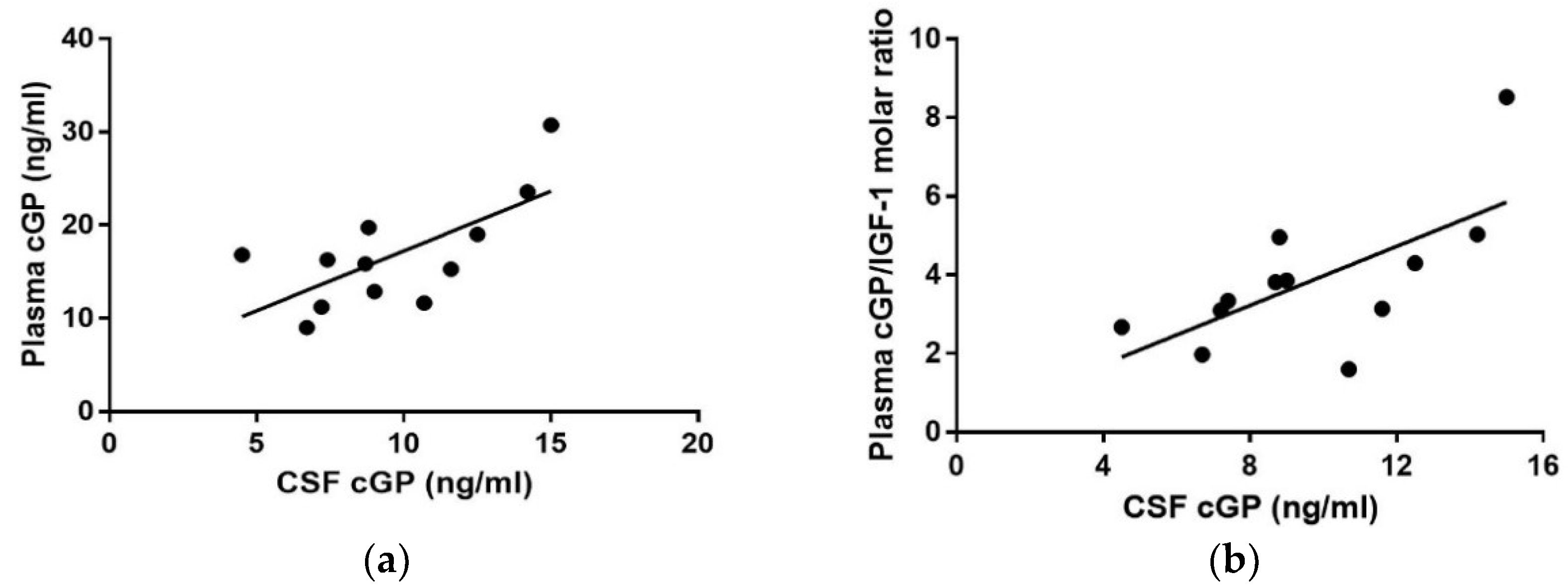
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Picillo, M.; Erro, R.; Santangelo, G.; Pivonello, R.; Longo, K.; Pivonello, C.; Vitale, C.; Amboni, M.; Moccia, M.; Colao, A.; et al. Insulin-like growth factor-1 and progression of motor symptoms in early, drug-naive Parkinson’s disease. J. Neurol. 2013, 260, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Antunes, H.K.; Tufik, S.; de Mello, M.T. Mood, anxiety, and serum IGF-1 in elderly men given 24 weeks of high resistance exercise. Percept. Mot. Skills 2010, 110, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Baldini, S.; Restani, L.; Baroncelli, L.; Coltelli, M.; Franco, R.; Cenni, M.C.; Maffei, L.; Berardi, N. Enriched early life experiences reduce adult anxiety-like behavior in rats: A role for insulin-like growth factor 1. J. Neurosci. 2013, 33, 11715–11723. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.Y. Rescue of alpha-synuclein cytotoxicity by insulin-like growth factors. Biochem. Biophys. Res. Commun. 2009, 385, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jiang, Q.; Xu, J.; Sun, Q.; Qiao, Y.; Chen, W.; Wu, Y.; Wang, Y.; Xiao, Q.; Liu, J.; et al. Plasma insulin-like growth factor 1 is associated with cognitive impairment in Parkinson’s disease. Dement. Geriatr. Cogn. Disord. 2015, 39, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Binoux, M. The IGF system in metabolism regulation. Diabete Metab. 1995, 21, 330–337. [Google Scholar] [PubMed]
- Guan, J.; Harris, P.; Brimble, M.; Lei, Y.; Lu, J.; Yang, Y.; Gunn, A.J. The role for IGF-1-derived small neuropeptides as a therapeutic target for neurological disorders. Expert Opin. Ther. Targets 2015, 19, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Singh-Mallah, G.; Liu, K.; Thorstensen, E.; Shorten, P.R.; Mitchell, E.A.; Taylor, R.; Harris, P.; Brimble, M.; Thompson, J.M.D.; et al. The role for cyclic Glycine-Proline, a biological regulator of Insulin-like Growth Factor-1 in Pregnancy-related Obesity and Weight changes. J. Biol. Regul. Homeost. Agents 2018, 32, 11–25. [Google Scholar]
- Singh-Mallah, G.; Singh, K.; McMahon, C.D.; Harris, P.; Brimble, M.A.; Thorstensen, E.; Guan, J. Maternally Administered Cyclic Glycine-Proline Increases Insulin-Like Growth Factor-1 Bioavailability and Novelty Recognition in Developing Offspring. Endocrinology 2016, 157, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, J.P.; Gerard, A. Role of insulin-like growth factor binding proteins in limitation of IGF-1 degradation into the N-methyl-D-aspartate receptor antagonist GPE: Evidence from gonadotrophin-releasing hormone secretion in vitro at two developmental stages. Brain Res. 1999, 847, 147–152. [Google Scholar] [CrossRef]
- Nilsson-Hakansson, L.; Civalero, I.; Zhang, X.; Carlsson-Skwirut, C.; Sara, V.R.; Nordberg, A. Effects of IGF-1, truncated IGF-1 and the tripeptide Gly-Pro-Glu on acetylcholine release from parietal cortex of rat brain. Neuroreport 1993, 4, 1111–1114. [Google Scholar] [PubMed]
- Sara, V.R.; Carlsson Skwirut, C.; Drakenberg, K.; Giacobini, M.B.; Olson, L.; Stahlbom, P.A.; Sandberg Nordqvist, A.C. Truncated IGF-1 in the CNS. Ann. N. Y. Acad. Sci. 1993, 692, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Murphy, L.J. Generation of des-(1-3) insulin-like growth factor-I in serum by an acid protease. Endocrinology 1994, 135, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Murphy, L.J. Enzymatic conversion of IGF-I to des(1-3)IGF-I in rat serum and tissues: A further potential site of growth hormone regulation of IGF-I action. J. Endocrinol. 1995, 146, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Gluckman, P.; Yang, P.; Krissansen, G.; Sun, X.; Zhou, Y.; Wen, J.; Phillips, G.; Shorten, P.R.; McMahon, C.D.; et al. Cyclic glycine-proline regulates IGF-1 homeostasis by altering the binding of IGFBP-3 to IGF-1. Sci. Rep. 2014, 4, 4388. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Mathai, S.; Harris, A.P.; Wen, J.Y.; Zhang, R.; Brimble, M.A.; Gluckman, P. Peripheral administration of a novel diketopiperazine, NNZ 2591, prevents brain injury and improves somatosensory-motor funcion following hypoxia-ischemia in adult rats. Neuropharmacology 2007, 53, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Krishnamurthi, R.; Waldvogel, H.J.; Faull, R.L.; Clark, R.; Gluckman, P. N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH positive neurons after 6-OHDA induced nigral lesion in rats. Brain Res. 2000, 859, 286–292. [Google Scholar] [CrossRef]
- Krishnamurthi, R.; Mathai, S.; Kim, H.; Zhang, R.; Guan, J. A novel diketopiperazine improves functional recovery given after the onset of 6-OHDA induced motor deficit in rats. Br. J. Pharmacol. 2008, 156, 662–672. [Google Scholar]
- Gao, X.; Cassidy, A.; Schwarzschild, M.; Rimm, E.; Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Alamri, Y.; MacAskill, M.; Anderson, T. Repeated Lumbar Punctures for Non-Clinical Indications: How Do Patients Feel? Eur. Neurol. 2016, 76, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Wang, G.J.; Fan, M.J.; Asokan Shibu, M.; Liu, Y.T.; Padma Viswanadha, V.; Lin, Y.L.; Lai, C.H.; Chen, Y.F.; Liao, H.E.; et al. Cellular apoptosis and cardiac dysfunction in STZ-induced diabetic rats attenuated by anthocyanins via activation of IGFI-R/PI3K/Akt survival signaling. Environ. Toxicol. 2017, 32, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Muller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, R.; Dale-Gandar, L.; Hodgkinson, S.; Vickers, M. NNZ-2591, a novel diketopiperazine, prevented scopolamine-induced acute memory impairment in the adult rat. Behav. Brain Res. 2010, 210, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., 2nd. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Pavlovic, D.; Waldvogel, H.; Dragunow, M.; Synek, B.; Turner, C.; Faull, R.; Guan, J. String Vessel Formation is Increased in the Brain of Parkinson Disease. J. Parkinson Dis. 2015, 5, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, D.; Inanc Tolun, F.; Toru, I. Serum insulin-like growth factor-1 and nitric oxide levels in Parkinson’s disease. Mediat. Inflamm. 2009, 2009, 132464. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Dong, M.; de la Monte, S.M. Brain insulin-like growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: Potential role of manganese neurotoxicity. J. Alzheimers Dis. 2009, 16, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Klempt, N.; Guan, J.; Mallard, C.; Sirimanne, E.; Dragunow, M.; Klempt, M.; Singh, K.; Williams, C.; Nikolics, K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem. Biophys. Res. Commun. 1992, 182, 593–599. [Google Scholar] [CrossRef]
- Singh-Mallah, G.; McMahon, C.D.; Guan, J.; Singh, K. Cyclic-glycine-proline accelerates mammary involution by promoting apoptosis and inhibiting IGF-1 function. J. Cell. Physiol. 2017, 232, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Fan, D.; Pitcher, T.L.; Dalrymple-Alford, J.C.; Anderson, T.; Alamri, Y. A novel biomarker for IGF-1 function: The application in aging and neurological conditions. In Proceedings of the Austrasia Winter Conference of Brain Research, Queenstown, New Zealand, 2–6 September 2017. [Google Scholar]

| Case | Age | Clinical Diagnosis | UPDRS III | MMSE | MoCA | HADS | PDQ-39 |
|---|---|---|---|---|---|---|---|
| BM02BC | 61 | idiopathic PD | 15 | 29 | 29 | 6 | 43.75 |
| LE14BC | 77 | idiopathic PD | 48 | 27 | 22 | 3 | 31.25 |
| SE07BC | 73 | idiopathic PD | 36 | 30 | 24 | 9 | 90.63 |
| YY03BC | 48 | idiopathic PD | 33 | 29 | 29 | 8 | 59.83 |
| EK05BC | 80 | idiopathic PD | 52 | 29 | 27 | 6 | 156.25 |
| GD08BC | 80 | idiopathic PD | 34 | 28 | 24 | 11 | 134.88 |
| ED12BC | 60 | idiopathic PD | 33 | 30 | 25 | 3 | 65.63 |
| EY17BC | 56 | idiopathic PD | 27 | 30 | 28 | 21 | 159.38 |
| TN15BC | 70 | idiopathic PD | 51 | 28 | 22 | 21 | 250 |
| KT16BC | 55 | idiopathic PD | 31 | 28 | 26 | 4 | 71.88 |
| Before the Supplementation | |||
| CSF (ng/mL) Mean ± SEM (n = 6) | Plasma (ng/mL) Mean ± SEM (n = 9–10) | Ratio of CSF to plasma (%) | |
| IGF-1 | 1.54 ± 0.26 | 179.04 ±14.89 | 0.86% |
| cGP | 7.27 ± 0.67 | 13.96 ± 1.33 | 52.01% |
| IGFBP-3 | 26.16 ± 2.79 | 3038.92 ± 111.90 | 0.86% |
| After the Supplementation | |||
| CSF (ng/mL) Mean ± SEM (n = 6) | Plasma (ng/mL) Mean ± SEM (n = 9–10) | Ratio of CSF to plasma | |
| IGF-1 | 2.29 ± 0.67 | 176.07 ± 14.13 | 1.30% |
| cGP | 12.12 ± 0.94 | 16.92 ± 2.79 | 71.63% |
| IGFBP-3 | 27.69 ± 3.53 | 3029.09 ± 59.35 | 0.91% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients 2018, 10, 714. https://doi.org/10.3390/nu10060714
Fan D, Alamri Y, Liu K, MacAskill M, Harris P, Brimble M, Dalrymple-Alford J, Prickett T, Menzies O, Laurenson A, et al. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients. 2018; 10(6):714. https://doi.org/10.3390/nu10060714
Chicago/Turabian StyleFan, Dawei, Yassar Alamri, Karen Liu, Michael MacAskill, Paul Harris, Margaret Brimble, John Dalrymple-Alford, Tim Prickett, Oliver Menzies, Andrew Laurenson, and et al. 2018. "Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function" Nutrients 10, no. 6: 714. https://doi.org/10.3390/nu10060714
APA StyleFan, D., Alamri, Y., Liu, K., MacAskill, M., Harris, P., Brimble, M., Dalrymple-Alford, J., Prickett, T., Menzies, O., Laurenson, A., Anderson, T., & Guan, J. (2018). Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients, 10(6), 714. https://doi.org/10.3390/nu10060714







