Maternal Circulating Vitamin Status and Colostrum Vitamin Composition in Healthy Lactating Women—A Systematic Approach
Abstract
1. Introduction
1.1. Colostrum, Transitional- and Mature Milk
1.2. Nutritional Constituents of Human Milk
1.3. Vitamins, Vital to Life
1.4. Maternal Nutritional Status and Milk Vitamin Composition
1.5. Factors Influencing Vitamin Status of Mother and Infant
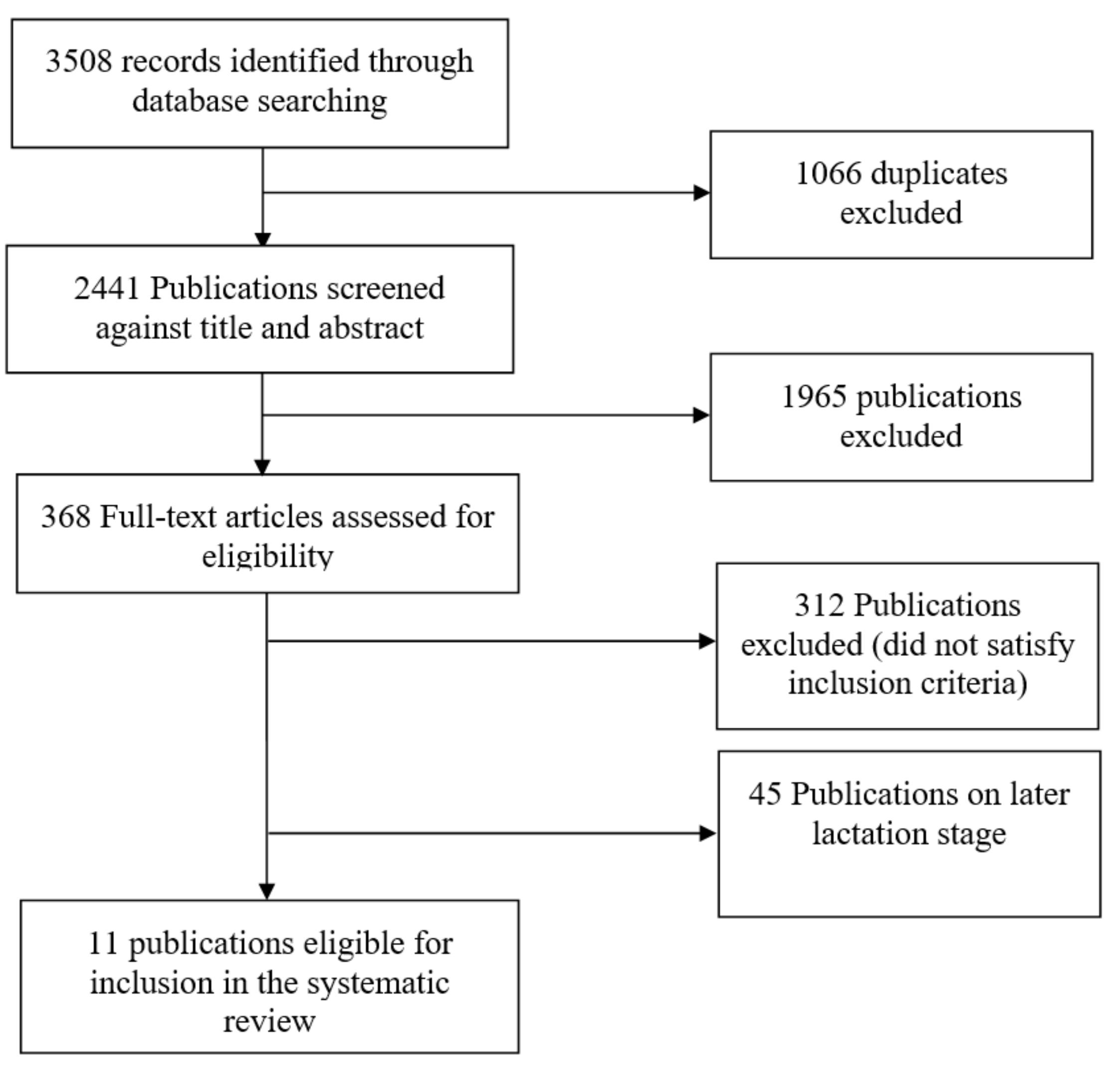
2. Literature Search Methods
2.1. Exclusion and Inclusion Criteria
2.2. Data Extraction
3. Results
3.1. Colostrum and Blood Vitamin Composition
3.2. Vitamin C
3.3. Vitamin D
3.4. Vitamin K
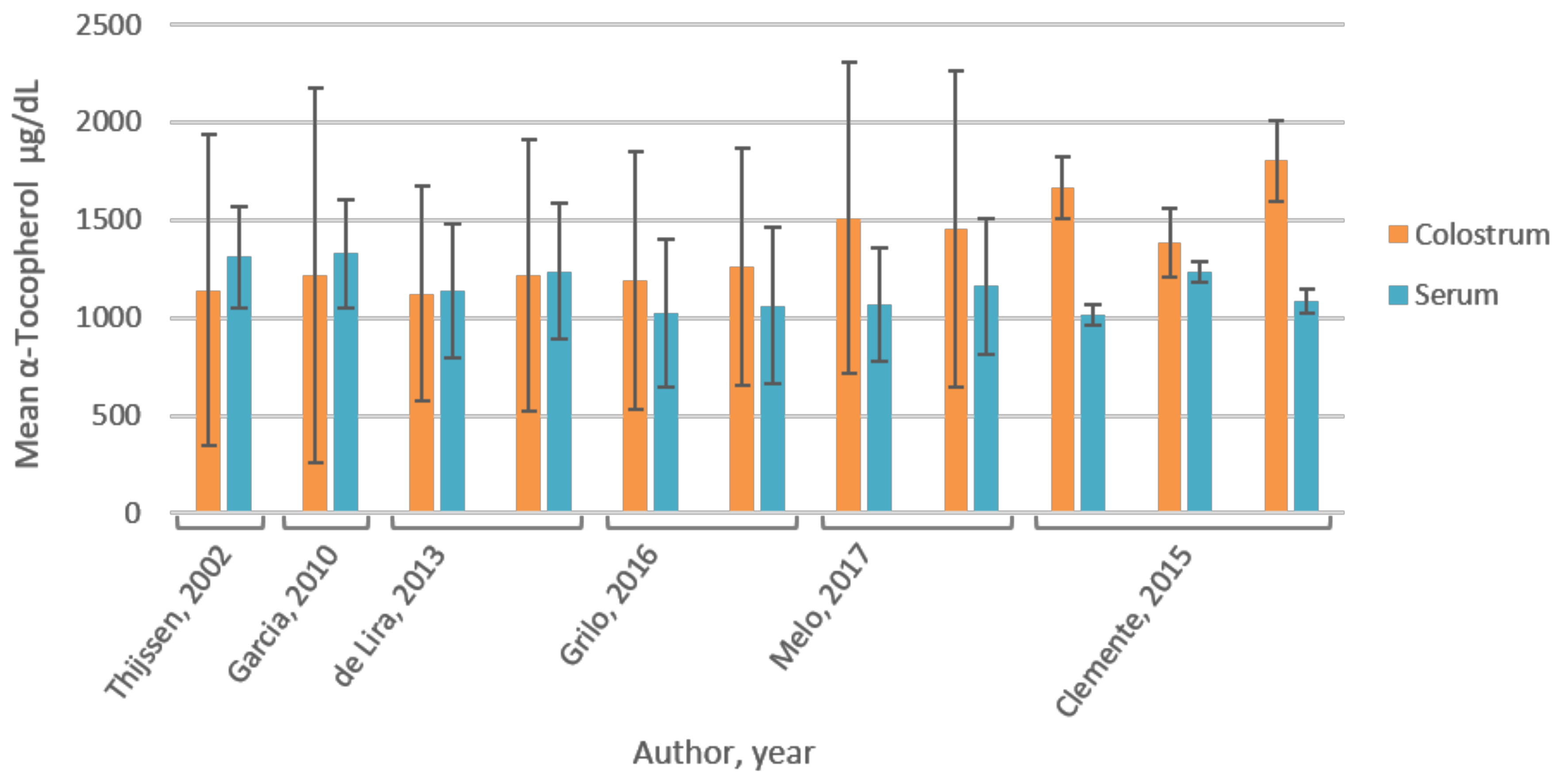
3.5. Vitamin E
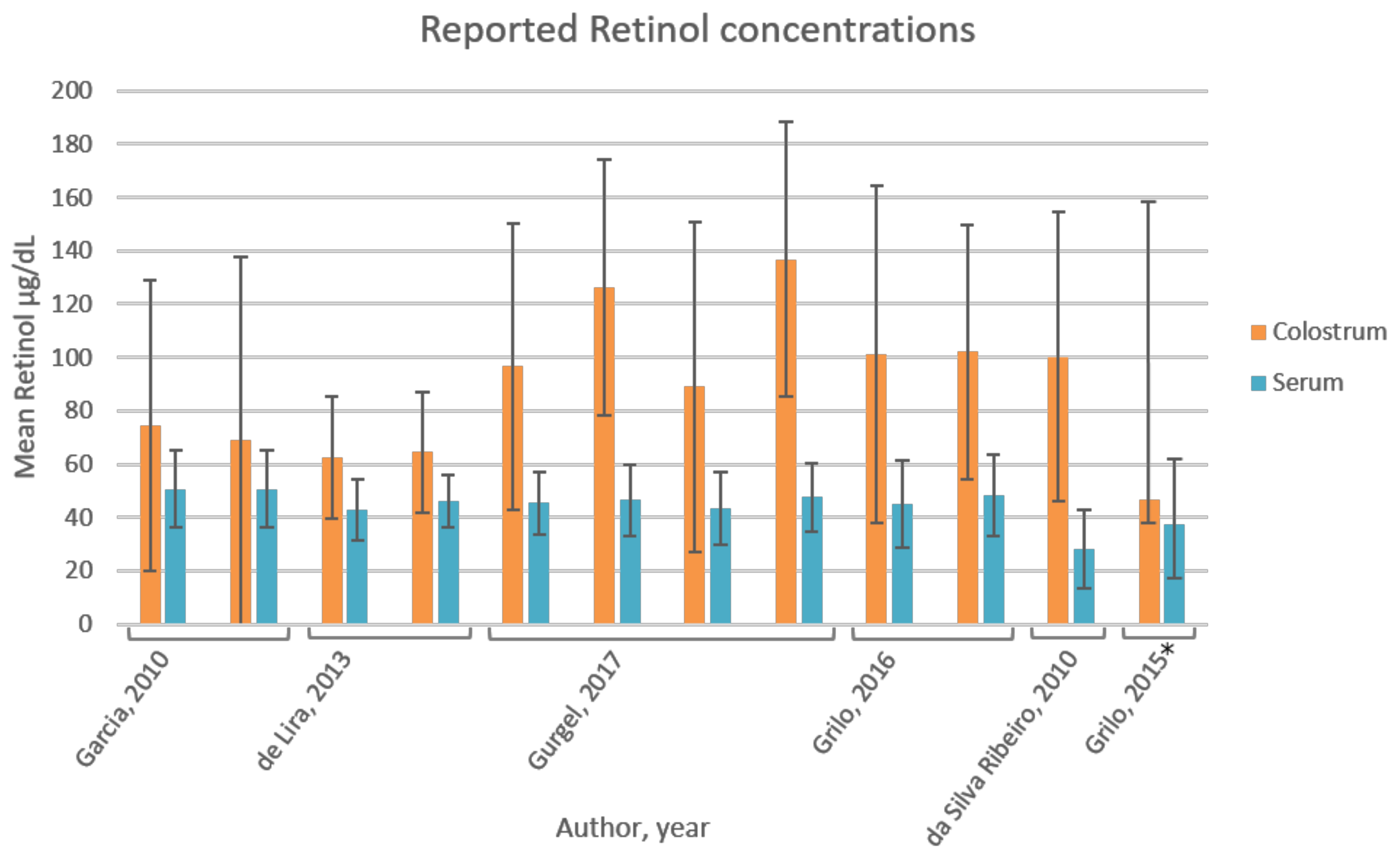
3.6. Vitamin A
3.7. Effect of (Serum) Retinol on Colostrum α-Tocopherol
4. Discussion
4.1. Correlation of Individual Vitamins in Blood and Colostrum
4.2. Vitamin C
4.3. Vitamin D
4.4. Vitamin K
4.5. Vitamin A
4.6. Vitamin E
4.7. Serum Retinol Affects Colostrum α-Tocopherol
5. Conclusions
6. Limitations and Strengths of the Performed Research
Recommendations
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar] [PubMed]
- World Health Organization; United Nations International Children’s Emergency Fund (UNICEF). Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Oftedal, O.T. The evolution of milk secretion and its ancient origins. Animal 2012, 6, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Bitman, J.; Wood, L.; Hamosh, M.; Hamosh, P.; Mehta, N.R. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. Am. J. Clin. Nutr. 1983, 38, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Villalpando, S.; Wong, W.W.; Flores-Huerta, S.; Hernandez-Beltran, M.d.J.; Smith, E.; Garza, C. Human milk intake and growth faltering of rural Mesoamerindian infants. Am. J. Clin. Nutr. 1992, 55, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-Y.; Bates, J.M., Jr.; Rennert, O.M. Comparative studies of manganese binding in human breast milk, bovine milk and infant formula. J. Nutr. 1982, 112, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, À. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Kulski, J.; Hartmann, P. Changes in human milk composition during the initiation of lactation. Aust. J. Exp. Biol. Med. Sci. 1981, 59, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.W.; Hartmann, P.E. Initiation of human lactation: Secretory differentiation and secretory activation. J. Mammary Gland Biol. Neoplasia 2007, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cavell, P.A.; Widdowson, E.M. Intakes and excretions of iron, copper, and zinc in the neonatal period. Arch. Dis. Child. 1964, 39, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.J.; Lönnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, K.Y.; Rechtman, D.J.; Lee, M.L.; Montoya, A.; Medo, E.T. Macronutrient analysis of a nationwide sample of donor breast milk. J. Am. Diet. Assoc. 2009, 109, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.R.; Barnett, D.; Gentles, E.; Cairns, L.; Simpson, J.H. Macronutrient content of donor human breast milk. Arch. Dis. Child.-Fetal Neonatal Ed. 2013, 98, F539–F541. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Skafte, L.; Badsberg, J.H.; Jørgensen, M. Variation in macronutrients in human bank milk: Influencing factors and implications for human milk banking. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem.-Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Lieben, L.; Carmeliet, G. Vitamin D signaling in osteocytes: Effects on bone and mineral homeostasis. Bone 2013, 54, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Raverdeau, M.; Mills, K.H. Modulation of T cell and innate immune responses by retinoic acid. J. Immunol. 2014, 192, 2953–2958. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Vitamin and Mineral Requirements; Report of a Joint FAO/WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Jeanes, Y.M.; Hall, W.L.; Ellard, S.; Lee, E.; Lodge, J.K. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br. J. Nutr. 2004, 92, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Lodge, J.K.; Hall, W.L.; Jeanes, Y.M.; Proteggente, A.R. Physiological factors influencing vitamin E biokinetics. Ann. N. Y. Acad. Sci. 2004, 1031, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Lodge, J.K. Vitamin E bioavailability in humans. J. Plant Physiol. 2005, 162, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; López-Sobaler, A.M.; Martínez, R.M.; Andrés, P.; Quintas, M.E. Influence of smoking on vitamin E status during the third trimester of pregnancy and on breast-milk tocopherol concentrations in Spanish women. Am. J. Clin. Nutr. 1998, 68, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Brot, C.; Jørgensen, N.R.; Sørensen, O.H. The influence of smoking on vitamin D status and calcium metabolism. Eur. J. Clin. Nutr. 1999, 53, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Schectman, G.; Byrd, J.C.; Gruchow, H.W. The influence of smoking on vitamin C status in adults. Am. J. Public Health 1989, 79, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Viteri, F.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Relton, C.L.; Pearce, M.S.; Parker, L. The influence of erythrocyte folate and serum vitamin B 12 status on birth weight. Br. J. Nutr. 2005, 93, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Bamji, M.; Iyengar, L. Effect of oral contraceptive agents on vitamin nutrition status. Am. J. Clin. Nutr. 1975, 28, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Debier, C.; Larondelle, Y. Vitamins A and E: Metabolism, roles and transfer to offspring. Br. J. Nutr. 2005, 93, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M. Vitamin K metabolism and nutriture. Blood Rev. 1992, 6, 92–104. [Google Scholar] [CrossRef]
- Greer, F.R. Vitamin K deficiency and hemorrhage in infancy. Clin. Perinatol. 1995, 22, 759–777. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Institute of Medicine and National Research Council. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Melo, L.R.; Clemente, H.A.; Bezerra, D.F.; Dantas, R.C.S.; Ramalho, H.M.M.; Dimenstein, R. Effect of maternal supplementation with vitamin E on the concentration of α-tocopherol in colostrum. J. Pediatr. 2017, 93, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sauberlich, H.E.; Dowdy, R.P.; Skala, J.H. Laboratory tests for the assessment of nutritional status. CRC Crit. Rev. Clin. Lab. Sci. 1973, 4, 215–340. [Google Scholar] [PubMed]
- Grilo, E.; Medeiros, W.; Silva, A.; Gurgel, C.; Ramalho, H.; Dimenstein, R. Maternal supplementation with a megadose of vitamin A reduces colostrum level of α-tocopherol: A randomised controlled trial. J. Hum. Nutr. Diet. 2016, 29, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Clemente, H.A.; Ramalho, H.M.; Lima, M.S.; Grilo, E.C.; Dimenstein, R. Maternal supplementation with natural or synthetic vitamin E and its levels in human colostrum. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, H.; Drittij, M.-J.; Vermeer, C.; Schoffelen, E. Menaquinone-4 in breast milk is derived from dietary phylloquinone. Br. J. Nutr. 2002, 87, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Ribeiro, K.; Araújo, K.; Pires, J.; Azevedo, G.; Dimenstein, R. Alpha-tocopherol concentration in the colostrum of nursing women supplemented with retinyl palmitate and alpha-tocopherol. J. Hum. Nutr. Diet. 2010, 23, 529–534. [Google Scholar] [PubMed]
- De Lira, L.Q.; Lima, M.S.R.; de Medeiros, J.M.S.; da Silva, I.F.; Dimenstein, R. Correlation of vitamin A nutritional status on alpha-tocopherol in the colostrum of lactating women. Matern. Child Nutr. 2013, 9, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, C.S.S.; de Araújo Pereira, L.A.; de Assis Costa, A.; da Silva Souza, M.A.; de Brito, P.A.; de Melo, L.R.M.; Dimenstein, R. Effect of routine prenatal supplementation on vitamin concentrations in maternal serum and breast milk. Nutrition 2017, 33, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Ribeiro, K.; De Araújo, K.; De Souza, H.; Soares, F.; da Costa Pereira, M.; Dimenstein, R. Nutritional vitamin A status in northeast Brazilian lactating mothers. J. Hum. Nutr. Diet. 2010, 23, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Cancela, L.; Le Boulch, N.; Miravet, L. Relationship between the vitamin D content of maternal milk and the vitamin D status of nursing women and breast-fed infants. J. Endocrinol. 1986, 110, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W. Individual quantitation of vitamin D2, vitamin D3, 25-hydroxyvitamin D2, and 25-hydroxyvitamin D3 in human milk. Anal. Biochem. 1983, 131, 211–219. [Google Scholar] [CrossRef]
- Ahmed, L.; Islam, S.; Khan, N.I.; Nahid, S.N. Vitamin C content in human milk (colostrum, transitional and mature) and serum of a sample of Bangladeshi mothers. Malays. J. Nutr. 2004, 10, 1–4. [Google Scholar] [PubMed]
- Grilo, E.C.; Lima, M.S.; Cunha, L.R.; Gurgel, C.S.; Clemente, H.A.; Dimenstein, R. Effect of maternal vitamin A supplementation on retinol concentration in colostrum. J. Pediatr. (Versão em Português) 2015, 91, 81–86. [Google Scholar] [CrossRef]
- Jelliffe, D.B.; Jelliffe, E.P. The volume and composition of human milk in poorly nourished communities. A review. Am. J. Clin. Nutr. 1978, 31, 492–515. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C. Scurvy: More than historical relevance. Am. Fam. Physician 1993, 48, 609–613. [Google Scholar] [PubMed]
- Baumbach, J. Scurvy by any other name: A case report. R. I. Med. 1994, 77, 24–25. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Malabanan, A.; Veronikis, I.E.; Holick, M.F. Redefining vitamin D insufficiency. Lancet 1998, 351, 805–806. [Google Scholar] [CrossRef]
- Thomas, M.K.; Lloyd-Jones, D.M.; Thadhani, R.I.; Shaw, A.C.; Deraska, D.J.; Kitch, B.T.; Vamvakas, E.C.; Dick, I.M.; Prince, R.L.; Finkelstein, J.S. Hypovitaminosis D in Medical Inpatients. N. Engl. J. Med. 1998, 338, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev. 2009, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, P.; Sheehy, P. Optimal nutrition: Vitamin E. Proc. Nutr. Soc. 1999, 58, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Horwitt, M.; Century, B.; Zeman, A. Erythrocyte survival time and reticulocyte levels after tocopherol depletion in man. Am. J. Clin. Nutr. 1963, 12, 99–106. [Google Scholar] [CrossRef] [PubMed]
- West, K.P. Extent of vitamin A deficiency among preschool children and women of reproductive age. J. Nutr. 2002, 132, 2857S–2866S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Macias, C.; Schweigert, F.J. Changes in the concentration of carotenoids, vitamin A, alpha-tocopherol and total lipids in human milk throughout early lactation. Ann. Nutr. Metab. 2001, 45, 82–85. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995-2005; WHO Global Database on Vitamin A Deficiency; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Stoltzfus, R.; Underwood, B.A. Breast-milk vitamin A as an indicator of the vitamin A status of women and infants. Bull. World Health Organ. 1995, 73, 703–711. [Google Scholar] [PubMed]
- Camarena, V.; Wang, G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. CMLS 2016, 73, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.; Richardson, D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption! Free Radic. Biol. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.; Al Nagdy, S.; Mourad, K.; El Azghal, H. Foetal maternal ascorbic acid gradient in normal Egyptian subjects. J. Trop. Pediatr. 1970, 16, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Salmenperä, L. Vitamin C nutrition during prolonged lactation: Optimal in infants while marginal in some mothers. Am. J. Clin. Nutr. 1984, 40, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Bates, C. Vitamin A in pregnancy and lactation. Proc. Nutr. Soc. 1983, 42, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Byerley, L.O.; Kirksey, A. Effects of different levels of vitamin C intake on the vitamin C concentration in human milk and the vitamin C intakes of breast-fed infants. Am. J. Clin. Nutr. 1985, 41, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Sneed, S.M.; Zane, C.; Thomas, M.R. The effects of ascorbic acid, vitamin B6, vitamin B12, and folic acid supplementation on the breast milk and maternal nutritional status of low socioeconomic lactating women. Am. J. Clin. Nutr. 1981, 34, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Brannon, P.M.; Picciano, M.F. Vitamin D in pregnancy and lactation in humans. Annu. Rev. Nutr. 2011, 31, 89–115. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D. Vitamin D and cancer: Current dilemmas and future research needs. Am. J. Clin. Nutr. 2008, 88, 565S–569S. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.; Roos, B.; Draper, H.; Lambert, P. Vitamin D and its metabolites in human and bovine milk. J. Nutr. 1981, 111, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Reeve, L.E.; Chesney, R.W.; DeLuca, H.F. Vitamin D of human milk: Identification of biologically active forms. Am. J. Clin. Nutr. 1982, 36, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Hollis, B.W.; Cripps, D.J.; Tsang, R.C. Effects of maternal ultraviolet B irradiation on vitamin D content of human milk. J. Pediatr. 1984, 105, 431–433. [Google Scholar] [CrossRef]
- Greer, F.R.; Hollis, B.W.; Napoli, J.L. High concentrations of vitamin D2 in human milk associated with pharmacologic doses of vitamin D2. J. Pediatr. 1984, 105, 61–64. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr. 2004, 80, 1752S–1758S. [Google Scholar] [CrossRef] [PubMed]
- Rowling, M.J.; Kemmis, C.M.; Taffany, D.A.; Welsh, J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J. Nutr. 2006, 136, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M.; Salomon, A.; Saupe, J.; Shearer, M.J. Transport of vitamin K to bone in humans. J. Nutr. 1996, 126, 1192S–1196S. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Sadowski, J.A.; Davidson, K.W.; O’Brien, M.E.; McNamara, J.R.; Schaefer, E.J. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am. J. Clin. Nutr. 1998, 67, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Marshall, S.P.; Foley, A.L.; Suttie, J.W. Improving the vitamin K status of breastfeeding infants with maternal vitamin K supplements. Pediatrics 1997, 99, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Gupta, J.; Graham, G.; Salonikas, C.; Naidoo, D. Vitamin K in preterm breastmilk with maternal supplementation. Acta Paediatri. 1998, 87, 960–962. [Google Scholar] [CrossRef]
- von Kries, R.; Shearer, M.; McCarthy, P.; Haug, M.; Harzer, G.; Göbel, U. Vitamin K1 content of maternal milk: Influence of the stage of lactation, lipid composition, and vitamin K1 supplements given to the mother. Pediatr. Res. 1987, 22, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R. Do breastfed infants need supplemental vitamins? Pediatr. Clin. N. Am. 2001, 48, 415–423. [Google Scholar] [CrossRef]
- Haskell, M.J.; Brown, K.H. Maternal vitamin A nutriture and the vitamin A content of human milk. J. Mammary Gland Biol. Neoplasia 1999, 4, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Pasatiempo, A.M.G.; Green, M.H. Chylomicron margination, lipolysis, and vitamin A uptake in the lactating rat mammary gland: Implications for milk retinoid content. Exp. Biol. Med. 2004, 229, 46–55. [Google Scholar] [CrossRef]
- Akohoue, S.A.; Green, J.B.; Green, M.H. Dietary vitamin A has both chronic and acute effects on vitamin A indices in lactating rats and their offspring. J. Nutr. 2006, 136, 128–132. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Kako, Y.; Deckelbaum, R.J.; Hansen, I.H.; Palczewski, K.; Goldberg, I.J.; Blaner, W.S. Multiple pathways ensure retinoid delivery to milk: Studies in genetically modified mice. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E862–E870. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Craft, N.; Handelman, G.; Nommsen-Rivers, I.; McCrory, M.; Dewey, K. Associations between serum and breast milk carotenoids, vitamins A and E. Proc. FASEB J. 1999, 13, A240. [Google Scholar]
- Meneses, F.; Trugo, N.M. Retinol, β-carotene, and lutein+ zeaxanthin in the milk of Brazilian nursing women: Associations with plasma concentrations and influences of maternal characteristics. Nutr. Res. 2005, 25, 443–451. [Google Scholar] [CrossRef]
- Green, M.H.; Green, J.B.; Akohoue, S.A.; Kelley, S.K. Vitamin A intake affects the contribution of chylomicrons vs. retinol-binding protein to milk vitamin A in lactating rats. J. Nutr. 2001, 131, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Suter, P.M.; Sahyoun, N.; Ribaya-Mercado, J.D.; Russell, R.M. Relation between beta-carotene intake and plasma and adipose tissue concentrations of carotenoids and retinoids. Am. J. Clin. Nutr. 1995, 62, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Gebre-Medhin, M.; Vahlquist, A.; Hofvander, Y.; Uppsäll, L.; Vahlquist, B. Breast milk composition in Ethiopian and Swedish mothers. I. Vitamin A and β-carotene. Am. J. Clin. Nutr. 1976, 29, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Canfield, L.; Giuliano, A.; Neilson, E.; Yap, H.; Graver, E.; Cui, H.; Blashill, B. Beta-carotene in breast milk and serum is increased after a single beta-carotene dose. Am. J. Clin. Nutr. 1997, 66, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Gossage, C.P.; Deyhim, M.; Yamini, S.; Douglass, L.W.; Moser-Veillon, P.B. Carotenoid composition of human milk during the first month postpartum and the response to β-carotene supplementation. Am. J. Clin. Nutr. 2002, 76, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.K.; Johannsen, A.K.B.; Hermansen, J.E. Quantitative secretion and maximal secretion capacity of retinol, β-carotene and α-tocopherol into cows’ milk. J. Dairy Res. 1999, 66, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Debier, C.; Pomeroy, P.; Van Wouwe, N.; Mignolet, E.; Baret, P.; Larondelle, Y. Dynamics of vitamin A in grey seal (Halichoerus grypus) mothers and pups throughout lactation. Can. J. Zool. 2002, 80, 1262–1273. [Google Scholar] [CrossRef]
- Schweigert, F. Effect of gestation and lactation on lipoprotein pattern and composition in dairy cows. J. Anim. Physiol. Anim. Nutr. 1990, 63, 75–83. [Google Scholar] [CrossRef]
- Bauer, J.; Gerss, J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin. Nutr. 2011, 30, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.E.; Francis, T.; Clandinin, M.T. Vitamin A and E content of human milk at early stages of lactation. Early Hum. Dev. 1985, 11, 157–167. [Google Scholar] [CrossRef]
- Lima, M.S.; Dimenstein, R.; Ribeiro, K.D. Vitamin E concentration in human milk and associated factors: A literature review. J. Pediatr. 2014, 90, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C.; Engel, H.; Jensen, S.K.; Craig, A.M.; Traber, M.G. Lactating sows and suckling piglets preferentially incorporate RRR-over all-rac-α-tocopherol into milk, plasma and tissues. J. Nutr. 2002, 132, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.; Kronfeld, D.; Grimsley-Cook, A.; Dascanio, J.; Ordakowski-Burk, A.; Splan, R.; Dunnington, E.; Sklan, D. Retinol, β-carotene and β-tocopherol concentrations in mare and foal plasma and in colostrum. J. Equine Vet. Sci. 2004, 24, 115–120. [Google Scholar] [CrossRef]
- Martinez, S.; Barbas, C.; Herrera, E. Uptake of (alpha)-tocopherol by the mammary gland but not by white adipose tissue is dependent on lipoprotein lipase activity around parturition and during lactation in the rat. Metab.-Clin. Exp. 2002, 51, 1444–1451. [Google Scholar] [PubMed]
- Mardones, P.; Rigotti, A. Cellular mechanisms of vitamin E uptake: Relevance in α-tocopherol metabolism and potential implications for disease. J. Nutr. Biochem. 2004, 15, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Goti, D.; Hrzenjak, A.; Levak-Frank, S.; Frank, S.; Van Der Westhuyzen, D.R.; Malle, E.; Sattler, W. Scavenger receptor class B, type I is expressed in porcine brain capillary endothelial cells and contributes to selective uptake of HDL-associated vitamin E. J. Neurochem. 2001, 76, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, F.J.; Gottwald, C. Effect of parturition on levels of vitamins A and E and of β-carotene in plasma and milk of mares. Equine Vet. J. 1999, 31, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Nonnecke, B.J.; Franklin, S.T.; Horst, R.L.; Bidlack, W.R.; Stuart, R.L.; Beitz, D.C. Dietary vitamin A modulates the concentrations of RRR-α-tocopherol in plasma lipoproteins from calves fed milk replacer. J. Nutr. 2000, 130, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Berthou, L.; Langouët, S.; Grudé, P.; Denèfle, P.; Branellec, D.; Guillouzo, A. Negative regulation of Apo AI gene expression by retinoic acid in rat hepatocytes maintained in a coculture system. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1998, 1391, 329–336. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta 2012, 1821, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Borel, P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011, 50, 388–402. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Dietary Reference Values for Nutrients Summary Report; EFSA Supporting Publications: Parma, Italy, 2017; p. e15121. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Dietary reference values for vitamin K. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015, 13, 4149. [Google Scholar] [CrossRef]
- Redeuil, K.M.; Longet, K.; Bénet, S.; Munari, C.; Campos-Giménez, E. Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A 2015, 1422, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, S.P.; Montoliu, I.; Da Silva, L.; Dayon, L.; Galindo, A.N.; Corthésy, J.; Kussmann, M.; Martin, F.-P. High-throughput and simultaneous quantitative analysis of homocysteine–methionine cycle metabolites and co-factors in blood plasma and cerebrospinal fluid by isotope dilution LC–MS/MS. Anal. Bioanal. Chem. 2017, 409, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kamao, M.; Tsugawa, N.; Suhara, Y.; Wada, A.; Mori, T.; Murata, K.; Nishino, R.; Ukita, T.; Uenishi, K.; Tanaka, K. Quantification of fat-soluble vitamins in human breast milk by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2007, 859, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
| Life Stage Group | Vit A (µg/day) a | Vit D (µg/day) b,c | Vit E (mg/day) d | Vit K (µg/day) | Vit C (mg/day) | Thiamin (mg/day) | Riboflavin (mg/day) | Niacin (mg/day) e | Vit B6 (mg/day) | Folate (µg/day) f | Vit B12 (µg/day) | Pantothenic Acid (mg/day) | Biotin (µg/day) | Choline (mg/day) g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infants | ||||||||||||||
| 0–6 month | 400 * | 5 * | 4 * | 2.0 * | 40 * | 0.2 * | 0.3 * | 2 * | 0.1 * | 65 * | 0.4 * | 1.7 * | 5 * | 125 * |
| Lactating women | ||||||||||||||
| 14–18 year | 1200 | 15 | 19 | 75 * | 115 | 1.4 | 1.6 | 17 | 2.0 | 500 | 2.8 | 7 * | 35 * | 550 * |
| 19–30 year | 1300 | 15 | 19 | 90 * | 120 | 1.4 | 1.6 | 17 | 2.0 | 500 | 2.8 | 7 * | 35 * | 550 * |
| 31–70 year | 1300 | 15 | 19 | 90 * | 120 | 1.4 | 1.6 | 17 | 2.0 | 500 | 2.8 | 7 * | 35 * | 550 * |
| Inclusion | Exclusion |
|---|---|
|
|
| First Author, Year [Ref] | Country | Study Design and Participants | Colostrum and Blood Samples | Method | Main Results | |
|---|---|---|---|---|---|---|
| Maternal Serum (Mean ± SD) | Colostrum (Mean ± SD) | |||||
| Melo, 2017 [36] | Brazil | RCT; 99 mothers; mean ± SD (range) age: 24 ± 6 (18–40) year; divided in CG (n = 39) and IG (n = 60); no maternal and infant pathologies, full-term healthy delivery. Mixed population based on socio-economic (marriage, education, income) and obstetric characteristics (maternal BMI, parity, way of delivery). Limitations: 51% normal pre-pregnancy weight; partly low SES; effect of these parameters on baseline vitamin E not assessed, postpartum day unknown. | Colostrum (2 mL) collected through manual expression of one breast, at end of feeding; foremilk was discarded; blood (5 mL) collected by venipuncture; in morning after overnight fast; baseline measurement; vitamin E was measured. | HPLC-UV spectro-photometry | α-Tocopherol:CG: 1066.6 ± 287.7 mg/dL *; IG: 1159.6 ± 350 mg/dL *; not significantly different between groups (p = 0.41); overall in 3.0% (n = 3) α-tocopherol deficiency (<499.6 mg/dL) [37] | α-Tocopherol: CG: 1509.3 ± 793.7 mg/dL *; individual analysis: 33% (n = 13) colostrum α-tocopherol levels too low to meet infant daily requirements (4 mg/day). IG: 1452.9 ± 808.6 mg/dL *; Individual analysis: 30% (n = 18) colostrum α-tocopherol levels too low to meet infant daily requirements (4 mg/day). α-Tocopherol not significantly different between groups (p = 0.74). |
| Grilo, 2016 [38] | Brazil | RCT; 88 mothers; mean ± SD age: 24 ± 7 year; divided in IG (n = 44) and CG (n = 44); total sample shows diversity in maternal, obstetric and newborn characteristics; similar for the two groups (p > 0.05). Limitations: 40% had pre-gestational BMI > 25; 40% had insufficient gestational weight gain; 14% low birth weight; effect of these parameters on baseline vitamin E not assessed. | Colostrum (2 mL) and blood (5 mL) collected after overnight (8–12 h) fast, 1 d postpartum; stored −20 °C (0–4 d) until analyses; baseline measurement; vitamin A and E were measured. | HPLC-UV spectro-photometry | Retinol: CG 44.8 ± 16.4 µg/dL; IG 48.3 ± 15.4 µg/dL; not significantly different between groups (p > 0.05). Total population 46.4 ± 15.9 µg/dL; 5.7% deficient (<20 µg/dL). | Retinol: CG 101.3 ± 63.3 µg/dL; IG: 102.1 ± 47.7 µg/dL; not significantly different between groups (p > 0.05) |
| α-Tocopherol: CG 1023.6 ± 380.4 µg/dL; IG: 1059.0 400.3 µg/dL; not significantly different between groups (p > 0.05); Total population 1023.6 ± 380.4 µg/dL; 9.1% deficient (<516 µg/dL) | α-Tocopherol: CG 1189.4 ± 660.6 µg/dL; IG: 1259.2 ± 608.2 µg/dL; not significantly different between groups (p > 0.05). | |||||
| Clemente, 2015 [39] | Brazil | RCT; 109 mothers; mean ± SD age: 24.1 ± 5.6 year; control group (CG; n = 36), natural supplemented group (GNAT; 400 IU of RRR-α-tocopherol; n = 40) and synthetic supplemented group (GSIN; 400 IU of all-rac-α-tocopherol; n = 33). | Colostrum (2 mL) collected through manual expression; foremilk was discarded; blood (5 mL) collected by venipuncture; after an overnight fast, ~12 h postpartum; baseline measurement; vitamin E was measured. | HPLC-UV spectro-photometry | α-Tocopherol: CG 1016 ± 52 µg/dL; GNAT 1236 ± 51 µg/dL; GSIN 1083 ± 61 µg/dL; not significantly different between groups (p = 0.546). | α-Tocopherol: CG 1665.2 ± 160.2 µg/dL; GNAT 1387.1 ± 176.5 µg/dL; GSIN 1802 ± 208.1 µg/dL; no difference between groups in the mean (p = 0.253) or the variance (p = 0.767). |
| Thijssen, 2017 [40] | The Netherlands | RCT; 31 mothers; no drug use or abuse, maternal/infant gastrointestinal dysfunction or low (<50 kg) or high (>90 kg) maternal body weight; randomly assigned to four treatment arms; no difference in baseline values between groups. Limitations: limited information on population characteristics. | Colostrum (5–10 mL) collected through manual pump device from the breast not used for the previous feed, first 10 mL was discarded; blood collected; between 8–11 am, 4 d postpartum; stored at −70 °C until analysis; baseline measurement; vitamin K and E were measured. | Vitamin K: fluorescence detection, HPLC separation and post-column reduction (Zn-column) Vitamin E: HPLC-UV spectro-photometry | Phylloquinone (plasma): Ranged from 0.5 to 7.2 nmol/L 95% CI (1.89, 3.28); (mean ± SD): 2.62 ± 1.91 nmol/L (1.18 ± 0.86 µg/L) (n = 31) | Phylloquinone: 5.84 ± 2.31 nmol/L (2.63 ± 1.04 µg/L) (n = 31) |
| Colostrum:plasma concentration ratio phylloquinone: 3.97 ± 3.11 (n = 31) | ||||||
| Menaquinone-4 (plasma): 0.25 ± 0.16 nmol/L (0.11 ± 0.07 µg/L); 95% confidence interval (0.14, 0.37); detected in 32.3% (n = 10) of the samples; | Menaquinone-4: 3.18 ± 1.53 nmol/L (1.41 ± 0.68 µg/L) (n = 31) | |||||
| Colostrum:plasma concentration ratio Menaquinone-4: 14.83 ± 8.3 (n = 10) | ||||||
| Plasma α- and γ- tocopherol: 30.4 ± 6.1 µmol/L (n = 31) | α- and γ- tocopherol: 26.5 ± 18.5 µmol/L (n = 31) | |||||
| Colostrum:plasma concentration ratio α- and γ- tocopherol: 0.97 ± 0.62 (n = 31) | ||||||
| Garcia, 2010 [41] | Brazil | Cross-sectional study; 73 mothers, free of pathologies, sufficient birthweight; divided in intervention group (IG; n = 36) and control group (CG; n = 37); age 14–18 year (n = 17); 19–36 year (n = 56). Limitations: limited information on population characteristics. | Colostrum (2 mL) collected by manual pressure of single breast not previously suckled; first ejection discarded; stored at −20 °C until analyses; 5 mL blood collected after overnight fast up to 16 h postpartum; baseline measurement; vitamin A and E were measured. | HPLC-UV spectro-photometry | Retinol (plasma): 1.77 ± 0.50 µmol/L (50.72 ± 14.33 µg/dL) (n = 73) | Retinol: CG: 2.6 ± 1.9 µmol/L (74.49 ± 54.44 µg/dL); IG 2.4 ± 2.4 µmol/L (68.77 ± 68.77 µg/dL); not significantly different between groups (p > 0.05) |
| No correlation between colostrum and plasma retinol (r = 0.39 and p = 0.74) | ||||||
| α-Tocopherol (plasma): 30.81 ± 6.46 µmol/L (1326.9 ± 278.2 µg/dL) (n = 73) | α-Tocopherol: 28.27 ± 22.29 µmol/L (1217.4 ± 959.9 µg/dL) (n = 73) | |||||
| No correlation between colostrum and plasma α-tocopherol (r = 0.74 and p = 0.54) | ||||||
| De Lira, 2013 [42] | Brazil | Cross-sectional study; 103 mothers, age range 14–41 year (24 ± 7 year), free of associated pathologies; mixed population based on obstetric, maternal and newborn parameters, but none of the variables was associated with retinol and α-tocopherol levels (p > 0.05). Subgroup (n = 87) without serum retinol deficiency (≥30 µg/dL). Subgroup (n = 88) without serum α-tocopherol deficiency [37]. Limitations: 36% pre-gestational BMI > 25, 20% exceeded gestational weight gain; 24% preterm deliveries; 40% and 7% infants had insufficient and excessive birth weight, respectively. | Colostrum (2 mL) collected after overnight fast for three consecutive days (1–3 d postpartum) to establish colostrum pool; blood (5 mL) collected after overnight fast 1 d postpartum; vitamin A and E were measured. | HPLC-UV spectro-photometry | Retinol: 1.49 ± 0.4 µmol/L (42.69 ± 11.46 µg/dL) (n = 103); 15.5% inadequate. Subgroup retinol: 1.61 ± 0.35 µmol/L (46.13 ± 10.03 µg/dL) (n = 87) | Retinol: 2.18 ± 0.8 µmol/L (62.46 ± 22.92 µg/dL) (n = 103) Subgroup retinol: 2.25 ± 0.79 µmol/L (64.47 ± 22.64 µg/dL) (n = 87); colostrum retinol deficiency (<60 µg/dL) in 34% (n = 30) |
| No correlation between serum and colostrum retinol (p = 0.11, r = 0.15) (n = 103) | ||||||
| α-Tocopherol: 26.4 ± 8.0 µmol/L (1137.0 ± 344.5 µg/dL) (n = 103); 16% inadequate (<500 µg/dL). Subgroup α-tocopherol : 28.7 ± 8.08 µmol/L (1236.0 ± 348.0 µg/dL) (n = 88) | α-Tocopherol: 26.1 ± 12.8 µmol/L (1124.0 ± 551.2 µg/dL) (n = 103); Subgroup α-tocopherol: 28.24 ± 16.1 µmol/L (1216.2 ± 693.4 µg/dL) (n = 88); colostrum α-tocopherol deficiency in 44% (<26.1 µmol/L). | |||||
| No correlation between serum and colostrum α-tocopherol (r = −0.12, p = 0.22) (n = 103). Inverse correlation between serum retinol and colostrum α-tocopherol (r = −0.28, p = 0.008) in retinol adequate subgroup (n = 87); No correlation between serum retinol and colostrum α-tocopherol in inadequate fraction of the population (n = 16). | ||||||
| Gurgel, 2017 [43] | Brazil | Cross-sectional study; 100 mothers; mean age 28.6 year; free of morbidities and healthy deliveries; CG no supplement (n = 25); intervention groups (n = 25 per group) had taken daily supplements from 16 wk gestation until delivery; IG1 (1500 IU retinol +1500 IU β-carotene, 112.5% RDI); IG2 (2700 IU β-carotene, 101% RDI); IG3 (2664 IU retinol, 99.9% RDI); maternal age, years of education, occupation and income was not significantly different between groups (p > 0.05); average vitamin A dietary intake in last trimester of pregnancy was not significantly different between groups (p > 0.05). Limitations: effect of these parameters on vitamin A levels not assessed, postpartum day unknown. | Colostrum (500 µL) collected through manual expression of a single breast at the start and end of the breastfeeding; blood (5 mL) collected through venipuncture; under fasting conditions; stored −20 °C until analyses; vitamin A was measured. | HPLC-UV spectro-photometry | Retinol: CG: 45.4 ± 11.8 µg/dL (1.58 ± 0.41 µmol/L), serum retinol inadequacy (<20 µg/dL) in 12% (n = 3); IG1: 46.5 ± 13.3 µg/dL (1.62 ± 0.46 µmol/L); IG2: 43.5 ± 13.7 µg/dL (1.52 ± 0.48 µmol/L); IG3: 47.5 ± 13.0 µg/dL (1.65 ± 0.45 µmol/L); no vitamin A inadequacy in supplemented groups; Serum retinol levels not significantly different between groups (p > 0.05). | Retinol: CG 96.6 ± 53.5 µg/dL (3.37 ± 1.87 µmol/L), colostrum retinol inadequacy (<60 µg/dL) in 20% (n = 5); IG1: 126.1 ± 48 µg/dL (4.40 ± 1.68 µmol/L), inadequacy in 4% (n = 1); IG2: 89 ± 61.9 µg/dL (3.11 ± 2.16 µmol/L), inadequacy in 40% (n = 10); IG3: 136.8 ± 51.7 µg/dL (4.47 ± 1.80 µmol/L), inadequacy in 4% (n = 1); Colostrum retinol levels significantly different between IG2 and IG3 (p < 0.05). |
| Da Silva Ribeiro, 2010 [44] | Brazil | Cross-sectional study; 86 mothers; mean ± SD (range) age: 25.4 ± 5.8 (18–40) year; free of pathologies with full term pregnancy; data were analysed for the entire group, as well as for groups divided according to the predominant source of dietary vitamin A (group A >50% preformed vitamin A (n = 37) and group B (>50% pro-vitamin A carotenoids (n = 49)); cut-off value retinol inadequacy colostrum ≤ 60 µg/dL; no differences in general characteristics (age, newborn birth weight, gestational age) between groups. Limitations: No information on maternal BMI. | Colostrum (1–3 mL) collected by manual expression from single full breast not suckled in previous feed; first milk ejection discarded; 5 mL blood sample by venipuncture; after overnight fast, up to 16 h postpartum; stored −20 °C until analyses; vitamin A was measured. | HPLC-UV spectro-photometry | Retinol: Group A: 1.4 ± 0.4 µmol/L, inadequacy in 5.4% (≤0.70 µmol/L or ≤ 20 µg/dL); Group B: 1.2 ± 0.6 µmol/L, inadequacy in 12.2%. A sig. higher than B (p = 0.033). Total population (n = 86): 1.3 ± 0.51 µmol/L (28 ± 14.6 µg/dL), below adequacy in 9.3%. | Retinol: Group A: 3.4 ± 1.7 µmol/L; inadequacy (<2.1 µmol/L) in 24.3%; Group B: 3.6 ± 1.9 µmol/L, inadequacy in 20.4%; inadequacy similar in both groups. Total population (n = 86): 3.5 ± 1.9 µmol/L (100.3 ± 54.4 µg/dL) below adequacy in 22.1%. |
| Cancela, 1986 [45] | France | Cross-sectional study; 12 mothers, full term pregnancy; data of n = 1 was individually analysed because of vitamin D supplementation (7–14 d postpartum). Limitations: lack of information on population characteristics; pooled colostrum samples (1–3 d postpartum). | Colostrum (>5 mL) collected by manual pump expression at the end of first morning feed; blood samples obtained; 3–5 d postpartum, stored −20 °C until analyses, vitamin D (25OHD and vitamin D in colostrum and 25OHD and 1,25-(OH)2D3 in serum) was measured. | HPLC-UV spectro-photometry, competitive protein binding assay [46] | 25OHD (mean ± SEM): 22.00 ± 2.61 µmol/L (n = 11); 20.0 µmol/L (n = 1) 1,25-(OH)2D3 (mean ± SEM): 0.194 ± 0.047 nmol/L (n = 11) 0.204 nmol/L (n = 1) | 25OHD (mean ± SEM): 0.50 ± 0.11 nmol/L (n = 11); 0.44 nmol/L (n = 1) vitamin D (mean ± SEM): 0.89 ± 0.32 nmol/L (n = 11); 0.21 nmol/L (n = 1) |
| Ahmed, 2004 [47] | Bangladesh | Cross-sectional study; 26 mothers, age 18–32 year; mixed general characteristics (parity, income, BMI) did not influence milk vitamin C content; subgroup (n = 7) with eligible data. Limitations: influence of general characteristics were not assessed for vitamin C serum outcome of the subgroup; infant gestational weight was unspecified. | Colostrum (2 mL) collected by manual expression; blood (1 mL); 2 d postpartum; vitamin C was measured. | Centrifuga-tion; TCA and DTC; UV spectropho-tometry | Vitamin C: 0.44 ± 0.29 mg/dL (n = 7) | Vitamin C: 3.50 ± 0.49 mg/dL (n = 7) |
| Grilo, 2015 [48] | Brazil | Quasi-experimental study; 33 mothers, age 18–35 year, free of morbidities and no unhealthy, preterm births. Limitations: limited information on population characteristics, postpartum day unknown. | Colostrum (2 mL) collected by manual expression of a single breast at the beginning and end of breastfeeding; blood (5 mL) by venipuncture; after overnight fast; stored −20 °C until analyses, vitamin A was measured. | HPLC-UV spectropho-tometry | Retinol median (range): 37.3 (16.8 to 62.2) µg/dL | Retinol median (range): 46.8 (29.7 to 158.9) µg/dL |
| No correlation between serum and colostrum retinol. | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Y. de Vries, J.; Pundir, S.; Mckenzie, E.; Keijer, J.; Kussmann, M. Maternal Circulating Vitamin Status and Colostrum Vitamin Composition in Healthy Lactating Women—A Systematic Approach. Nutrients 2018, 10, 687. https://doi.org/10.3390/nu10060687
Y. de Vries J, Pundir S, Mckenzie E, Keijer J, Kussmann M. Maternal Circulating Vitamin Status and Colostrum Vitamin Composition in Healthy Lactating Women—A Systematic Approach. Nutrients. 2018; 10(6):687. https://doi.org/10.3390/nu10060687
Chicago/Turabian StyleY. de Vries, Jasmijn, Shikha Pundir, Elizabeth Mckenzie, Jaap Keijer, and Martin Kussmann. 2018. "Maternal Circulating Vitamin Status and Colostrum Vitamin Composition in Healthy Lactating Women—A Systematic Approach" Nutrients 10, no. 6: 687. https://doi.org/10.3390/nu10060687
APA StyleY. de Vries, J., Pundir, S., Mckenzie, E., Keijer, J., & Kussmann, M. (2018). Maternal Circulating Vitamin Status and Colostrum Vitamin Composition in Healthy Lactating Women—A Systematic Approach. Nutrients, 10(6), 687. https://doi.org/10.3390/nu10060687





