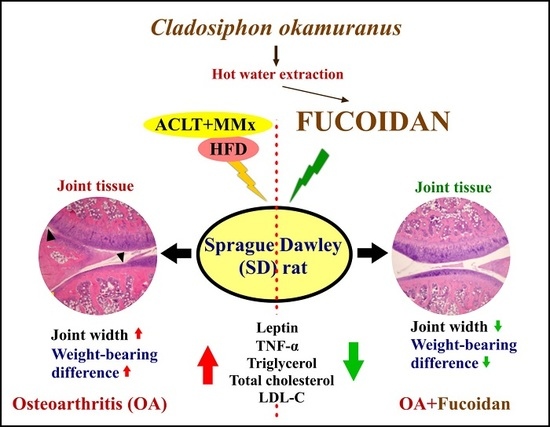
Effect of Fucoidan on Anterior Cruciate Ligament Transection and Medial Meniscectomy Induced Osteoarthritis in High-Fat Diet-Induced Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Fucoidan
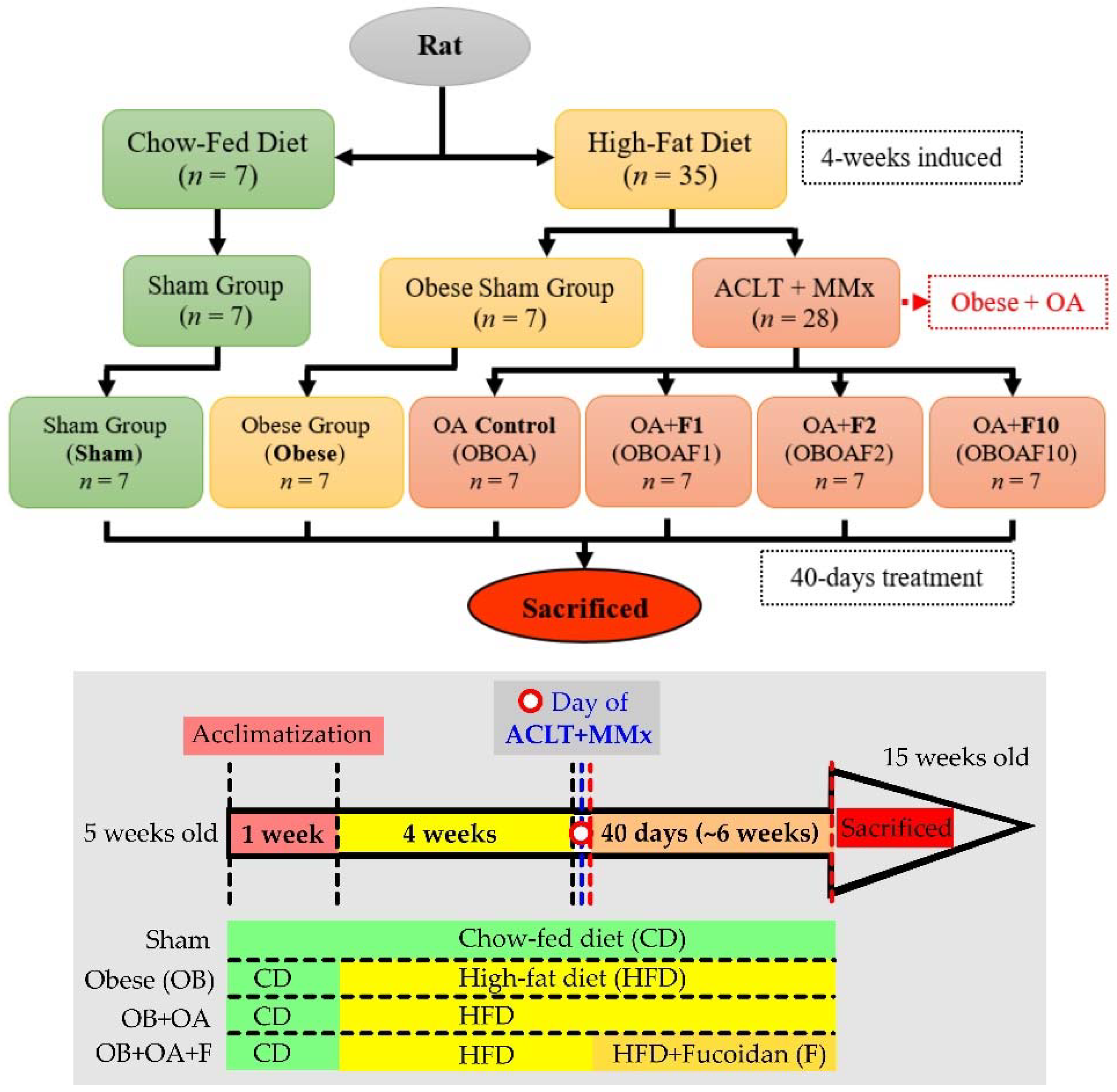
2.2. Animal Model
2.3. Measurement of Plasma Biochemical Parameters
2.4. Weight-Bearing Distribution Assessment
2.5. Knee Width and Joint Histopathology
2.6. Statistical Analysis
3. Results
3.1. Reduction of Body Weight and Body Lipid by Fucoidan
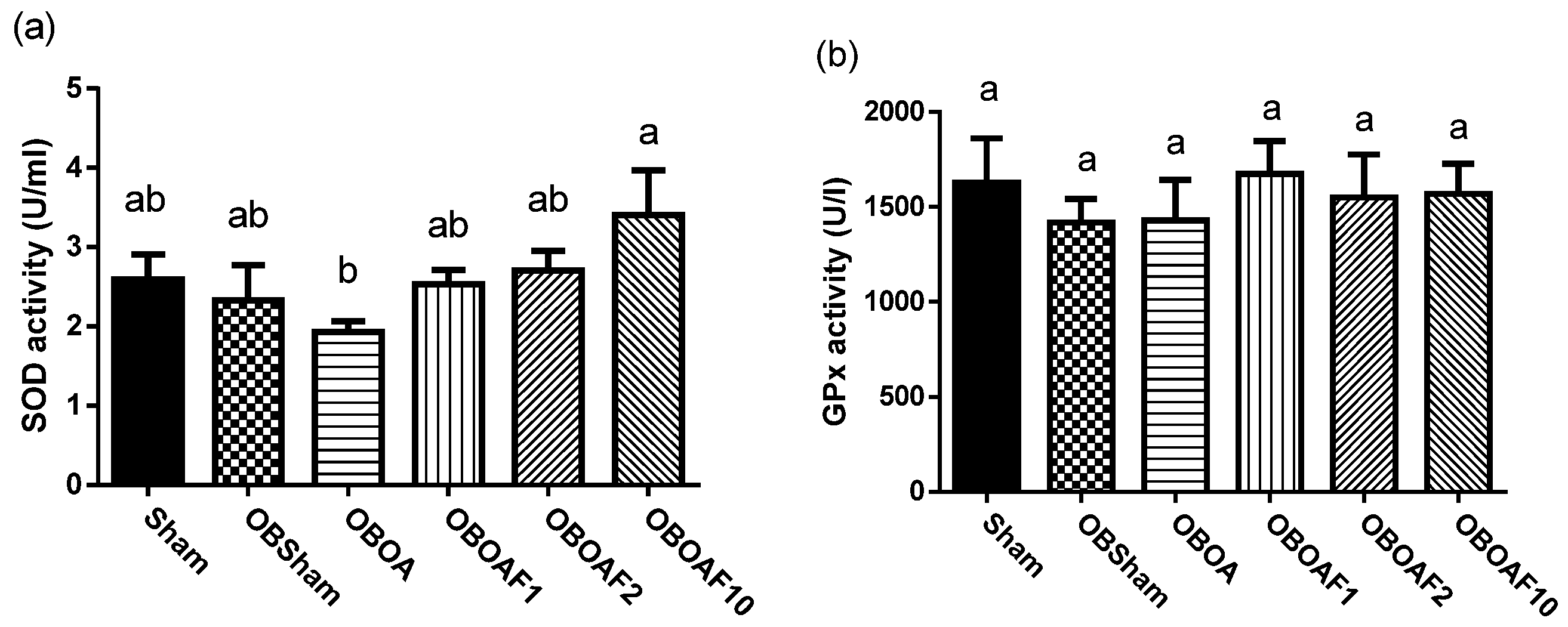
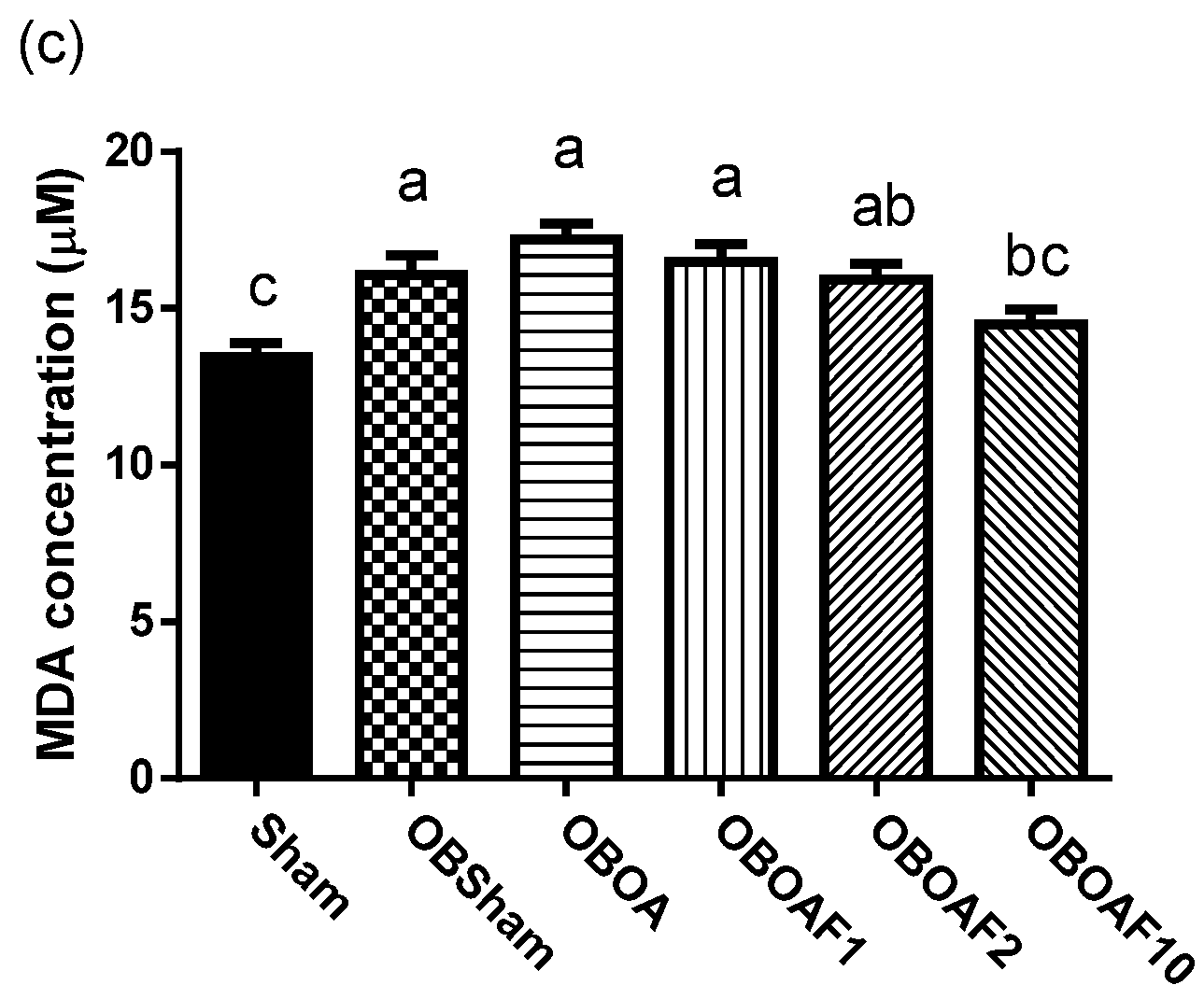
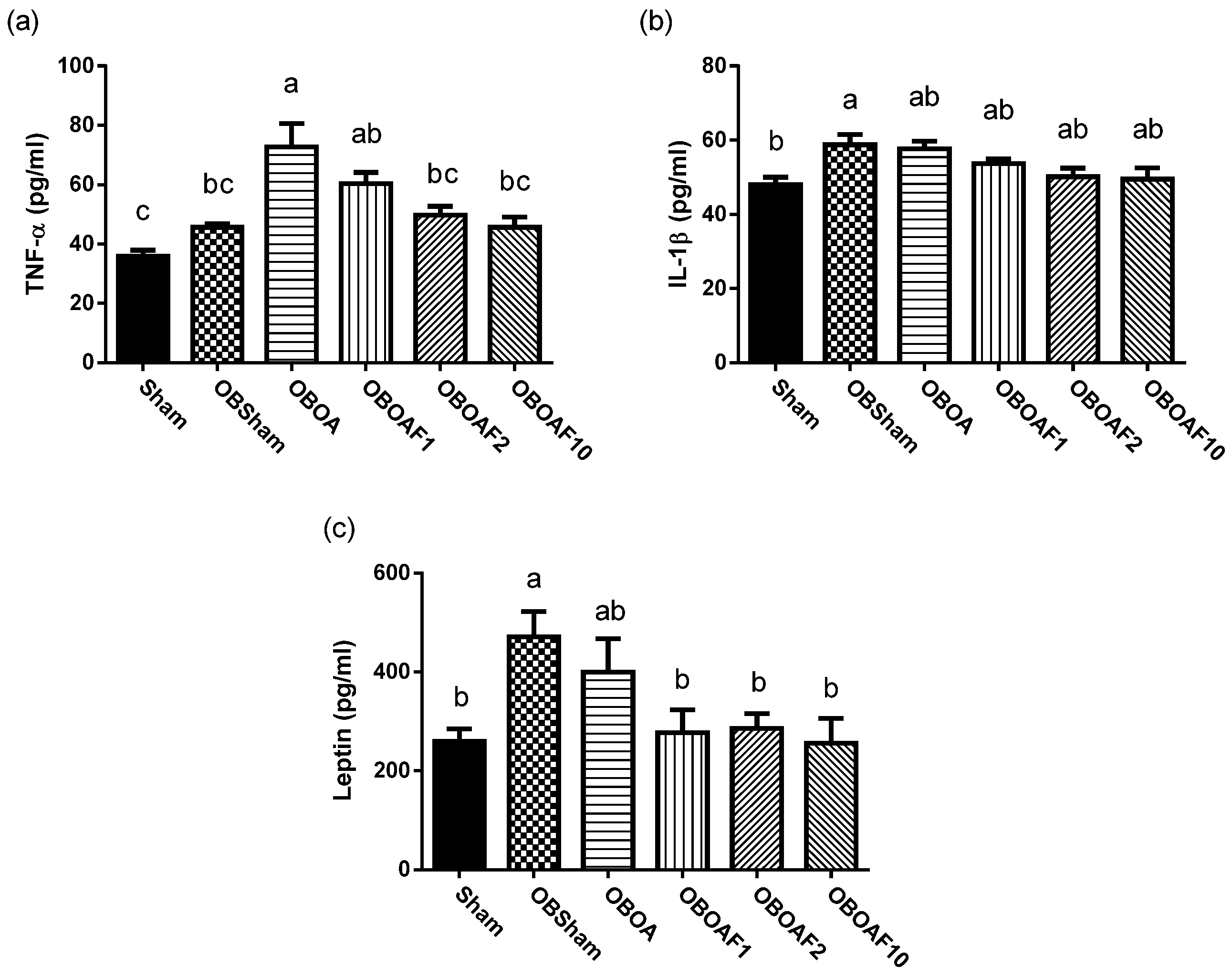
3.2. The Effect of Fucoidan on the Antioxidant Properties and Anti-Inflammatory Properties
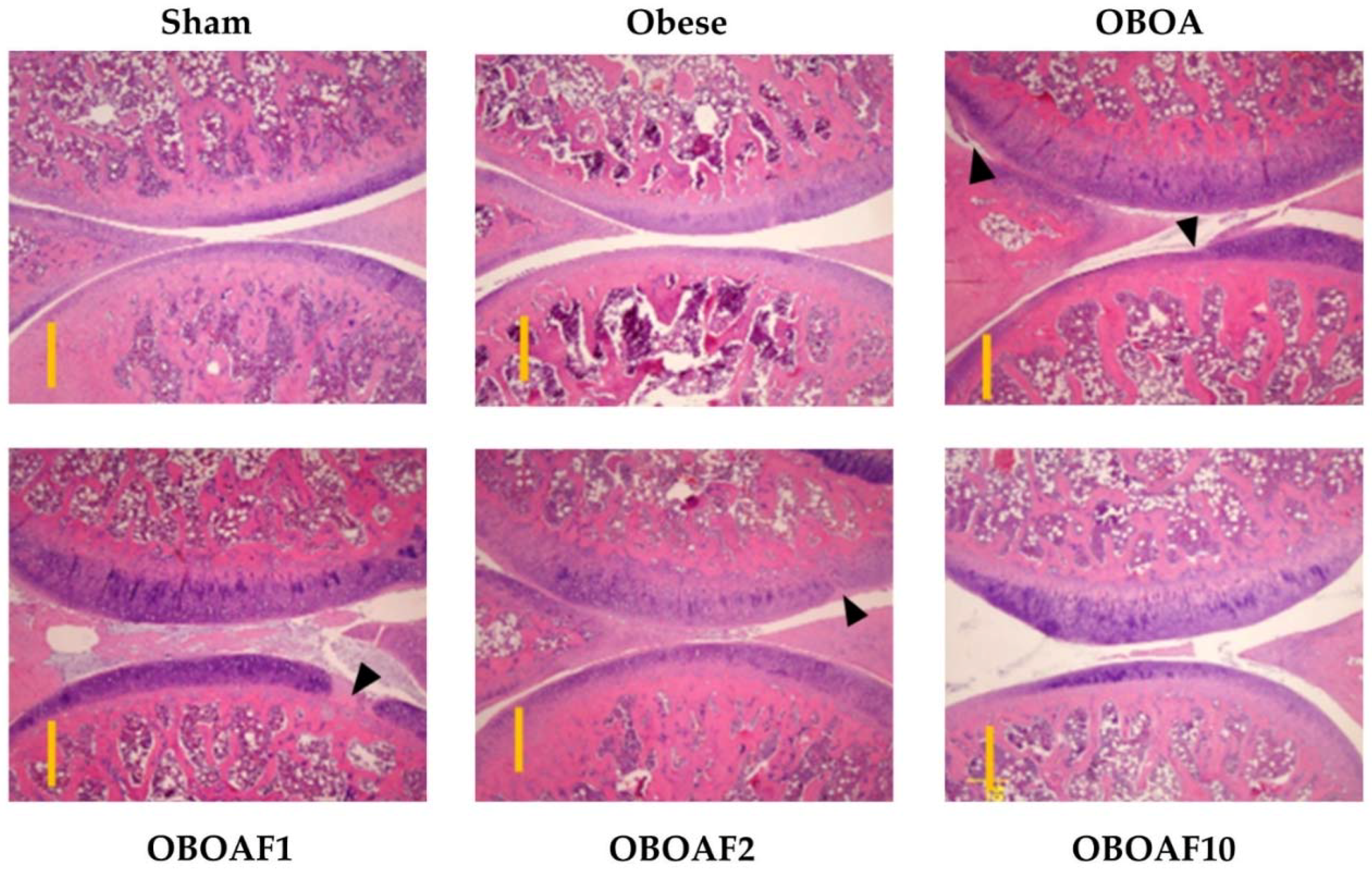
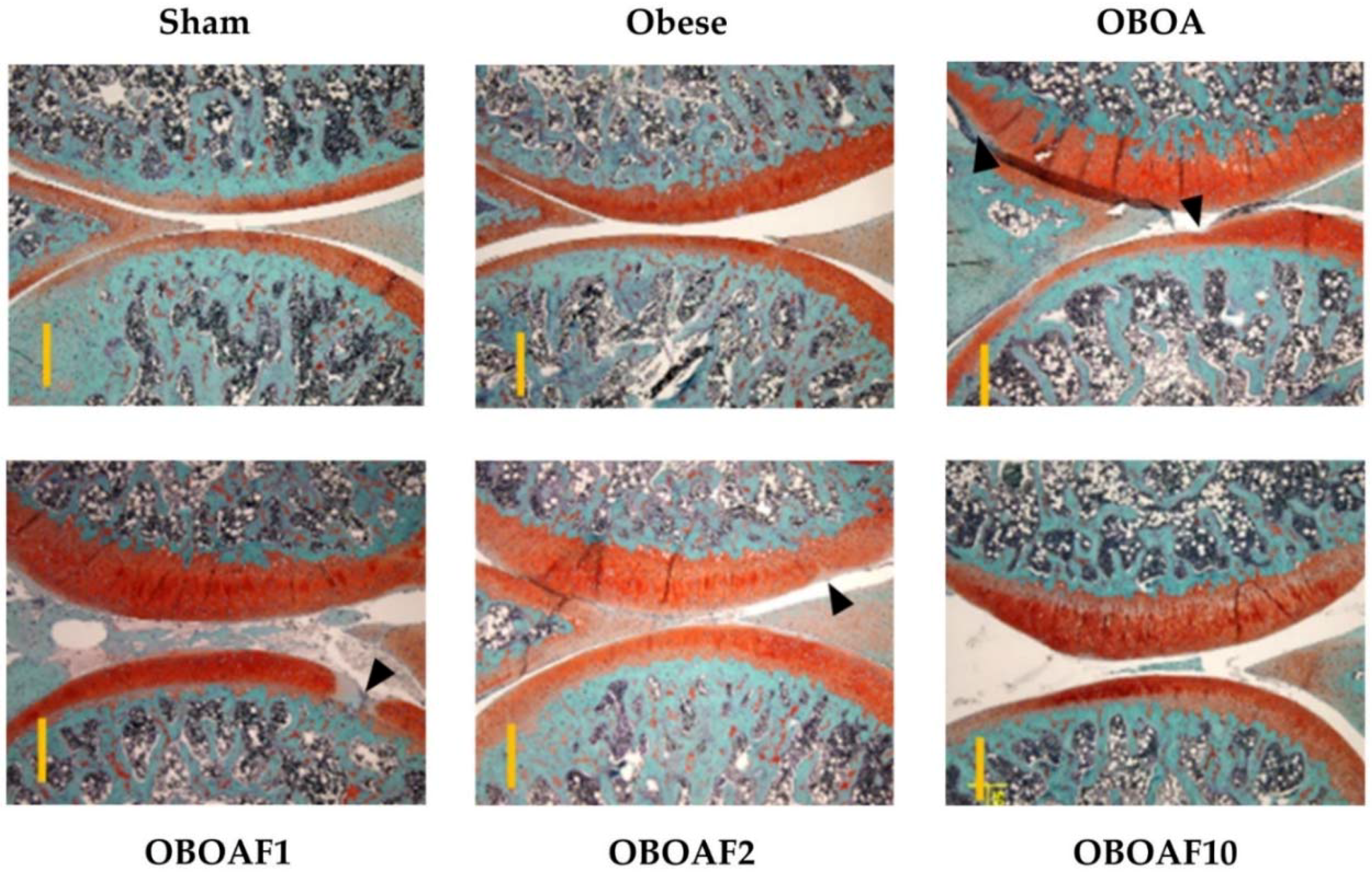
3.3. Effects Fucoidan on Joint Histology
3.4. Fucoidan Attenuate OA Caused Pain and Damage
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Golightly, Y.M. Epidemiology of osteoarthritis: State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Barve, R.A.; Minnerly, J.C.; Weiss, D.J.; Meyer, D.M.; Aguiar, D.J.; Sullivan, P.M.; Weinrich, S.L.; Head, R.D. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: Relevance to human disease. Osteoarthr. Cartil. 2007, 15, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Kurz, B. Antioxidant to treat osteoarthritis: Dream or reality? Curr. Drug Targets 2007, 8, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Krasnokutsky, S.; Attur, M.; Palmer, G.; Samuels, J.; Abramson, S.B. Current concepts in the pathogenesis of osteoarthritis. Osteoarthr. Cartil. 2008, 16, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-G.; Park, S.-Y.; Chung, W.-S.; Park, J.-H.; Hwang, E.; Mavlonov, G.T.; Kim, I.-H.; Kim, K.-Y.; Yi, T.-H. Fucoidan prevents the progression of osteoarthritis in rats. J. Med. Food 2015, 18, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Poonpet, T. Adipokines: Biomarkers for osteoarthritis? World J. Orthop. 2014, 5, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Int. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Koonce, R.C.; Bravman, J.T. Obesity and osteoarthritis: More than just wear and tear. J. Am. Acad. Orthop. Surg. 2013, 21, 161–169. [Google Scholar] [PubMed]
- Yusuf, E.; Nelissen, R.G.; Ioan-Facsinay, A.; Stojanovic-Susulic, V.; DeGroot, J.; van Osch, G.; Middeldorp, S.; Huizinga, T.W.J.; Kloppenburg, M. Association between weight or body mass index and hand osteoarthritis: A systematic review. Ann. Rheum. Dis. 2009, 69, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Pottie, P.; Presle, N.; Terlain, B.; Netter, P.; Mainard, D.; Berenbaum, F. Obesity and osteoarthritis: More complex than predicted. Ann. Rheum. Dis. 2006, 65, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Vuolteenaho, K.; Koskinen, A.; Kukkonen, M.; Nieminen, R.; Päivärinta, U.; Moilanen, T.; Moilanen, E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage—Mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediat. Inflamm. 2009, 2009, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Distel, E.; Cadoudal, T.; Durant, S.; Poignard, A.; Chevalier, X.; Benelli, C. The infrapatellar fat pad in knee osteoarthritis: An important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009, 60, 3374–3377. [Google Scholar] [CrossRef] [PubMed]
- Neuman, P.; Englund, M.; Kostogiannis, I.; Friden, T.; Roos, H.; Dahlberg, L.E. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury. Am. J. Sports Med. 2017, 36, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.P.; Appleyard, R.C.; Mahajan, S.; Murrell, G.A.C. Meniscal and chondral loss in the anterior cruciate ligament injured knee. Sports Med. 2003, 33, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Louboutin, H.; Debarge, R.; Richou, J.; Selmi, T.A.S.; Donell, S.T.; Neyret, P.; Dubrana, F. Osteoarthritis in patients with anterior cruciate ligament rupture: A review of risk factors. Knee 2009, 16, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Mascarenhas, R.; Saltzman, B.M.; Rollins, M.; Bach, B.R.; MacDonald, P. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv. Orthop. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Reimer, R.A.; Seerattan, R.A.; Leonard, T.R.; Herzog, W. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthr. Cartil. 2015, 23, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Hart, D.A.; Reimer, R.A.; Seerattan, R.A.; Herzog, W. Response to diet-induced obesity produces time-dependent induction and progression of metabolic osteoarthritis in rat knees. J. Orthop. Res. 2016, 34, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Herzog, W.; MacDonald, G.Z.; Reimer, R.A.; Rios, J.L.; Smith, I.C.; Zernicke, R.F.; Hart, D.A. Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory pathways suggest a central role for loss of muscle integrity. Front. Physiol. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Fibel, K.H.; Howard, J.H.; Brian, C.H. State-of-the-art management of knee osteoarthritis. World J. Clin. Cases 2015, 3, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Trelle, S.; Reichenbach, S.; Wandel, S.; Hildebrand, P.; Tschannen, B.; Villiger, P.M.; Egger, M.; Juni, P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ Clin. Res. 2011, 342, c7086. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.W.P.; Schlüter-Brust, K.U.; Peer, E. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch. Ärzteblatt Int. 2010, 107, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Wan Aida, W.M.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mazita Mohd, D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Phull, A.-R.; Majid, M.; Haq, I.-U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, Q.; Kong, Y.; Xie, B.; Gao, M.; Tao, Y.; Xu, H.; Zhan, F.; Dai, B.; Shi, J.; et al. Antitumor activity of fucoidan against diffuse large B cell lymphomain vitroandin vivo. Acta Biochim. Biophys. Sin. 2015, 47, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Jeon, J.; Lee, J.-S. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-B.; Chun, K.-R.; Kim, J.-K.; Suk, K.; Jung, Y.-M.; Lee, W.-H. The differential effect of high and low molecular weight fucoidans on the severity of collagen-induced arthritis in mice. Phytother. Res. 2010, 24, 1384–1391. [Google Scholar] [CrossRef]
- Fitton, J.; Stringer, D.; Karpiniec, S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, M. For researchers on obesity: Historical review of extra body weight definitions. J. Obes. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, W.; Perino, G.; Gilbert, S.L.; Maher, S.A.; Windhager, R.; Boettner, F. Oarsi osteoarthritis cartilage histopathology assessment system: A biomechanical evaluation in the human knee. J. Orthop. Res. 2016, 34, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.J.; Colditz, G.A. The epidemic of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2522–2525. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Gutekunst, D.J.; Davis, C.; DeVita, P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005, 52, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Puett, D.W.; Griffin, M.R. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Ann. Intern. Med. 1994, 121, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Jacquet, J.; Solinas, G.; Montani, J.P.; Schutz, Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int. J. Obes. 2010, 34, S4–S17. [Google Scholar] [CrossRef] [PubMed]
- Adeneye, A.A.; Adeyemi, O.O.; Agbaje, E.O. Anti-obesity and antihyperlipidaemic effect of Hunteria umbellata seed extract in experimental hyperlipidaemia. J. Ethnopharmacol. 2010, 130, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, M.-S.; Jo, K.; Hwang, J.-K. Piperidine alkaloids from Piper retrofractum Vahl. Protect against high-fat diet-induced obesity by regulating lipid metabolism and activating amp-activated protein kinase. Biochem. Biophys. Res. Commun. 2011, 411, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kang, N.; Ko, S.-C.; Kim, Y.-B.; Jeon, Y.-J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Ciotola, M.; Schisano, B.; Misso, L.; Giannetti, G.; Ceriello, A.; Giugliano, D. Oxidative stress in the metabolic syndrome. J. Endocrinol. Investig. 2014, 29, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Ozata, M.; Mergen, M.; Oktenli, C.; Aydin, A.; Yavuz Sanisoglu, S.; Bolu, E.; Yilmaz, M.I.; Sayal, A.; Isimer, A.; Ozdemir, I.C. Increased oxidative stress and hypozincemia in male obesity. Clin. Biochem. 2002, 35, 627–631. [Google Scholar] [CrossRef]
- Sabitha, K.; Venugopal, B.; Rafi, M.; V Ramana, K. Role of antioxidant enzymes in glucose and lipid metabolism in association with obesityand type 2 diabetes. Am. J. Med. Sci. Med. 2014, 2, 21–24. [Google Scholar] [CrossRef]
- Patel, M.D.; Kishore, K.; Patel, D.J. Valuation of oxidative stress and serum magnesium levels in south Indian obese males. Int. J. Sci. Res. 2014, 3, 229–230. [Google Scholar]
- Agrawal, N.; Singh, S.K. Obesity: An independent risk factor for oxidative stress. Int. J. Adv. Med. 2017, 4, 718–721. [Google Scholar] [CrossRef]
- Higdon, J.V. Obesity and oxidative stress: A direct link to CVD? Arterioscler. Thromb. Vasc. Biol. 2003, 23, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Bogdanowicz, P.; Domagala, F.; Ficheux, H.; Pujol, J.-P. Rhein inhibits interleukin-1β-induced activation of MEK/ERK pathway and DNA binding of NF-κB and AP-1 in chondrocytes cultured in hypoxia: A potential mechanism for its disease-modifying effect in osteoarthritis. Inflammation 2003, 27, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Figenschau, Y.; Knutsen, G.; Shahazeydi, S.; Johansen, O.; Sveinbjörnsson, B. Human articular chondrocytes express functional leptin receptors. Biochem. Biophys. Res. Commun. 2001, 287, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Dumond, H.; Presle, N.; Terlain, B.; Mainard, D.; Loeuille, D.; Netter, P.; Pottie, P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003, 48, 3118–3129. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliezer, M.; Phillip, M.; Gat-Yablonski, G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine 2007, 32, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, K.; Nishino, T.; Fujihara, M.; Nagumo, T. Isolation and preliminary characterization of fucose-containing sulfated polysaccharides with blood-anticoagulant activity from the brown seaweed hizikia fusiforme. Carbohydr. Res. 1989, 194, 315–320. [Google Scholar] [CrossRef]
- Millet, J.; Jouault, S.C.; Vidal, B.; Sternberg, C.; Theveniaux, J.; Mauray, S.; Fischer, A.M. Antithrombotic and anticoagulant activities of a low molecular weight fucoidan by the subcutaneous route. Thromb. Haemost. 1999, 81, 391–395. [Google Scholar] [PubMed]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.-Y.; Kim, G.-Y.; Choi, I.-W.; Kim, N.D.; Nam, T.-J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and AKT activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Yoon, S.Y.; Oh, S.J.; Kim, S.K.; Kang, K.W. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem. Biophys. Res. Commun. 2006, 346, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonicain hyperlipidemic rats. Pharmaceutical Biology 2010, 48, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of nk cells in antitumor activity of dietary fucoidan from Undaria pinnatifida Sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.-N.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan extracted from cladosiphon okamuranus tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, J.; Song, B.; Zhang, L.; Shao, Q.; Liu, Y.; Yuan, D.; Zhang, Y.; Qu, X. Fucoidan inhibits CCL22 production through NF-κB pathway in m2 macrophages: A potential therapeutic strategy for cancer. Sci. Rep. 2016, 6, 35855. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-O.; Zhang, W.; Du, J.-Y.; Wong, K.-W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar] [CrossRef] [PubMed]
- Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum. 2012, 64, 3220–3230. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef] [PubMed]

| Group | Sham | Obese | Obese + OA | |||
|---|---|---|---|---|---|---|
| Control | F1 | F2 | F10 | |||
| Body Weight (g) | ||||||
| Initial | 136.24 ± 1.58 a | 139.15 ± 2.98 a | 141.82 ± 4.61 a | 137.71 ± 1.43 a | 135.19 ± 2.34 a | 140.93 ± 2.83 a |
| Final | 385.47 ± 16.50 c | 530.89 ± 33.53 a | 537.94 ± 36.55 a | 477.98 ± 19.75 b | 489.45 ± 22.23 b | 477.61 ± 35.41 b |
| Adipose Tissue Weight (g/100 g Body Weight) | ||||||
| Perirenal | 1.54 ± 0.15 c | 3.23 ± 0.54 a | 2.54 ± 0.37 b | 2.23 ± 0.35 b | 2.17 ± 0.33 b | 2.05 ± 0.43 b,c |
| Epididymal | 1.02 ± 0.09 c | 2.21 ± 0.33 a | 2.06 ± 0.27 a,b | 1.80 ± 0.12 b | 1.85 ± 0.27 a,b | 1.87 ± 0.36 a,b |
| Group | Sham | Obese | Obese + OA | |||
|---|---|---|---|---|---|---|
| Control | F1 | F2 | F10 | |||
| TG | 70.83 ± 3.37 b | 81.59 ± 4.61 a,b | 95.26 ± 8.04 a | 79.94 ± 5.68 a,b | 77.08 ± 7.19 a | 70.30 ± 3.57 a |
| TC | 89.43 ± 7.83 b | 120.13 ± 9.92 a | 125.81 ± 7.08 a | 98.29 ± 4.91 b | 91.52 ± 3.45 b | 88.57 ± 3.16 b |
| HDL-C | 39.23 ± 1.66 a | 41.44 ± 2.98 a | 39.69 ± 2.02 a | 41.86 ± 2.52 a | 38.65 ± 2.51 a | 38.60 ± 3.16 a |
| LDL-C | 36.03 ± 7.70 b | 62.38 ± 9.84 a | 67.07 ± 6.90 a | 40.45 ± 6.76 b | 37.46 ± 4.17 b | 35.91 ± 2.23 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudirman, S.; Ong, A.D.; Chang, H.-W.; Kong, Z.-L. Effect of Fucoidan on Anterior Cruciate Ligament Transection and Medial Meniscectomy Induced Osteoarthritis in High-Fat Diet-Induced Obese Rats. Nutrients 2018, 10, 686. https://doi.org/10.3390/nu10060686
Sudirman S, Ong AD, Chang H-W, Kong Z-L. Effect of Fucoidan on Anterior Cruciate Ligament Transection and Medial Meniscectomy Induced Osteoarthritis in High-Fat Diet-Induced Obese Rats. Nutrients. 2018; 10(6):686. https://doi.org/10.3390/nu10060686
Chicago/Turabian StyleSudirman, Sabri, Alan Darmasaputra Ong, Heng-Wei Chang, and Zwe-Ling Kong. 2018. "Effect of Fucoidan on Anterior Cruciate Ligament Transection and Medial Meniscectomy Induced Osteoarthritis in High-Fat Diet-Induced Obese Rats" Nutrients 10, no. 6: 686. https://doi.org/10.3390/nu10060686
APA StyleSudirman, S., Ong, A. D., Chang, H.-W., & Kong, Z.-L. (2018). Effect of Fucoidan on Anterior Cruciate Ligament Transection and Medial Meniscectomy Induced Osteoarthritis in High-Fat Diet-Induced Obese Rats. Nutrients, 10(6), 686. https://doi.org/10.3390/nu10060686