Implementation of Low Glycemic Index Diet Together with Cornstarch in Post-Gastric Bypass Hypoglycemia: Two Case Reports
Abstract
:1. Introduction
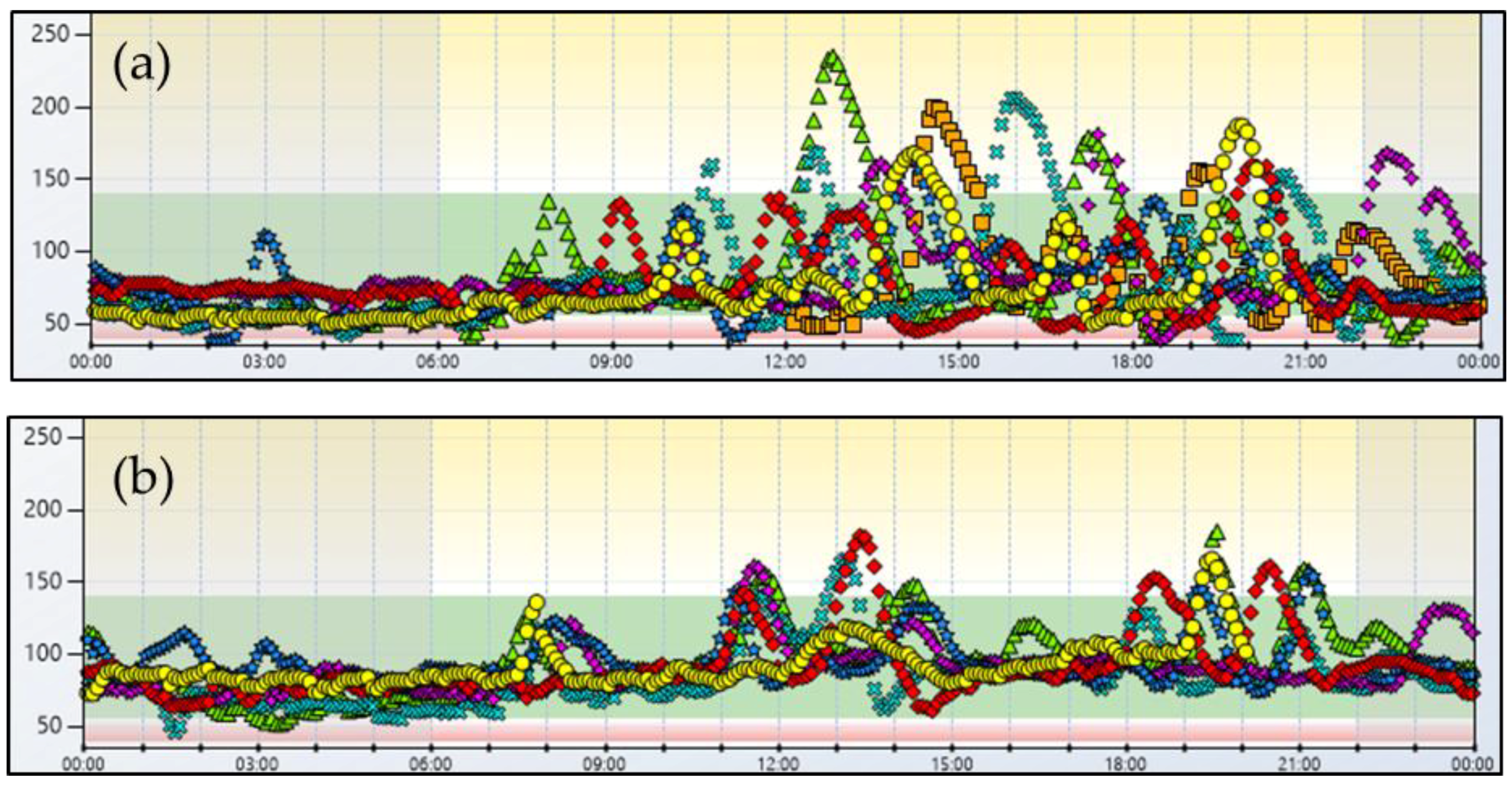
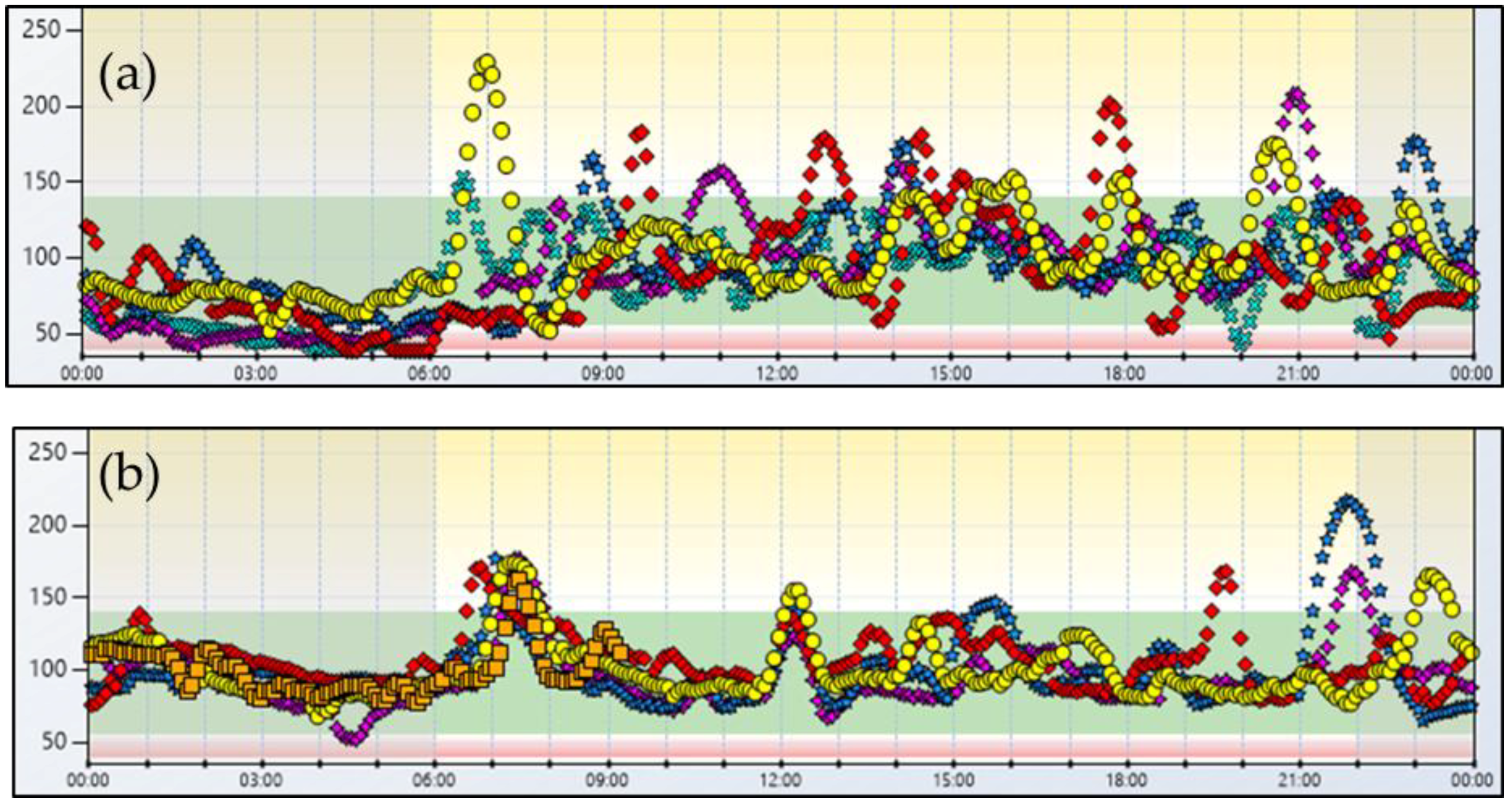
2. Case Report No. 1
3. Case Report No. 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. High-Fiber Low-Glycemic Index Diet for the Nutritional Management of Patients with Post-Bariatric Hypoglycemia (PBH)
Appendix A.2. An Example Day
- Breakfast
- -
- Coffee or tea or herbal teas or skimmed yogurt 125 g + cornflakes (or oatmeal or wholemeal rusks) 30 g.
- Lunch
- -
- Pasta (or brown rice) 40 g + legumes (lentils, beans, chickpeas) 50 g + parmesan cheese 10 g,
- -
- Vegetable (spinach, zucchini, beets, green beans, tomato, pumpkin, lettuce) 150 g,
- -
- Fresh fruit (apple, pear, peach, apricot, orange, kiwi) 150 g.
- Dinner
- -
- Meat 120 g,
- -
- Vegetables (spinach, zucchini, beets, green beans, tomato, pumpkin, lettuce) 150 g,
- -
- Whole brain bread 80 g.
- 3 Snacks (mid-day, mid-afternoon, bedtime)
- -
- Fresh fruit 150–200 g,
- -
- Crackers 25 g,
- -
- 2 toast bread + speck (or fresh cheese or tuna fish) 30 g.
- Seasoning for the day
- -
- Extra-virgin olive oil (30 g).
References
- Eisenberg, D.; Azagury, D.E.; Ghiassi, S.; Grover, B.T.; Kim, J.J. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg. Obes. Relat. Dis. 2017, 13, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Marsk, R.; Jonas, E.; Rasmussen, F.; Näslund, E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia 2010, 53, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, H.; Chapman, W.H.; Pender, J.R.; Ivanescu, A.; Drake, A.J.; Pories, W.J.; Dar, M.S. Hypoglycemia after Roux-en-Y gastric bypass: The BOLD experience. Obes. Surg. 2014, 24, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Clark, J.M.; Schweitzer, M.; Magnuson, T.; Steele, K.; Koerner, O.; Brown, T.T. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity 2015, 23, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Emous, M.; Ubels, F.L.; Beek, A.P. Diagnostic tools for post-gastric bypass hypoglycaemia. Obes. Rev. 2015, 16, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Kefurt, R.; Langer, F.B.; Schindler, K.; Shakeri-Leidenmühler, S.; Ludvik, B.; Prager, G. Hypoglycemia after Roux-En-Y gastric bypass: Detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg. Obes. Relat. Dis. 2015, 11, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Nosso, G.; Lupoli, R.; Saldalamacchia, G.; Griffo, E.; Cotugno, M.; Costabile, G.; Riccardi, G.; Capaldo, B. Diabetes remission after bariatric surgery is characterized by high glycemic variability and high oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Salkind, S.J.; Huizenga, R.; Fonda, S.J.; Walker, M.S.; Vigersky, R.A. Glycemic variability in nondiabetic morbidly obese persons: Results of an observational study and review of the literature. J. Diabetes Sci. Technol. 2014, 8, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Zagury, L.; Moreira, R.O.; Guedes, E.P.; Coutinho, W.F.; Appolinario, J.C. Insulinoma misdiagnosed as dumping syndrome after bariatric surgery. Obes. Surg. 2004, 14, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Mulla, C.M.; Storino, A.; Yee, E.U.; Lautz, D.; Sawnhey, M.S.; Moser, A.J.; Patti, M.E. Insulinoma after bariatric surgery: Diagnostic dilemma and therapeutic approaches. Obes. Surg. 2016, 26, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Suhl, E.; Anderson-Haynes, S.E.; Mulla, C.; Patti, M.E. Medical nutrition therapy for post-bariatric hypoglycemia: Practical insights. Surg. Obes. Relat. Dis. 2017, 13, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.O.; Moreira, R.B.; Machado, N.A.; Goncalves, T.B.; Coutinho, W.F. Postprandial hypoglycemia after bariatic surgery: Pharmacological treatment with verapamil and acarbose. Obes. Surg. 2008, 18, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.; Worona, L.; Consuelo, A. Nutritional therapy for glycogen storage diseases. J. Pediatr. Gastroenterol. Nutr. 2008, 47, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Band, M.; Tester, R.; Piggott, J.; Hurel, S.J. Use of slow release starch (SRS) to treat hypoglycaemia in type 1 diabetics. J. Nutr. Food Sci. 2010, 40, 228–234. [Google Scholar] [CrossRef]
- Lechner, K.; Aulinger, B.; Brand, S.; Waldmann, E.; Parhofer, K.G. Hydrothermally modified slow release corn starch: A potential new therapeutic option for treating hypoglycemia in autoimmune hypoglycemia (Hirata’s disease). Eur. J. Clin. Nutr. 2015, 69, 1369–1370. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of obesity: Weight loss and bariatric surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Di Minno, M.N.D.; Guidone, C.; Cefalo, C.; Capaldo, B.; Riccardi, G.; Mingrone, G. Effects of bariatric surgery on markers of subclinical atherosclerosis and endothelial function: A meta-analysis of literature studies. Int. J. Obes. 2016, 40, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Griffo, E.; Nosso, G.; Lupoli, R.; Cotugno, M.; Saldalamacchia, G.; Vitolo, G.; Angrisani, L.; Cutolo, P.P.; Rivellese, A.A.; Capaldo, B. Early improvement of postprandial lipemia after bariatric surgery in obese type 2 diabetic patients. Obes. Surg. 2014, 24, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Halperin, F.; Patti, M.E.; Skow, M.; Bajwa, M.; Goldfine, A.B. Continuous glucose monitoring for evaluation of glycemic excursions after gastric bypass. J. Obes. 2011, 2011, 869536. [Google Scholar] [CrossRef] [PubMed]
- Shantavasinkul, P.C.; Torquati, A.; Corsino, L. Post-gastric bypass hypoglycaemia: A review. Clin. Endocrinol. 2016, 85, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, A.; Magruder, J.T.; Salas Carillo, R.; Carlson, O.; Egan, J.M.; Askin, S.B.; Elahi, D.; Andersen, D.K. Hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: Unraveling the role of gut hormonal and pancreatic endocrine dysfunction. J. Surg. Res. 2011, 167, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Prigeon, R.L.; D’Alessio, D.A. Gastric Bypass Surgery Enhances Glucagon-Like Peptide 1-Stimulated Postprandial Insulin Secretion in Humans. Diabetes 2011, 60, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Li, P.; Goldfine, A.B. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity 2015, 23, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Butler, A.E.; Galasso, R.; Butler, P.C. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care 2006, 29, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Nosso, G.; Griffo, E.; Cotugno, M.; Saldalamacchia, G.; Lupoli, R.; Pacini, G.; Riccardi, G.; Angrisani, L.; Capaldo, B. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: A one-year prospective study. Horm. Metab. Res. 2016, 48, 312–317. [Google Scholar] [PubMed]
- Salehi, M.; Gastaldelli, A.; D’alessio, D.A. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J. Clin. Endocrinol. Metab. 2014, 99, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Goldfine, A.B. Hypoglycemia after gastric bypass: The dark side of GLP-1. Gastroenterology 2014, 146, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Laferrère, B.; Reilly, D.; Arias, S.; Swerdlow, N.; Gorroochurn, P.; Bawa, B.; Bose, M.; Teixeira, J.; Stevens, R.D.; Wenner, B.R.; et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med. 2011, 3, 80re2. [Google Scholar] [CrossRef] [PubMed]
| Case 1 | Case 2 | |||||
|---|---|---|---|---|---|---|
| Before | After | Δ % | Before | After | Δ % | |
| Mean IG 1 (mg/dL) | 81 | 92 | +13.6 | 93 | 101 | +8.6 |
| % time > 140 mg/dL | 7 | 4 | −3 | 8 | 7 | −1 |
| % time in range 70–140 mg/dL | 49 | 88 | +39 | 72 | 92 | +20 |
| % time ≥ 55 and <70 mg/dL | 32 | 7 | −25 | 9 | 1 | −8 |
| % time < 55 mg/dL | 12 | 1 | −11 | 11 | 0 | −11 |
| % nightime < 55 mg/dL | 23 | 1 | −22 | 33 | 1 | −32 |
| SD 2 (mg/dL) | 32 | 21 | −34.4 | 32 | 23 | −28.1 |
| CV 3 (%) | 39 | 23 | −16 | 34 | 22 | −12 |
| MAGE 4 (mg/dL) | 35.4 | 23.6 | −33.3 | 35.6 | 24.6 | −30.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lembo, E.; Lupoli, R.; Ciciola, P.; Creanza, A.; Silvestri, E.; Saldalamacchia, G.; Capaldo, B. Implementation of Low Glycemic Index Diet Together with Cornstarch in Post-Gastric Bypass Hypoglycemia: Two Case Reports. Nutrients 2018, 10, 670. https://doi.org/10.3390/nu10060670
Lembo E, Lupoli R, Ciciola P, Creanza A, Silvestri E, Saldalamacchia G, Capaldo B. Implementation of Low Glycemic Index Diet Together with Cornstarch in Post-Gastric Bypass Hypoglycemia: Two Case Reports. Nutrients. 2018; 10(6):670. https://doi.org/10.3390/nu10060670
Chicago/Turabian StyleLembo, Erminia, Roberta Lupoli, Paola Ciciola, Annalisa Creanza, Eufemia Silvestri, Gennaro Saldalamacchia, and Brunella Capaldo. 2018. "Implementation of Low Glycemic Index Diet Together with Cornstarch in Post-Gastric Bypass Hypoglycemia: Two Case Reports" Nutrients 10, no. 6: 670. https://doi.org/10.3390/nu10060670
APA StyleLembo, E., Lupoli, R., Ciciola, P., Creanza, A., Silvestri, E., Saldalamacchia, G., & Capaldo, B. (2018). Implementation of Low Glycemic Index Diet Together with Cornstarch in Post-Gastric Bypass Hypoglycemia: Two Case Reports. Nutrients, 10(6), 670. https://doi.org/10.3390/nu10060670





