The Biological Activities of Oleocanthal from a Molecular Perspective
Abstract
1. Introduction
2. Literature Search
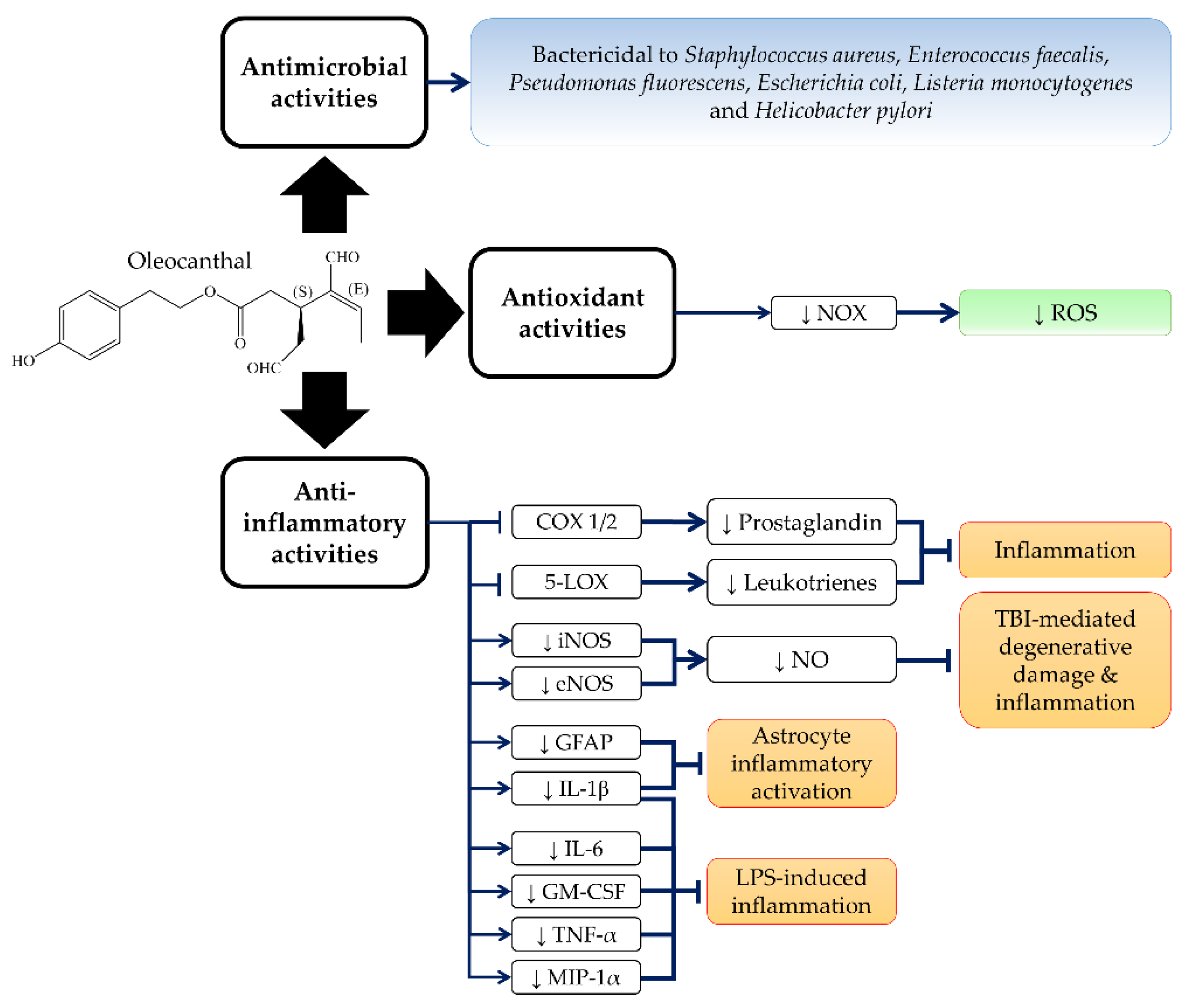
3. Anti-Inflammatory, Antioxidant and Antimicrobial Activities of OC
4. Anticancer Properties of OC
4.1. Melanoma
4.2. Breast Cancer
4.3. Liver Cancer
4.4. Colon Cancer
4.5. Other Cancer Types
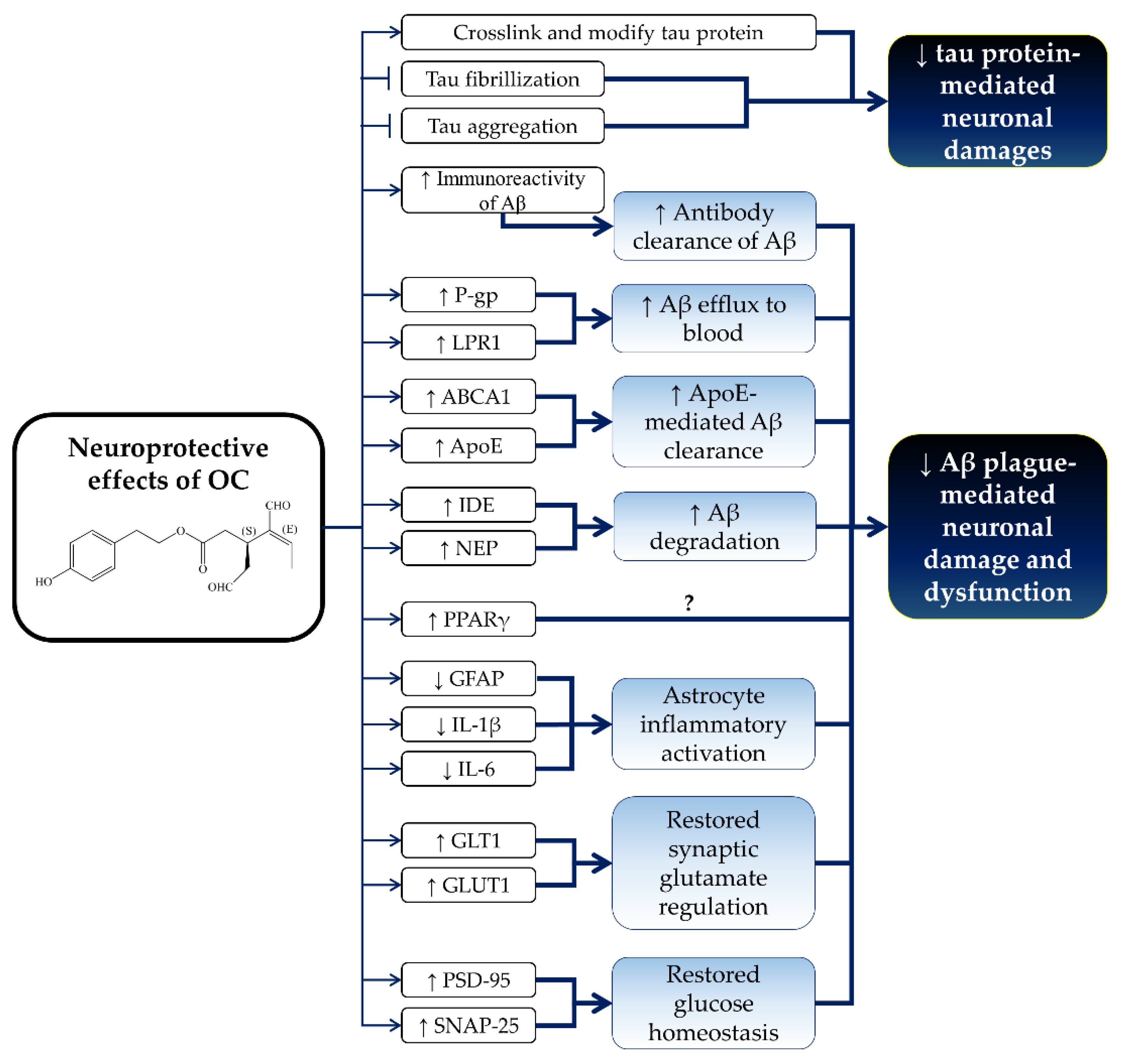
5. Neuroprotective Effects of OC
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fogliano, V.; Sacchi, R. Oleocanthal in olive oil: Between myth and reality. Mol. Nutr. Food Res. 2006, 50, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. Hum. Nutr. Metab. 2001, 132, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Bosetti, C.; Lipworth, L.; La Vecchia, C. Olive oil and cancer risk: An update of epidemiological findings through 2010. Curr. Pharm. Des. 2011, 17, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.M.; Arnoni, Y.; Aviram, M. The middle eastern and biblical origins of the Mediterranean diet. Public Health Nutr. 2011, 14, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Luchsinger, J.A.; Schupf, N.; Brickman, A.M.; Cosentino, S.; Tang, M.X.; Stern, Y. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Martinez-Gonzalez, M.A. Olive oil in the primary prevention of cardiovascular disease. Maturitas 2011, 68, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L. Therapeutic effects of olive and its derivatives on osteoarthritis: From bench to bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. Olives and bone: A green osteoporosis prevention option. Int. J. Environ. Res. Public Health 2016, 13, 755. [Google Scholar] [CrossRef] [PubMed]
- Maalej, A.; Mahmoudi, A.; Bouallagui, Z.; Fki, I.; Marrekchi, R.; Sayadi, S. Olive phenolic compounds attenuate deltamethrin-induced liver and kidney toxicity through regulating oxidative stress, inflammation and apoptosis. Food Chem. Toxicol. 2017, 106, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Takashima, T.; Sakata, Y.; Iwakiri, R.; Shiraishi, R.; Oda, Y.; Inoue, N.; Nakayama, A.; Toda, S.; Fujimoto, K. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J. Nutr. Biochem. 2014, 25, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Rangel-Zuniga, O.A.; Haro, C.; Meza-Miranda, E.R.; Pena-Orihuela, P.; Meneses, M.E.; Marin, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Delgado-Lista, J.; et al. Olive oil phenolic compounds decrease the postprandial inflammatory response by reducing postprandial plasma lipopolysaccharide levels. Food Chem. 2014, 162, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Iuliano, L.; Pratico, D. Extra-virgin olive oil ameliorates cognition and neuropathology of the 3xTg mice: Role of autophagy. Ann. Clin. Transl. Neurol. 2017, 4, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, C.M.; Mesa, M.D.; Ramirez-Tortosa, M.C.; Nestares, M.T.; Ros, E.; Gil, A. Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clin. Nutr. 2004, 23, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Majo, D.D.; Giammanco, S.; Guardia, M.L. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Conlan, X.A.; Barnett, N.W.; Sinclair, A.J.; Keast, R.S. Influence of heat on biological activity and concentration of oleocanthal—A natural anti-inflammatory agent in virgin olive oil. J. Agric. Food Chem. 2009, 57, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 2. Initial characterization of the hydrolyzable fraction. J. Agric. Food Chem. 1992, 40, 1577–1580. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Smith, A.B.; Han, Q.; Breslin, P.A.S.; Beauchamp, G.K. Synthesis and assignment of absolute configuration of (−)-oleocanthal: A potent, naturally occurring non-steroidal anti-inflammatory and anti-oxidant agent derived from extra virgin olive oils. Org. Lett. 2005, 7, 5075–5078. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B., 3rd; Sperry, J.B.; Han, Q. Syntheses of (−)-oleocanthal, a natural NSAID found in extra virgin olive oil, the (−)-deacetoxy-oleuropein aglycone, and related analogues. J. Org. Chem. 2007, 72, 6891–6900. [Google Scholar] [CrossRef] [PubMed]
- Valli, M.; Peviani, E.G.; Porta, A.; D’Alfonso, A.; Zanoni, G.; Vidari, G. A concise and efficient total synthesis of oleocanthal. Eur. J. Org. Chem. 2013, 2013, 4332–4336. [Google Scholar] [CrossRef]
- English, B.J.; Williams, R.M. Synthesis of (+/−)-oleocanthal via a tandem intramolecular Michael cyclization-HWE olefination. Tetrahedron Lett. 2009, 50, 2713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, K.; Morita, H.; Honda, T. Formal synthesis of (−)-oleocanthal by means of a SmI2-promoted intramolecular coupling of bromoalkyne with α,β-unsaturated ester. Tetrahedron Lett. 2012, 53, 3342–3345. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Breslin, P.A.; Beauchamp, G.K.; Keast, R.S. Sensory characterization of the irritant properties of oleocanthal, a natural anti-inflammatory agent in extra virgin olive oils. Chem. Senses 2009, 34, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: In vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Des Gachons, C.P.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B., 3rd; Beauchamp, G.K.; Breslin, P.A. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Nevedomskaya, E.; Mayboroda, O.A.; Deelder, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Exploratory analysis of human urine by LC–ESI-TOF MS after high intake of olive oil: Understanding the metabolism of polyphenols. Anal. Bioanal. Chem. 2010, 398, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Qin, H.; Selgrad, M.; Boland, C.R.; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Casamenti, F.; Stefani, M. Olive polyphenols: New promising agents to combat aging-associated neurodegeneration. Expert Rev. Neurother. 2017, 17, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Medina, E.; Vargas, J.; Brenes, M.; De Castro, A. In vitro activity of olive oil polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2007, 55, 680–686. [Google Scholar] [CrossRef] [PubMed]
- ChemSpider. Oleocanthal. Available online: http://www.chemspider.com/Chemical-Structure.9827154.html (accessed on 25 March 2018).
- Fito, M.; de la Torre, R.; Farre-Albaladejo, M.; Khymenetz, O.; Marrugat, J.; Covas, M.-I. Bioavailability and antioxidant effects of olive oil phenolic compounds in humans: A review. Annali dell'Istituto Superiore Di Sanita 2007, 43, 375–381. [Google Scholar] [PubMed]
- Corona, G.; Tzounis, X.; Dessi, M.A.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, P.; Busch, J.L.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory properties of virgin olive oil polyphenols: Identification of deacetoxy-ligstroside aglycon as a key contributor to pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhe, V.; Plee-Gautier, E.; Carre, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernandez-Pena, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef] [PubMed]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Vougogiannopoulou, K.; Lemus, C.; Halabalaki, M.; Pergola, C.; Werz, O.; Smith, A.B., 3rd; Michel, S.; Skaltsounis, L.; Deguin, B. One-step semisynthesis of oleacein and the determination as a 5-lipoxygenase inhibitor. J. Nat. Prod. 2014, 77, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Pergola, C.; Werz, O. 5-lipoxygenase inhibitors: A review of recent developments and patents. Expert Opin. Ther. Pat. 2010, 20, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.C.; Gijon, M.A. Biosynthesis and metabolism of leukotrienes. Biochem. J. 2007, 405, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; Ottani, A.; Bertolini, A. Dual acting anti-inflammatory drugs. Curr. Top. Med. Chem. 2007, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Burnett, B.P.; Levy, R.M. 5-Lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Adv. Ther. 2012, 29, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, T.M.; Boyce, J.A. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol. Allergy Clin. N. Am. 2013, 33, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.J.; Lago, F.; Smith, A.B., 3rd; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1α and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Gomez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gomez-Reino, J.J.; Smith, A.B., 3rd; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheumatol. 2010, 62, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal enhances amyloid-β clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Mete, M.; Aydemir, I.; Unsal, U.U.; Collu, F.; Vatandas, G.; Gurcu, B.; Duransoy, Y.K.; Taneli, F.; Tugrul, M.I.; Selcuki, M. Neuroprotective effects of oleocanthal, a compound in virgin olive oil, in a rat model of traumatic brain injury. Turk. Neurosurg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; McKeever, L.C.; Malik, N.S. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; de Castro, A.; Romero, C.; Ramirez, E.; Brenes, M. Effect of antimicrobial compounds from olive products on microorganisms related to health, food and agriculture. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 2, pp. 1087–1094. [Google Scholar]
- Medina, E.; Brenes, M.; Garcia, A.; Romero, C.; De Castro, A. Bactericidal activity of glutaraldehyde-like compounds from olive products. J. Food Prot. 2009, 72, 2611–2614. [Google Scholar] [CrossRef] [PubMed]
- Tagliafierro, L.; Officioso, A.; Sorbo, S.; Basile, A.; Manna, C. The protective role of olive oil hydroxytyrosol against oxidative alterations induced by mercury in human erythrocytes. Food Chem. Toxicol. 2015, 82, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvan, I.; Dicson, S.M.; Kasi, P.D. Olive oil and its phenolic constituent tyrosol attenuates dioxin-induced toxicity in peripheral blood mononuclear cells via an antioxidant-dependent mechanism. Nat. Prod. Res. 2015, 29, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; La Fauci, L.; Graziani, G.; Ferracane, R.; Masella, R.; Di Giacomo, C.; Scacco, A.; D’Archivio, M.; Vanella, L.; Galvano, G. Phenolic compounds and antioxidant activity of Italian extra virgin olive oil Monti Iblei. J. Med. Food 2007, 10, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Dunn, L.; Liu, L.; Hasan, A.; Vincent, K.; Brackstone, M.; Hess, D.; Lala, P.K. COX-2 induces oncogenic micro RNA miR655 in human breast cancer. Sci Rep. 2018, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, M.P.; Lightfoot, T.; Speirs, V.; Horgan, K.; Gooderham, N.J. Expression of COX-2, NF-κB-p65, NF-κB-p50 and IKKα in malignant and adjacent normal human colorectal tissue. Br. J. Cancer 2009, 101, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic activity of oleocanthal isolated from virgin olive oil on human melanoma cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, J.; Peng, L. (−)-Oleocanthal exerts anti-melanoma activities and inhibits STAT3 signaling pathway. Oncol. Rep. 2017, 37, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Elnagar, A.Y.; Sylvester, P.W.; El Sayed, K.A. (−)-Oleocanthal as a c-Met inhibitor for the control of metastatic breast and prostate cancers. Planta Med. 2011, 77, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Busnena, B.A.; Foudah, A.I.; Melancon, T.; El Sayed, K.A. Olive secoiridoids and semisynthetic bioisostere analogues for the control of metastatic breast cancer. Bioorg. Med. Chem. 2013, 21, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, M.M.; Akl, M.R.; Ebrahim, H.Y.; Dragoi, A.M.; Dykes, S.; Cardelli, J.A.; El Sayed, K.A. The oleocanthal-based homovanillyl sinapate as a novel c-Met inhibitor. Oncotarget 2016, 7, 32247–32273. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, M.M.; Busnena, B.A.; Akl, M.R.; Dragoi, A.M.; Cardelli, J.A.; El Sayed, K.A. Novel c-Met inhibitory olive secoiridoid semisynthetic analogs for the control of invasive breast cancer. Eur. J. Med. Chem. 2016, 118, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.Y.; Sayed, K.A. Olive phenolics as c-Met inhibitors: (−)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; El Sayed, K.A. Olive oil-derived oleocanthal as potent inhibitor of mammalian target of rapamycin: Biological evaluation and molecular modeling studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (−)-Oleocanthal inhibits growth and metastasis by blocking activation of STAT3 in human hepatocellular carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; McCubrey, J.A.; Montalto, G.; et al. Oleocanthal exerts antitumor effects on human liver and colon cancer cells through ROS generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Oh, W.K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.G.; Kwon, S.M.; Choi, H.K.; Choi, H.S. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.J.; Lago, F.; Smith, A.B., 3rd; Gualillo, O. Oleocanthal inhibits proliferation and MIP-1α expression in human multiple myeloma cells. Curr. Med. Chem. 2013, 20, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Selvaggini, R.; Montedoro, G.F.; Di Saverio, C.; Morozzi, G. Virgin olive oil phenols inhibit proliferation of human promyelocytic leukemia cells (HL60) by inducing apoptosis and differentiation. Nutr. Dis. 2006, 136, 614–619. [Google Scholar] [CrossRef] [PubMed]
- LeGendre, O.; Breslin, P.A.; Foster, D.A. (−)-Oleocanthal rapidly and selectively induces cancer cell death via lysosomal membrane permeabilization. Mol. Cell. Oncol. 2015, 2, e1006077. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (−)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Margarucci, L.; Monti, M.C.; Cassiano, C.; Mozzicafreddo, M.; Angeletti, M.; Riccio, R.; Tosco, A.; Casapullo, A. Chemical proteomics-driven discovery of oleocanthal as an Hsp90 inhibitor. Chem. Commun. 2013, 49, 5844–5846. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, C.; Casapullo, A.; Tosco, A.; Monti, M.C.; Riccio, R. In cell interactome of oleocanthal, an extra virgin olive oil bioactive component. Nat. Prod. Commun. 2015, 10, 1013–1016. [Google Scholar] [PubMed]
- Keiler, A.M.; Zierau, O.; Bernhardt, R.; Scharnweber, D.; Lemonakis, N.; Termetzi, A.; Skaltsounis, L.; Vollmer, G.; Halabalaki, M. Impact of a functionalized olive oil extract on the uterus and the bone in a model of postmenopausal osteoporosis. Eur. J. Nutr. 2014, 53, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Djiogue, S.; Ehrhardt, T.; Zierau, O.; Skaltsounis, L.; Halabalaki, M.; Vollmer, G. Oleocanthal modulates estradiol-induced gene expression involving estrogen receptor α. Planta Med. 2015, 81, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Abuznait, A.H.; Qosa, H.; O’Connell, N.D.; Akbarian-Tefaghi, J.; Sylvester, P.W.; El Sayed, K.A.; Kaddoumi, A. Induction of expression and functional activity of P-glycoprotein efflux transporter by bioactive plant natural products. Food Chem.Toxicol. 2011, 49, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.X.; Xu, J.H.; Zhang, K.Z.; Lin, Q.; Huang, X.W.; Wen, C.X.; Chen, Y.Z. Disruption of the Bcr-Abl/Hsp90 protein complex: A possible mechanism to inhibit Bcr-Abl-positive human leukemic blasts by novobiocin. Leukemia 2008, 22, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, R.; Garabedian, M.J. Role for Hsp90-associated cochaperone p23 in estrogen receptor signal transduction. Mol. Cell. Biol. 1999, 19, 3748–3759. [Google Scholar] [CrossRef] [PubMed]
- Fliss, A.E.; Benzeno, S.; Rao, J.; Caplan, A.J. Control of estrogen receptor ligand binding by Hsp90. J. Steroid Biochem. Mol. Biol. 2000, 72, 223–230. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The Hsp90 chaperone machinery. Nat. Rev. Mol. Cell. Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ma, Q.; Ren, Y.; He, J.; Zhang, Y.; Zhang, Y.; Chen, W. Geldanamycin destabilizes HER2 tyrosine kinase and suppresses Wnt/β-catenin signaling in HER2 overexpressing human breast cancer cells. Oncol. Rep. 2007, 17, 89–96. [Google Scholar] [CrossRef] [PubMed]
- McCleese, J.K.; Bear, M.D.; Fossey, S.L.; Mihalek, R.M.; Foley, K.P.; Ying, W.; Barsoum, J.; London, C.A. The novel Hsp90 inhibitor STA-1474 exhibits biologic activity against osteosarcoma cell lines. Int. J. Cancer 2009, 125, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.P.; Hose, C.D.; Koochekpour, S.; Jeffers, M.; Oskarsson, M.; Sausville, E.; Monks, A.; Woude, G.F.V. The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-Met-urokinase plasminogen activator-plasmin proteolytic network. Cancer Res. 2000, 60, 342–349. [Google Scholar] [PubMed]
- Wang, S.; Pashtan, I.; Tsutsumi, S.; Xu, W.; Neckers, L. Cancer cells harboring MET gene amplification activate alternative signaling pathways to escape MET inhibition but remain sensitive to Hsp90 inhibitors. Cell Cycle 2009, 8, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, C.E.; Kasembeli, M.M.; Roh, S.H.; Tweardy, D.J. Contribution of chaperones to STAT pathway signaling. JAKSTAT 2014, 3, e970459. [Google Scholar] [CrossRef] [PubMed]
- Subik, K.; Lee, J.-F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.-C.; Bonfiglio, T.; Hicks, D.G.; et al. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer Basic Clin. Res. 2010, 4, 35–41. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Cortes, J. Breast cancer and Hsp90 inhibitors: Is there a role beyond the HER2-positive subtype? Breast 2012, 21, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Dai, Q.; Sun, D.F.; Xiong, H.; Tian, X.Q.; Gao, F.H.; Xu, M.H.; Chen, G.Q.; Han, Z.G.; Fang, J.Y. mTOR signaling pathway is a target for the treatment of colorectal cancer. Ann. Surg. Oncol. 2009, 16, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Tian, X.Q.; Sun, D.F.; Zhao, S.L.; Xiong, H.; Fang, J.Y. Combined inhibition of MEK and mTOR signaling inhibits initiation and progression of colorectal cancer. Cancer Investig. 2009, 27, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, X.F. mTOR-independent 4E-BP1 phosphorylation is associated with cancer resistance to mTOR kinase inhibitors. Cell Cycle 2012, 11, 594–603. [Google Scholar] [CrossRef] [PubMed]
- van Assema, D.M.; Lubberink, M.; Bauer, M.; van der Flier, W.M.; Schuit, R.C.; Windhorst, A.D.; Comans, E.F.; Hoetjes, N.J.; Tolboom, N.; Langer, O.; et al. Blood-brain barrier P-glycoprotein function in Alzheimer's disease. Brain 2012, 135, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Miceli, T.S.; Colson, K.; Faiman, B.M.; Miller, K.; Tariman, J.D.; International Myeloma Foundation Nurse Leadership, B. Maintaining bone health in patients with multiple myeloma: Survivorship care plan of the International Myeloma Foundation Nurse Leadership Board. Clin. J. Oncol. Nurs. 2011, 15, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Politou, M.; Viniou, N.; Rahemtulla, A. Significance of macrophage inflammatory protein-1 alpha (MIP-1α) in multiple myeloma. Leuk Lymphoma 2005, 46, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Kato, C.; Manno, M.; Ogaki, M.; Satou, T.; Itoh, T.; Kusunoki, T.; Tanimori, Y.; Fujiwara, K.; Matsuoka, H.; et al. Macrophage inflammatory protein-1α (MIP-1α) enhances a receptor activator of nuclear factor κB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol. Cell. Biochem. 2007, 304, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Cruz, J.C.; Craig, F.; Chung, H.; Devlin, R.D.; Roodman, G.D.; Alsina, M. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood 2000, 96, 671–675. [Google Scholar] [PubMed]
- Li, W.; Sperry, J.B.; Crowe, A.; Trojanowski, J.Q.; Smith, A.B., 3rd; Lee, V.M. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J. Neurochem. 2009, 110, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Tosco, A.; Riccio, R.; Casapullo, A. New insights on the interaction mechanism between tau protein and oleocanthal, an extra-virgin olive-oil bioactive component. Food Funct. 2011, 2, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Casapullo, A. Modulation of tau protein fibrillization by oleocanthal. J. Nat. Prod. 2012, 75, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Deane, R.; Fagan, A.M.; Spinner, M.L.; Parsadanian, M.; Finn, M.B.; Jiang, H.; Prior, J.L.; Sagare, A.; Bales, K.R.; et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-βdeposition in an Alzheimer disease mouse model. J. Clin. Investig. 2005, 115, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hashimoto, T.; Yabuki, C.; Nagae, Y.; Tachikawa, M.; Strickland, D.K.; Liu, Q.; Bu, G.; Basak, J.M.; Holtzman, D.M.; et al. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid βpeptides in an in vitro model of the blood-brain barrier cells. J. Biol. Chem. 2008, 283, 34554–34562. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.; Roth, W.; Lacor, P.; Smith, A.B.; Blankenship, M.; Velasco, P.; De Felice, F.; Breslin, P.; Klein, W.L. Alzheimer's-associated aβ oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol. Appl. Pharmacol. 2009, 240, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, Y.S.; Mohamed, L.A.; Al Rihani, S.B.; Mousa, Y.M.; Siddique, A.B.; El Sayed, K.A.; Kaddoumi, A. Oleocanthal ameliorates amyloid-βoligomers' toxicity on astrocytes and neuronal cells: In vitro studies. Neuroscience 2017, 352, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008, 192, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Katsouri, L.; Parr, C.; Bogdanovic, N.; Willem, M.; Sastre, M. PPARγ co-activator-1α (PGC-1α) reduces amyloid-β generation through a PPARγ-dependent mechanism. J. Alzheimers Dis. 2011, 25, 151–162. [Google Scholar] [PubMed]
- Heneka, M.T.; Reyes-Irisarri, E.; Hüll, M.; Kummer, M.P. Impact and therapeutic potential of PPARs in Alzheimer's disease. Curr. Neuropharmacol. 2011, 9, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Sastre, M.; Dewachter, I.; Landreth, G.E.; Willson, T.M.; Klockgether, T.; van Leuven, F.; Heneka, M.T. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-γ agonists modulate immunostimulated processing of amyloid precursor protein through regulation of β-secretase. J. Neurosci. 2003, 23, 9796–9804. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.-L.; Ferran, A.; Bousquet-Mélou, A. Species differences in pharmacokinetics and pharmacodynamics. In Comparative and Veterinary Pharmacology; Cunningham, F., Elliott, J., Lees, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 19–48. [Google Scholar]
- Musther, H.; Olivares-Morales, A.; Hatley, O.J.; Liu, B.; Rostami Hodjegan, A. Animal versus human oral drug bioavailability: Do they correlate? Eur. J. Pharm. Sci. 2014, 57, 280–291. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef] [PubMed]
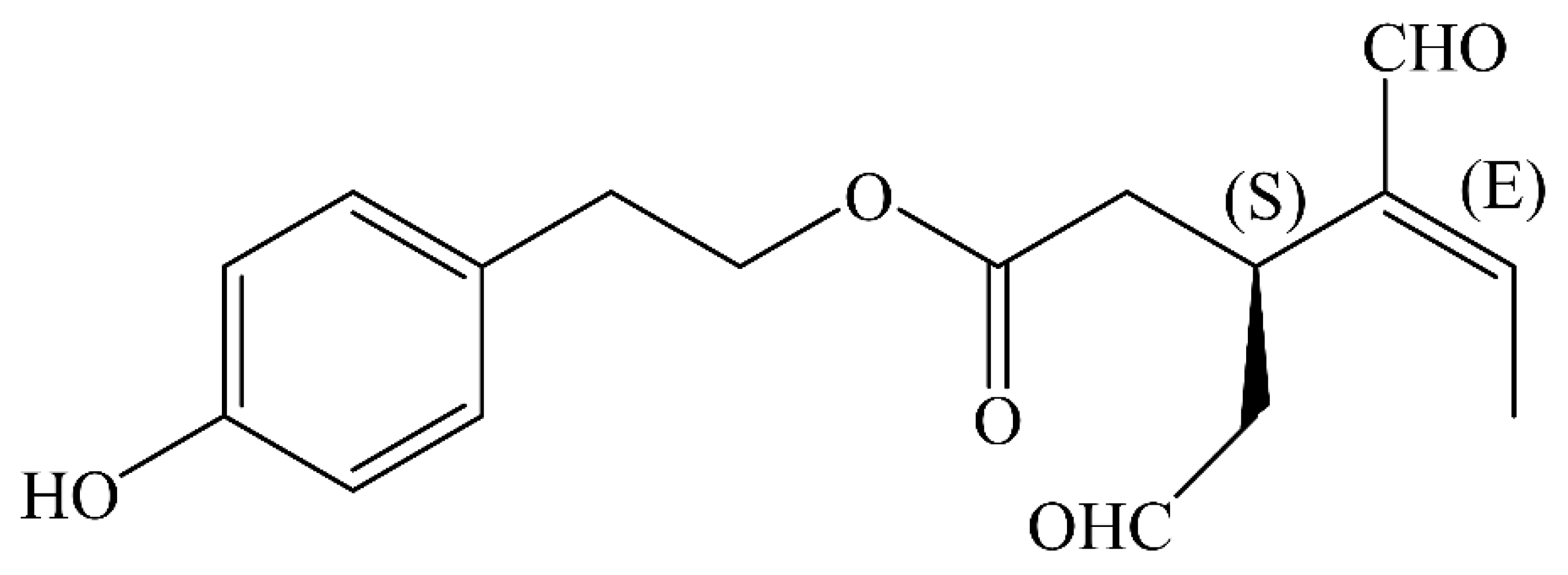

| Model | Molecular Action | Reference |
|---|---|---|
| Human melanoma A375 cells and 501Mel cells |
| [64] |
| Human melanoma A375 cells and A2058 cells |
| [65] |
| HUVEC cells |
| [65] |
| Endothelial colony forming cells |
| [66] |
| Human breast cancer MDA-MB-231 and MCF-7 cells Human prostate cancer PC-3 cells |
| [66] |
| Human breast cancer MDA-MB-231 cells |
| [67] |
| Human breast cancer MDA-MB-231, MCF-7, and BT-474 cells |
| [70] |
| Human breast cancer MDA-MB-231, MCF-7, and T-47D cells |
| [71] |
| Human breast cancer MDA-MB-231, MDA-MB-468, MCF-7, BT-474 and T-47D cells |
| [68] |
| Human breast cancer MDA-MB-231 and MDA-MB-468 cells |
| [69] |
| Human breast cancer MDA-MB-231 3D spheroids |
| [69] |
| Human breast cancer MCF-7, BT-474, and T-47D cells |
| [78] |
| Molecular docking analysis |
| [66,67] |
| [71] | |
| [78] | |
| Z’-LYTE mTOR kinase assay, Invitrogen, Carlsbad, CA |
| [71] |
| Z’-LYTE c-MET kinase assay, Invitrogen, Carlsbad, CA |
| [66,67,68,69] |
| Omnia® c-MET kinase assay, Invitrogen, Carlsbad, CA |
| [67] |
| Surface plasmon resonance, molecular docking analysis and in vitro immobilized OC pull-down assay |
| [79] |
| In vitro immobilized OC pulldown assay |
| [80] |
| Human histiocytic lymphoma U937 cells |
| [79] |
| PolarScreenTM ERα and ERβ competitor assay, from Life Technologies, Darmstadt, Germany |
| [81] |
| Human MVLN cells with ERα Human RNDA cells with ERβ |
| [82] |
| Human osteosarcoma U2OS cells |
| [82] |
| Human hepatocellular carcinoma Huh-7, HepG2, and HCCLM3 cells |
| [72] |
| Human hepatocellular carcinoma HepG2, Hep3B, Huh-7 and PLC/PRF/5 cells Human colorectal carcinoma HT-29 and SW480 cells |
| [73] |
| Human colon cancer HT-29 and HCT116 cells |
| [74] |
| Human myeloma ARH-77 cells Murine myeloma MOPC-31C cells |
| [75] |
| Human prostate cancer PC-3 cells Human breast cancer MDA-MB-231 cells Human pancreas adenocarcinoma BxPC3 cells |
| [77] |
| Human acute promyelocytic leukaemia HL-60 cells |
| [76] |
| HT-29 cells inoculation in chorioallantoic membrane assay |
| [74] |
| Human colon adenocarcinoma LS-180 cells |
| [83] |
| Human colorectal adenocarcinoma Caco-2 cells Human cervical cancer HeLa cells |
| [71] |
| Non-tumorigenic human adult dermal fibroblast HDFa cells |
| [64] |
| Non-tumorigenic human mammary epithelial MCF10A cells |
| [70] |
| Non-tumorigenic human liver LO2 cells |
| [72] |
| Murine macrophage J774A.1 cells |
| [50] |
| Isolated primary human hepatocytes |
| [73] |
| Non-tumorigenic human fibroblast BJ cells Rat fibroblast 3Y1 cells Human lung fibroblast IMR90 cells |
| [77] |
| Non-tumorigenic mouse epidermis JB6 CI41 |
| [74] |
| Murine chondrocyte ATDC-5 cells |
| [51] |
| Subcutaneous A375 xenograft on male BALB/c athymic nude mice |
| [65] |
| Injected A375 xenograft on the tail vein of male BALB/c athymic nude mice |
| [65] |
| Mammary gland subcutaneous MDA-MB-231 xenograft in female athymic nude mice |
| [70] |
| Mammary gland subcutaneous BT-474 xenograft in female athymic nude mice with 17β-oestradiol releasing pellets preimplantation |
| [78] |
| HCCLM3 xenograft in male BALB/c athymic nude mice |
| [72] |
| Hepatocellular carcinoma patient-derived xenograft in the liver of male BALB/c athymic nude mice |
| [72] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.-L.; Chin, K.-Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. https://doi.org/10.3390/nu10050570
Pang K-L, Chin K-Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients. 2018; 10(5):570. https://doi.org/10.3390/nu10050570
Chicago/Turabian StylePang, Kok-Lun, and Kok-Yong Chin. 2018. "The Biological Activities of Oleocanthal from a Molecular Perspective" Nutrients 10, no. 5: 570. https://doi.org/10.3390/nu10050570
APA StylePang, K.-L., & Chin, K.-Y. (2018). The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients, 10(5), 570. https://doi.org/10.3390/nu10050570






