The Vitamin D–Folate Hypothesis as an Evolutionary Model for Skin Pigmentation: An Update and Integration of Current Ideas
Abstract
1. Introduction
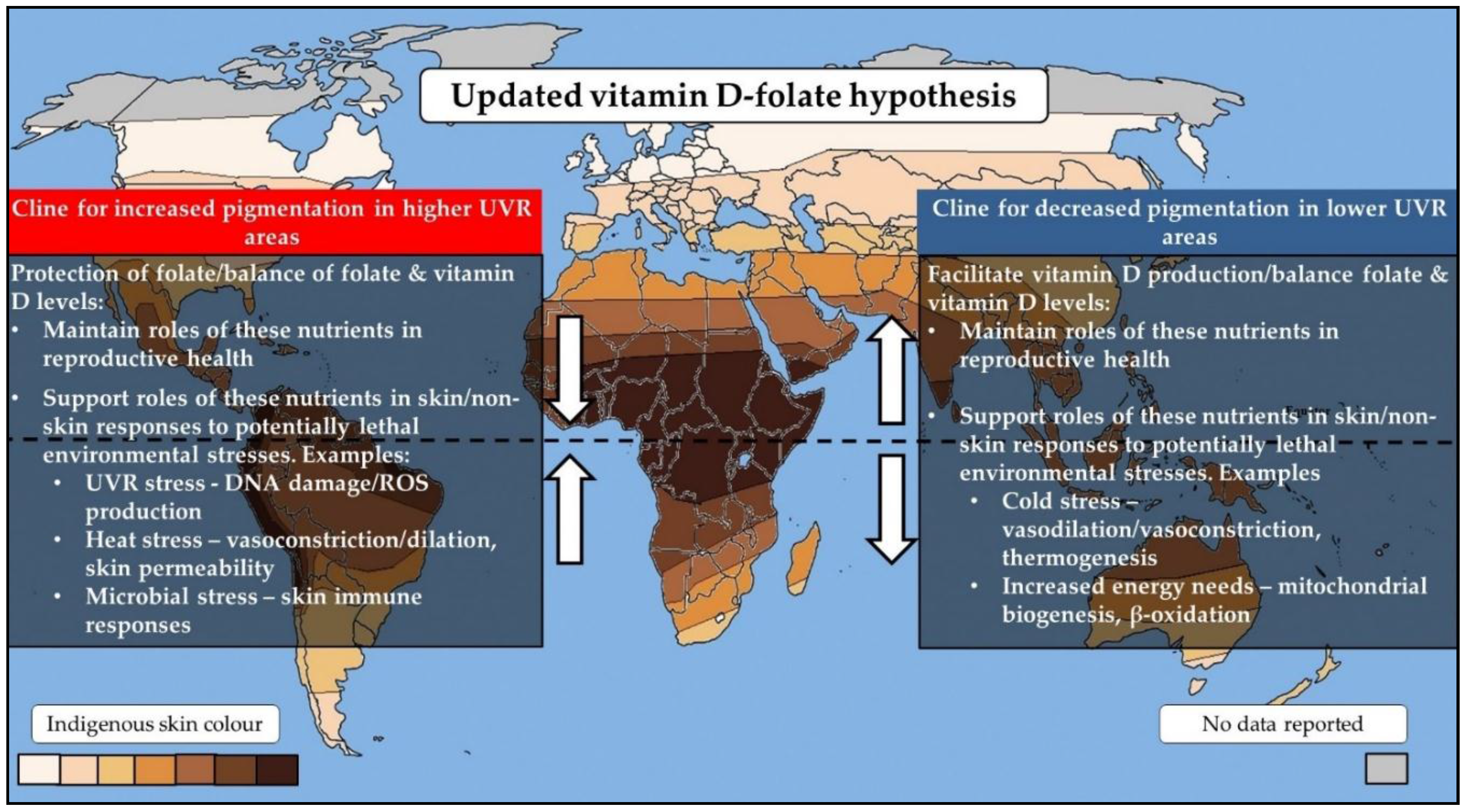
2. The Vitamin D–Folate Hypothesis
3. Alternative Theories for the Evolution of Skin Pigmentation
3.1. The Skin Mutagenesis Hypothesis
3.2. The Skin Barrier Hypothesis
3.3. The Metabolic Conservation Hypothesis
4. New Evidence Supporting the Vitamin D–Folate Hypothesis
5. Integrating Current Theories
6. Relevance to Public Health
Acknowledgments
Conflicts of Interest
References
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689s–1696s. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, S.; Vaisanen, S.; Pehkonen, P.; Seuter, S.; Benes, V.; Carlberg, C. Nuclear hormone 1alpha,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011, 39, 9181–9193. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrell, A.; Handunnetthi, L.; Handel, A.E.; Disanto, G.; Orton, S.M.; et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.E.; Sandve, G.K.; Disanto, G.; Berlanga-Taylor, A.J.; Gallone, G.; Hanwell, H.; Drablos, F.; Giovannoni, G.; Ebers, G.C.; Ramagopalan, S.V. Vitamin D receptor ChIP-seq in primary CD4+ cells: Relationship to serum 25-hydroxyvitamin D levels and autoimmune disease. BMC Med. 2013, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. VDR/RXR and TCF4/beta-catenin cistromes in colonic cells of colorectal tumor origin: Impact on c-FOS and c-MYC gene expression. Mol. Endocrinol. 2012, 26, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Prié, D.; Friedlander, G. Reciprocal Control of 1,25-Dihydroxyvitamin D and FGF23 Formation Involving the FGF23/Klotho System. Clin. J. Am. Soc. Nephrol. 2010, 5, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. Vitamin D as a promising anticancer agent. Indian J. Pharmacol. 2011, 43, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and Cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Thompson, J.R. The Regulation of Parathyroid Hormone Secretion and Synthesis. J. Am. Soc. Nephrol. JASN 2011, 22, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Metabolism and Function in the Skin. Mol. Cell. Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kollias, N.; Sayre, R.M.; Zeise, L.; Chedekel, M.R. Photoprotection by melanin. J. Photochem. Photobiol. B Biol. 1991, 9, 135–160. [Google Scholar] [CrossRef]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982, 1, 74–76. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. The evolution of human skin coloration. J. Hum. Evol. 2000, 39, 57–106. [Google Scholar] [CrossRef] [PubMed]
- Shane, B. Folate Chemistry and Metabolism. In Folate in Health and Disease, 2nd ed.; Bailey, L.B., Ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Off, M.K.; Steindal, A.E.; Porojnicu, A.C.; Juzeniene, A.; Vorobey, A.; Johnsson, A.; Moan, J. Ultraviolet photodegradation of folic acid. J. Photochem. Photobiol. B Biol. 2005, 80, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Thu Tam, T.T.; Iani, V.; Moan, J. 5-Methyltetrahydrofolate can be photodegraded by endogenous photosensitizers. Free Radic Biol. Med. 2009, 47, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Steindal, A.H.; Tam, T.T.T.; Lu, X.Y.; Juzeniene, A.; Moan, J. 5-Methyltetrahydrofolate is photosensitive in the presence of riboflavin. Photochem. Photobiol. Sci. 2008, 7, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.T.T.; Juzeniene, A.; Steindal, A.H.; Iani, V.; Moan, J. Photodegradation of 5-methyltetrahydrofolate in the presence of Uroporphyrin. J. Photochem. Photobiol. B Biol. 2009, 94, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; DeCosta, B.R.; Holick, M.F. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 1989, 68, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Williams, M.L. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: The barrier and metabolic conservation hypotheses revisited. Am. J. Phys. Anthropol. 2016, 161, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Was skin cancer a selective force for black pigmentation in early hominin evolution? Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G.; Chaplin, G. Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 2010, 107, 8962–8968. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and fertility: A systematic review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Picciano, M.F. Folate and human reproduction. Am. J. Clin. Nutr. 2006, 83, 993–1016. [Google Scholar] [CrossRef] [PubMed]
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000, 141, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Tsujimura, A.; Ueda, T.; Fujita, K.; Matsuoka, Y.; Takao, T.; Miyagawa, Y.; Koike, N.; Okuyama, A. Effect of 1,25-dihydroxyvitamin d on testicular morphology and gene expression in experimental cryptorchid mouse: Testis specific cDNA microarray analysis and potential implication in male infertility. J. Urol. 2009, 181, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, R.M. Folate and neural tube defects. Am. J. Clin. Nutr. 2007, 85, 285S–288S. [Google Scholar] [CrossRef] [PubMed]
- Boxmeer, J.C.; Smit, M.; Utomo, E.; Romijn, J.C.; Eijkemans, M.J.; Lindemans, J.; Laven, J.S.; Macklon, N.S.; Steegers, E.A.; Steegers-Theunissen, R.P. Low folate in seminal plasma is associated with increased sperm DNA damage. Fertil. Steril. 2009, 92, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ravid, A.; Rubinstein, E.; Gamady, A.; Rotem, C.; Liberman, U.A.; Koren, R. Vitamin D inhibits the activation of stress-activated protein kinases by physiological and environmental stresses in keratinocytes. J. Endocrinol. 2002, 173, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Youn, J.I. The photoprotective effect of 1,25-dihydroxyvitamin D3 on ultraviolet light B-induced damage in keratinocyte and its mechanism of action. J. Dermatol. Sci. 1998, 18, 11–18. [Google Scholar] [CrossRef]
- Dixon, K.M.; Deo, S.S.; Norman, A.W.; Bishop, J.E.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J. Steroid Biochem. Mol. Biol. 2007, 103, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Sequeira, V.B.; Dixon, K.M.; Gordon-Thomson, C.; Pobre, K.; Dilley, A.; Mizwicki, M.T.; Norman, A.W.; Feldman, D.; Halliday, G.M.; et al. Photoprotection by 1alpha,25-dihydroxyvitamin D and analogs: Further studies on mechanisms and implications for UV-damage. J. Steroid Biochem. Mol. Biol. 2010, 121, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Gupta, R.; Dixon, K.M.; Deo, S.S.; Choong, S.M.; Halliday, G.M.; Bishop, J.E.; Ishizuka, S.; Norman, A.W.; Posner, G.H.; et al. 1,25-Dihydroxyvitamin D and three low-calcemic analogs decrease UV-induced DNA damage via the rapid response pathway. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dixon, K.M.; Deo, S.S.; Holliday, C.J.; Slater, M.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J. Investig. Dermatol. 2007, 127, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Jacobson, M.K. Photobiological Implications of Folate Depletion and Repletion in Cultured Human Keratinocytes. J. Photochem. Photobiol. B Biol. 2010, 99, 49–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williams, J.D.; Jacobson, E.L.; Kim, H.; Kim, M.; Jacobson, M.K. Folate in skin cancer prevention. Subcell. Biochem. 2012, 56, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G.; Chaplin, G. Skin cancer was not a potent selective force in the evolution of protective pigmentation in early hominins. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140517. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J. Geography and skin colour. Nature 2005, 435, 283. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, K. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 2003, 15, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, R.; Schurer, N.Y.; Shoo, B.A.; Celli, A.; Hachem, J.P.; Crumrine, D.; Sirimanna, G.; Feingold, K.R.; Mauro, T.M.; Elias, P.M. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J. Investig. Dermatol. 2009, 129, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, W.Z.; Hegazy, R.A. Vitamin D and the skin: Focus on a complex relationship: A review. J. Adv. Res. 2015, 6, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Uchida, Y.; Moradian, S.; Crumrine, D.; Elias, P.M.; Bikle, D.D. Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J. Investig. Dermatol. 2009, 129, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Gallo, R.L. The vitamin D pathway: A new target for control of the skin’s immune response? Exp. Dermatol. 2008, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, C.; Elson-Schwab, I.; Mashiyama, S.T.; Kerry, N.; Ames, B.N. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J. Immunol. 2004, 173, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Horgan, G.; de Roos, B.; Rucklidge, G.; Reid, M.; Duncan, G.; Pirie, L.; Basten, G.P.; Powers, H.J. Blood folate status and expression of proteins involved in immune function, inflammation, and coagulation: Biochemical and proteomic changes in the plasma of humans in response to long-term synthetic folic acid supplementation. J. Proteome Res. 2010, 9, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wen, J.; Wang, X.; Xiao, C. Highdose folic acid improves endothelial function by increasing tetrahydrobiopterin and decreasing homocysteine levels. Mol. Med. Rep. 2014, 10, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Chalupsky, K.; Kračun, D.; Kanchev, I.; Bertram, K.; Görlach, A. Folic Acid Promotes Recycling of Tetrahydrobiopterin and Protects against Hypoxia-Induced Pulmonary Hypertension by Recoupling Endothelial Nitric Oxide Synthase. Antioxid. Redox Sign. 2015, 23, 1076–1091. [Google Scholar] [CrossRef] [PubMed]
- Chalupsky, K.; Cai, H. Endothelial dihydrofolate reductase: Critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2005, 102, 9056–9061. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Shirodaria, C.; Warrick, N.; Cai, S.; de Bono, J.; Lee, J.; Leeson, P.; Neubauer, S.; Ratnatunga, C.; Pillai, R.; et al. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: Effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 2006, 114, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.; Pawelek, J. L-tyrosine and L-DOPA as hormone-like regulators of melanocytes functions. Pigm. Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F. Central control of body temperature. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Mata-Greenwood, E.; Chen, D.-B. Racial Differences in Nitric Oxide-Dependent Vasorelaxation. Reprod. Sci. 2008, 15, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ajabshir, S.; Asif, A.; Nayer, A. The effects of vitamin D on the renin-angiotensin system. J. Nephropathol. 2014, 3, 41–43. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Szeto, F.L.; Zhang, W.; Ye, H.; Kong, J.; Zhang, Z.; Sun, X.J.; Li, Y.C. Involvement of the vitamin D receptor in energy metabolism: Regulation of uncoupling proteins. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E820–E828. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Post, P.W.; Daniels, F., Jr.; Binford, R.T., Jr. Cold injury and the evolution of “white” skin. Hum. Biol. 1975, 47, 65–80. [Google Scholar] [PubMed]
- Maley, M.J.; Eglin, C.M.; House, J.R.; Tipton, M.J. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur. J. Appl. Physiol. 2014, 114, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.E.; Macfarlane, F. Retrospective analysis of the ethnic origins of male British army soldiers with peripheral cold weather injury. J. R. Army Med. Corps 2009, 155, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Branda, R.F.; Eaton, J.W. Skin color and nutrient photolysis: An evolutionary hypothesis. Science 1978, 201, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G. The Evolution of Human Skin and Skin Color. Annu. Rev. Anthropol. 2004, 33, 585–623. [Google Scholar] [CrossRef]
- Zmuda, J.M.; Cauley, J.A.; Ferrell, R.E. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol. Rev. 2000, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; van Meurs Joyce, B.J.; d’Alesio, A.; Jhamai, M.; Zhao, H.; Rivadeneira, F.; Hofman, A.; van Leeuwen Johannes, P.T.; Jehan, F.; Pols Huibert, A.P.; et al. Promoter and 3′-Untranslated-Region Haplotypes in the Vitamin D Receptor Gene Predispose to Osteoporotic Fracture: The Rotterdam Study. Am. J. Hum. Genet. 2005, 77, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Audi, L.; Climer, S.; Zhang, W.; Templeton, A.R.; Fernández-Cancio, M.; Gershoni-Baruch, R.; Sánchez-Muro, J.M.; El Kholy, M.; Hochberg, Z. Latitudinal Clines of the Human Vitamin D Receptor and Skin Color Genes. G3 Genes Genomes Genet. 2016, 6, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Lucock, M.; Jones, P.; Martin, C.; Beckett, E.; Yates, Z.; Furst, J.; Veysey, M. Vitamin D: Beyond Metabolism. J. Evid. Based Complement. Altern. Med. 2015, 20, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Kanazawa, Y.; Motohashi, Y.; Yamada, S.; Maruyama, T.; Ikegami, H.; Awata, T.; Kawasaki, E.; Kobayashi, T.; Nakanishi, K.; et al. Evidence for association between vitamin D receptor BsmI polymorphism and type 1 diabetes in Japanese. J. Autoimmun. 2008, 30, 207–211. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.; Verlinden, L.; Giulietti, A.; Ramos-Lopez, E.; Branisteanu, D.D.; Ferreira, G.B.; Overbergh, L.; Verstuyf, A.; Bouillon, R.; Roep, B.O.; et al. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur. J. Immunol. 2007, 37, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Agliardi, C.; Guerini, F.R.; Saresella, M.; Caputo, D.; Leone, M.A.; Zanzottera, M.; Bolognesi, E.; Marventano, I.; Barizzone, N.; Fasano, M.E.; et al. Vitamin D receptor (VDR) gene SNPs influence VDR expression and modulate protection from multiple sclerosis in HLA-DRB1*15-positive individuals. Brain Behav. Immun. 2011, 25, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, Q.; Yang, L.; Li, X.; Liu, L.; Wang, L.; Li, Q.; Wang, G.; Li, C.Y.; Gao, T.W. The association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with generalized vitiligo. Br. J. Dermatol. 2012, 167, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; van Meurs, J.B.J.; Pols, H.A.P.; van Leeuwen, J.P.T.M. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Hypponen, E. Determinants of vitamin D status: Focus on genetic variations. Curr. Opin. Nephrol. Hypertens. 2011, 20, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Nissen, J.; Vogel, U.; Ravn-Haren, G.; Andersen, E.W.; Madsen, K.H.; Nexo, B.A.; Andersen, R.; Mejborn, H.; Bjerrum, P.J.; Rasmussen, L.B.; et al. Common variants in CYP2R1 and GC genes are both determinants of serum 25-hydroxyvitamin D concentrations after UVB irradiation and after consumption of vitamin D(3)-fortified bread and milk during winter in Denmark. Am. J. Clin. Nutr. 2015, 101, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D–Binding Protein and Vitamin D Status of Black Americans and White Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Shin, W.K.; Caudill, M.A.; Stover, P.J. A UV-responsive internal ribosome entry site enhances serine hydroxymethyltransferase 1 expression for DNA damage repair. J. Biol. Chem. 2009, 284, 31097–31108. [Google Scholar] [CrossRef] [PubMed]
- Yafei, W.; Lijun, P.; Jinfeng, W.; Xiaoying, Z. Is the prevalence of MTHFR C677T polymorphism associated with ultraviolet radiation in Eurasia? J. Hum. Genet. 2012, 57, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Beckett, E.; Yates, Z.; Veysey, M.; Lucock, M. Converging Evolutionary, Environmental and Clinical Ideas on Folate Metabolism. ERHM 2016, 1. [Google Scholar] [CrossRef]
- Jones, P.; Lucock, M.; Veysey, M.; Jablonski, N.; Chaplin, G.; Beckett, E. Frequency of folate-related polymorphisms varies by skin pigmentation. Am. J. Hum. Biol. 2018, 30, e23079. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, G. Geographic distribution of environmental factors influencing human skin coloration. Am. J. Phys. Anthropol. 2004, 125, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J. Physiology of folate and vitamin B12 in health and disease. Nutr. Rev. 2004, 62, S3–S12; discussion S13. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Marti-Soler, H.; Gonseth, S.; Gubelmann, C.; Stringhini, S.; Bovet, P.; Chen, P.C.; Wojtyniak, B.; Paccaud, F.; Tsai, D.H.; Zdrojewski, T.; et al. Seasonal variation of overall and cardiovascular mortality: A study in 19 countries from different geographic locations. PLoS ONE 2014, 9, e113500. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin d in the united states: Data from the third national health and nutrition examination survey. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Garland, F.C. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 2008, 51, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Roychoudhuri, R.; Peto, J.; Schwartz, G.; Baade, P.; Moller, H. Cancer survival is dependent on season of diagnosis and sunlight exposure. Int. J. Cancer 2006, 119, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Gabriel, A.; Bhatnagar, A.; Etienne, D.; Loukas, M. Seasonality pattern of breast, colorectal, and prostate cancer is dependent on latitude. Med. Sci. Monit. 2014, 20, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Lee, J.A.H. Seasonal Variation in Leukaemia Incidence. Br. Med. J. 1964, 1, 57. [Google Scholar] [CrossRef]
- Lee, J.A. Seasonal variation in the clinical onset of leukaemia in young people. Br. Med. J. 1962, 1, 1737–1738. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, P.; Lucock, M.; Veysey, M.; Beckett, E. The Vitamin D–Folate Hypothesis as an Evolutionary Model for Skin Pigmentation: An Update and Integration of Current Ideas. Nutrients 2018, 10, 554. https://doi.org/10.3390/nu10050554
Jones P, Lucock M, Veysey M, Beckett E. The Vitamin D–Folate Hypothesis as an Evolutionary Model for Skin Pigmentation: An Update and Integration of Current Ideas. Nutrients. 2018; 10(5):554. https://doi.org/10.3390/nu10050554
Chicago/Turabian StyleJones, Patrice, Mark Lucock, Martin Veysey, and Emma Beckett. 2018. "The Vitamin D–Folate Hypothesis as an Evolutionary Model for Skin Pigmentation: An Update and Integration of Current Ideas" Nutrients 10, no. 5: 554. https://doi.org/10.3390/nu10050554
APA StyleJones, P., Lucock, M., Veysey, M., & Beckett, E. (2018). The Vitamin D–Folate Hypothesis as an Evolutionary Model for Skin Pigmentation: An Update and Integration of Current Ideas. Nutrients, 10(5), 554. https://doi.org/10.3390/nu10050554




