Maternal DHA Status during Pregnancy Has a Positive Impact on Infant Problem Solving: A Norwegian Prospective Observation Study
Abstract
1. Introduction
2. Materials and Methods
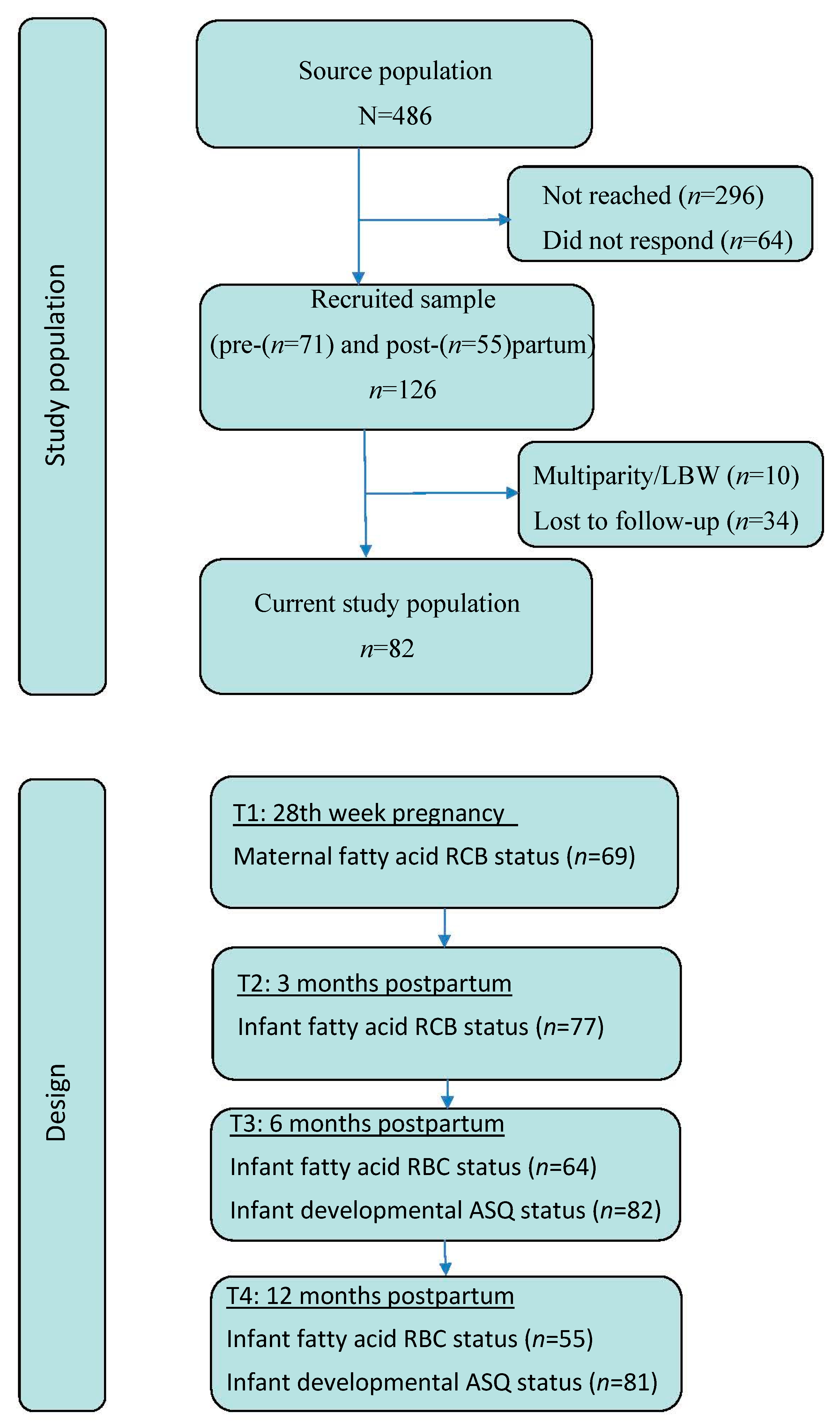
2.1. Design and Study Population
2.2. Blood Sampling and Fatty Acid Analyses
2.3. Infant Developmental Status
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Fatty Acid Composition in RBC
3.2. Associations between Maternal and Infant Fatty Acids
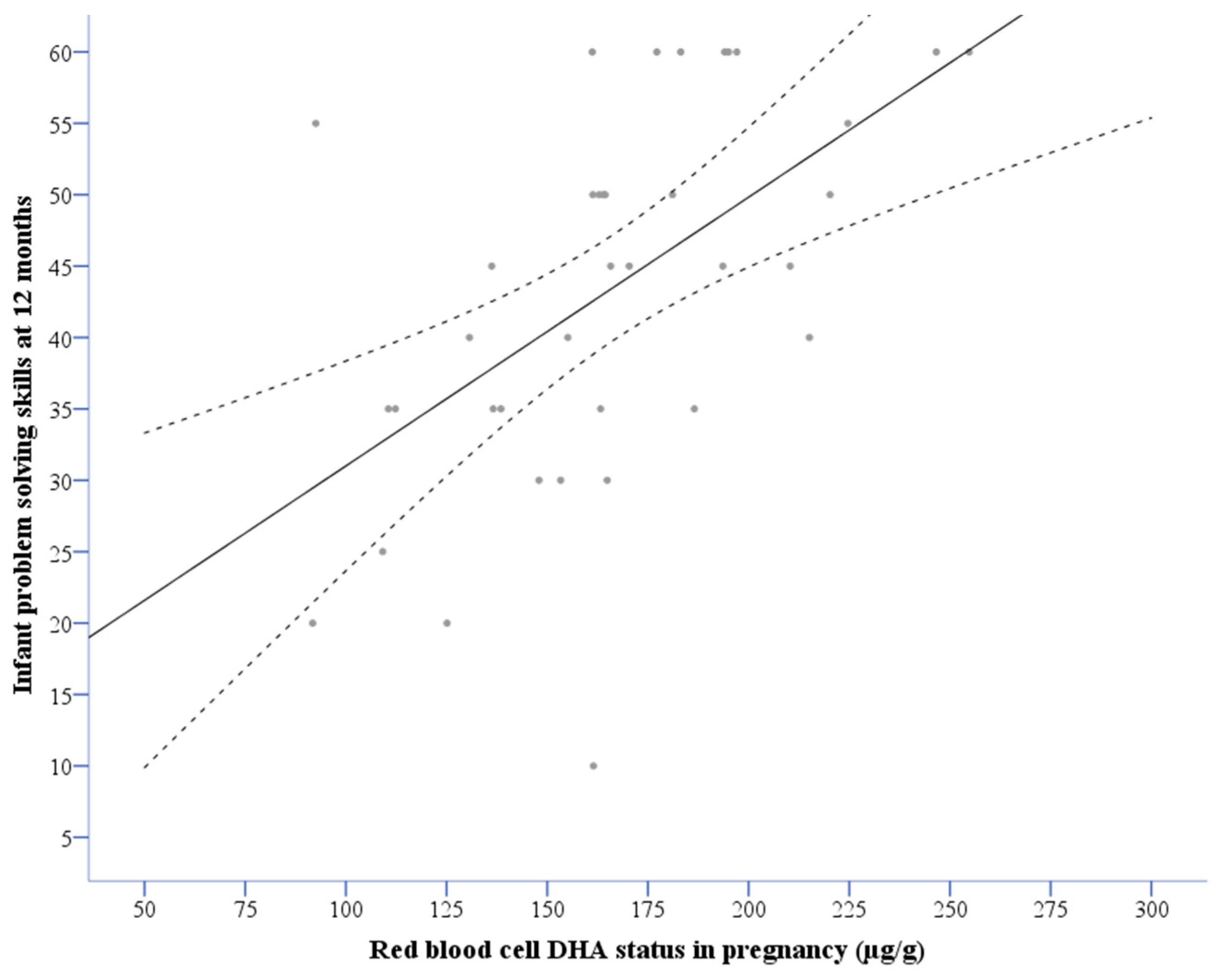
3.3. Associations between DHA Status and Problem Solving Scores
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wainwright, P.E. Dietary essential fatty acids and brain function: A developmental perspective on mechanisms. Proc. Nutr. Soc. 2002, 61, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B. Lipid supply and metabolism in infancy. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Hansen, H.S.; Jørgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Willatts, P.; Forsyth, J.S. The role of long-chain polyunsaturated fatty acids in infant cognitive development. Prostaglandins Leuk. Essent. Fat. Acids 2000, 63, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Julvez, J.; Mendez, M.; Fernandez-Barres, S.; Romaguera, D.; Vioque, J.; Llop, S.; Ibarluzea, J.; Guxens, M.; Avella-Garcia, C.; Tardon, A.; et al. Maternal consumption of seafood in pregnancy and child neuropsychological development: A longitudinal study based on a population with high consumption levels. Am. J. Epidemiol. 2016, 183, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.; Mitchell, A.; Steffenson, N. Mothers, infants, and DHA: Implications for nursing practice. Am. J. Matern./Child Nurs. 2000, 25, 71–75. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Van Elst, K.; Bruining, H.; Birtoli, B.; Terreaux, C.; Buitelaar, J.K.; Kas, M.J. Food for thought: Dietary changes in essential fatty acid ratios and the increase in autism spectrum disorders. Neurosci. Biobehav. Rev. 2014, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hornstra, G.; Al, M.D.M.; Vonhouwelingen, A.C.; Foremanvandrongelen, M. Essential fatty-acids in pregnancy and early human-development. Eur. J. Obstet. Gyn. Reprod. Biol. 1995, 61, 57–62. [Google Scholar] [CrossRef]
- Markhus, M.W.; Rasinger, J.D.; Malde, M.K.; Froyland, L.; Skotheim, S.; Braarud, H.C.; Stormark, K.M.; Graff, I.E. Docosahexaenoic acid status in pregnancy determines the maternal docosahexaenoic acid status 3-, 6-and 12 months postpartum. Results from a longitudinal observational study. PLoS ONE 2015, 10, e0136409. [Google Scholar] [CrossRef] [PubMed]
- FDA. Fish: What Pregnant Women and Parents Should Know, June 2014 ed.; U.S. Food and Drug Administration, 2017. Available online: https://www.epa.gov/fish-tech/2017-epa-fda-advice-about-eating-fish-and-shellfish (accessed on 22 March 2018).
- Norwegian Directorate of Health. Recommendation of Food, Nutrition and Physical Activity, 04.03.2014 ed.; Norwegian Directorate of Health, 2014. Available online: www.helsedirektoratet.no (accessed on 13 February 2018).
- Daniels, J.L.; Longnecker, M.P.; Rowland, A.S.; Golding, J.; ALSPAC Study Team. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology 2004, 15, 394–402. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel. Scientific opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. EFSA J. 2014, 12, 88. [Google Scholar]
- Markhus, M.W.; Graff, I.E.; Dahl, L.; Seldal, C.F.; Skotheim, S.; Braarud, H.C.; Stormark, K.M.; Malde, M.K. Establishment of a seafood index to assess the seafood consumption in pregnant women. Food Nutr. Res. 2013, 57, 19272. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Noakes, P.S.; Kremmyda, L.S.; Vlachava, M.; Diaper, N.D.; Rosenlund, G.; Urwin, H.; Yaqoob, P.; Rossary, A.; Farges, M.C.; et al. The salmon in pregnancy study: Study design, subject characteristics, maternal fish and marine n-3 fatty acid intake, and marine n-3 fatty acid status in maternal and umbilical cord blood. Am. J. Clin. Nutr. 2011, 94, 1986S–1992S. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Oken, E.; Østerdal, M.L.; Gillman, M.W.; Knudsen, V.K.; Halldorsson, T.I.; Strøm, M.; Bellinger, D.C.; Hadders-Algra, M.; Michaelsen, K.F.; Olsen, S.F. Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: A study from the danish national birth cohort. Am. J. Clin. Nutr. 2008, 88, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Radesky, J.S.; Wright, R.O.; Bellinger, D.C.; Amarasiriwardena, C.J.; Kleinman, K.P.; Hu, H.; Gillman, M.W. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 2008, 167, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Smithers, L.G.; Makrides, M. The effect of maternal omega-3 (n-3) lcpufa supplementation during pregnancy on early childhood cognitive and visual development: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Trojan, S.; Bellu, R.; Riva, E.; Giovannini, M. Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice: The role of long-chain polyunsaturated fatty acids. Pediatr. Res. 1995, 38, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Zuccotti, G.V.; Radaelli, G.; Besana, R.; Podestà, A.; Sterpa, A.; Rottoli, A.; Riva, E.; Giovannini, M. Docosahexaenoic acid supplementation and time at achievement of gross motor milestones in healthy infants: A randomized, prospective, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2009, 89, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Willatts, P.; Forsyth, J.S.; DiModugno, M.K.; Varma, S.; Colvin, M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet 1998, 352, 688–691. [Google Scholar] [CrossRef]
- Henriksen, C.; Haugholt, K.; Lindgren, M.; Aurvåg, A.K.; Rønnestad, A.; Grønn, M.; Solberg, R.; Moen, A.; Nakstad, B.; Berge, R.K.; et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008, 121, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [PubMed]
- Makrides, M.; Gould, J.F.; Gawlik, N.R.; Yelland, L.N.; Smithers, L.G.; Anderson, P.J.; Gibson, R.A. Four-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA 2014, 311, 1802–1804. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shulkin, M.; Pimpin, L.; Bellinger, D.; Kranz, S.; Fawzi, W.; Duggan, C.; Mozaffarian, D. N–3 fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: A systematic review and meta-analysis. J. Nutr. 2018, 148, 409–418. [Google Scholar] [PubMed]
- Bouthoorn, S.H.; van Lenthe, F.J.; Hokken-Koelega, A.C.S.; Moll, H.A.; Tiemeier, H.; Hofman, A.; Mackenbach, J.P.; Jaddoe, V.W.V.; Raat, H. Head circumference of infants born to mothers with different educational levels; the generation R study. PLoS ONE 2012, 7, e39798. [Google Scholar] [CrossRef] [PubMed]
- Handal, A.J.; Lozoff, B.; Breilh, J.; Harlow, S.D. Sociodemographic and nutritional correlates of neurobehavioral development: A study of young children in a rural region of Ecuador. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2007, 21, 292–300. [Google Scholar] [CrossRef]
- Keim, S.A.; Daniels, J.L.; Siega-Riz, A.M.; Herring, A.H.; Dole, N.; Scheidt, P.C. Breastfeeding and long-chain polyunsaturated fatty acid intake in the first 4 post-natal months and infant cognitive development: An observational study. Matern. Child Nutr. 2012, 8, 471–482. [Google Scholar] [PubMed]
- Sameroff, A. Environmental context of child development. J. Pediatr. 1986, 109, 192–200. [Google Scholar] [CrossRef]
- Markhus, M.W.; Skotheim, S.; Graff, I.E.; Frøyland, L.; Braarud, H.C.; Stormark, K.M.; Malde, M.K. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS ONE 2013, 8, e67617. [Google Scholar] [CrossRef] [PubMed]
- Kuratko, C.N.; Salem, N., Jr. Biomarkers of DHA status. Prostaglandins Leuk. Essent. Fat. Acids 2009, 81, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.; Von Schacky, C. The omega-3 index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.; Nguyen, T.T.; Froyland, L.; Wang, J.; Kang, J.X. Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J. Chromatogr. A 2008, 1212, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.; Bricker, D.; Potter, L. Revision of a parent-completed developmental screening tool: Ages and stages questionnaires. J. Pediatr. Psychol. 1997, 22, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Janson, H.; Squires, J. Parent-completed developmental screening in a norwegian population sample: A comparison with US normative data. Acta Paediatr. 2004, 93, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.K.; Potter, L.; Bricker, D.D.; Lamorey, S. Parent-completed developmental questionnaires: Effectiveness with low and middle income parents. Early Child. Res. Q. 1998, 13, 345–354. [Google Scholar] [CrossRef]
- Janson, H.; Smith, L. Norsk Manualsupplement til Ages and Stages Questionnaires; R.BUP Regionsenter for barne og ungdomspsykiatri, Helseregion Øst/Sør: Oslo, Norway, 2003. [Google Scholar]
- Richter, J.; Janson, H. A validation study of the norwegian version of the ages and stages questionnaires. Acta Paediatr. 2007, 96, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Bøe, T.; Sivertsen, B.; Heiervang, E.; Goodman, R.; Lundervold, A.J.; Hysing, M. Socioeconomic status and child mental health: The role of parental emotional well-being and parenting practices. J. Abnormal Child Psychol. 2014, 42, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Braveman, P.A.; Cubbin, C.; Egerter, S.; Chideya, S.; Marchi, K.S.; Metzler, M.; Posner, S. Socioeconomic status in health research: One size does not fit all. JAMA 2005, 294, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.L.; Voigt, R.G.; Prager, T.C.; Zou, Y.L.; Fraley, J.K.; Rozelle, J.C.; Turcich, M.R.; Llorente, A.M.; Anderson, R.E.; Heird, W.C. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am. J. Clin. Nutr. 2005, 82, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.E.; Garfield, S.; Hoffman, D.R.; Uauy, R.; Birch, D.G. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000, 42, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Prevor, M.B.; Callender, G.; Druin, D.P. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monogr. Soc. Res. Child Dev. 1997, 62, i-206. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.; Nelson, C. Neurobiology of fetal and infant development. In Handbook of Infant Mental Health, 3rd ed.; Zeanah, C.H., Ed.; The Guilford Press: New York, NY, USA, 2009; pp. 40–59. [Google Scholar]
- Brantsaeter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of probiotic food and risk of preeclampsia in primiparous women. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Klamer, A.; Lando, A.; Pinborg, A.; Greisen, G. Ages and stages questionnaire used to measure cognitive deficit in children born extremely preterm. Acta Paediatr. 2005, 94, 1327–1329. [Google Scholar] [CrossRef] [PubMed]
- Slater, A. Individual differences in infancy and later IQ. J. Child Psychol. Psychiatry 1995, 36, 69–112. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Value (Min–Max) | n |
|---|---|---|
| Sex (% boys) | 52.4 | 80 |
| Gestational age (weeks) | 39.7 (37–42) | 72 |
| Infant birth weight (g) | 3611 (2520–4980) | 80 |
| Infant head circumference (cm) | 35 (32–39) | 47 |
| Breastfed at 3 months (%) | 67.2 | 61 |
| Duration of breastfeeding (months) | 3.6 (0–6) | 61 |
| Maternal age (years) | 31 (19–42) | 75 |
| Mother’s highest educational level (%) | ||
| Nine-year primary school | 4.0 | 3 |
| High school | 30.7 | 23 |
| 1–3 years College/University | 34.7 | 26 |
| 4 or more years College/University | 30.7 | 23 |
| Pregnancy | Infants 3 Months | Infants 6 Months | Infants 12 Months | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 32) | (n = 60) | (n = 53) | (n = 47) | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| µg/g | % | µg/g | % | µg/g | % | µg/g | % | |
| Total SAFA | 1103 (107) | 39 (2) | 1160 (140) | 40 (2) | 1093 (134) | 38 (2) | 931 (80) | 37 (3) |
| Total MUFA | 526 (105) | 18 (2) | 565 (156) | 19 (3) | 504 (82) | 18 (2) | 492 (90) | 19 (2) |
| Total PUFA | 1107 (136) | 39 (3) | 1082 (115) | 38 (2) | 1134 (125) | 40 (3) | 1013 (126) | 40 (3) |
| Total n-6 PUFA | 816 (123) | 29 (2) | 825 (102) | 29 (2) | 801 (118) | 28 (3) | 739 (114) | 29 (3) |
| Total n-3 PUFA | 291 (61) | 10 (2) | 258 (61) | 9 (2) | 332 (53) | 12 (2) | 273 (45) | 11 (2) |
| 18:2 n-6 (LA) | 374 (93) | 13 (2) | 332 (80) | 11 (2) | 335 (54) | 12 (2) | 319 (76) | 13 (2) |
| 18:3 n-3 (ALA) | 284 (70) | 0.3 (0.1) | 6 (3) | 0.1 (0.1) | 6 (2) | 0.2 (0.1) | 7 (5) | 0.3 (0.2) |
| 20:4 n-6 (AA) | 319 (49) | 11 (2) | 372 (51) | 13 (2) | 348 (54) | 12 (1) | 290 (48) | 12 (2) |
| 20:5 n-3 (EPA) | 25 (17) | 0.9 (0.6) | 16 (15) | 0.6 (0.5) | 18 (16) | 0.7 (0.6) | 13 (12) | 0.6 (0.5) |
| 22:5 n-3 (DPA) | 53 (13) | 1.9 (0.5) | 43 (12) | 1.5 (0.5) | 52 (10) | 1.5 (0.4) | 38 (9) | 1.5 (0.4) |
| 22:6 n-3 (DHA) | 168 (37) | 6 (1) | 182 (36) | 6 (1) | 218 (40) | 8 (1) | 160 (30) | 6 (1) |
| n-3 index % * | 6.8 (1.9) | 6.9 (1.6) | 8.3 (1.7) | 7.0 (1.7) | ||||
| ASQ Problem Solving 95% Confidence Interval (CI) | ||||
|---|---|---|---|---|
| Coeff. | Lower Bound | Upper Bound | p | |
| DHA (µg/g RBC) in pregnancy | 5.68 a | 1.32 | 10.08 | 0.012 |
| Nine-year | −9.94 | −19.95 | 0.07 | 0.051 |
| High school | −4.46 | −14.48 | 5.36 | 0.36 |
| 1–3 year of college/university | 0 b | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braarud, H.C.; Markhus, M.W.; Skotheim, S.; Stormark, K.M.; Frøyland, L.; Graff, I.E.; Kjellevold, M. Maternal DHA Status during Pregnancy Has a Positive Impact on Infant Problem Solving: A Norwegian Prospective Observation Study. Nutrients 2018, 10, 529. https://doi.org/10.3390/nu10050529
Braarud HC, Markhus MW, Skotheim S, Stormark KM, Frøyland L, Graff IE, Kjellevold M. Maternal DHA Status during Pregnancy Has a Positive Impact on Infant Problem Solving: A Norwegian Prospective Observation Study. Nutrients. 2018; 10(5):529. https://doi.org/10.3390/nu10050529
Chicago/Turabian StyleBraarud, Hanne Cecilie, Maria Wik Markhus, Siv Skotheim, Kjell Morten Stormark, Livar Frøyland, Ingvild Eide Graff, and Marian Kjellevold. 2018. "Maternal DHA Status during Pregnancy Has a Positive Impact on Infant Problem Solving: A Norwegian Prospective Observation Study" Nutrients 10, no. 5: 529. https://doi.org/10.3390/nu10050529
APA StyleBraarud, H. C., Markhus, M. W., Skotheim, S., Stormark, K. M., Frøyland, L., Graff, I. E., & Kjellevold, M. (2018). Maternal DHA Status during Pregnancy Has a Positive Impact on Infant Problem Solving: A Norwegian Prospective Observation Study. Nutrients, 10(5), 529. https://doi.org/10.3390/nu10050529





