Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions
Abstract
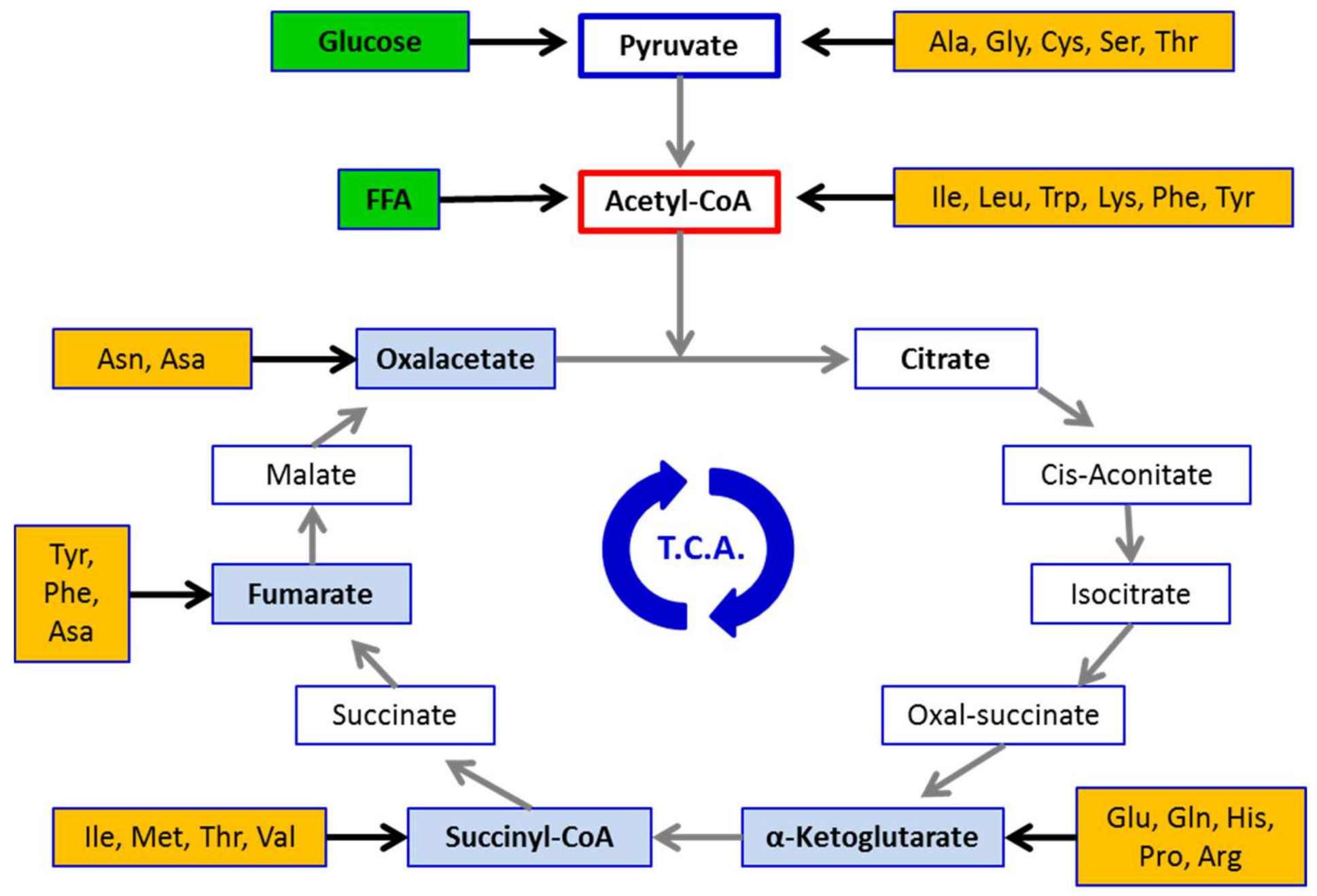
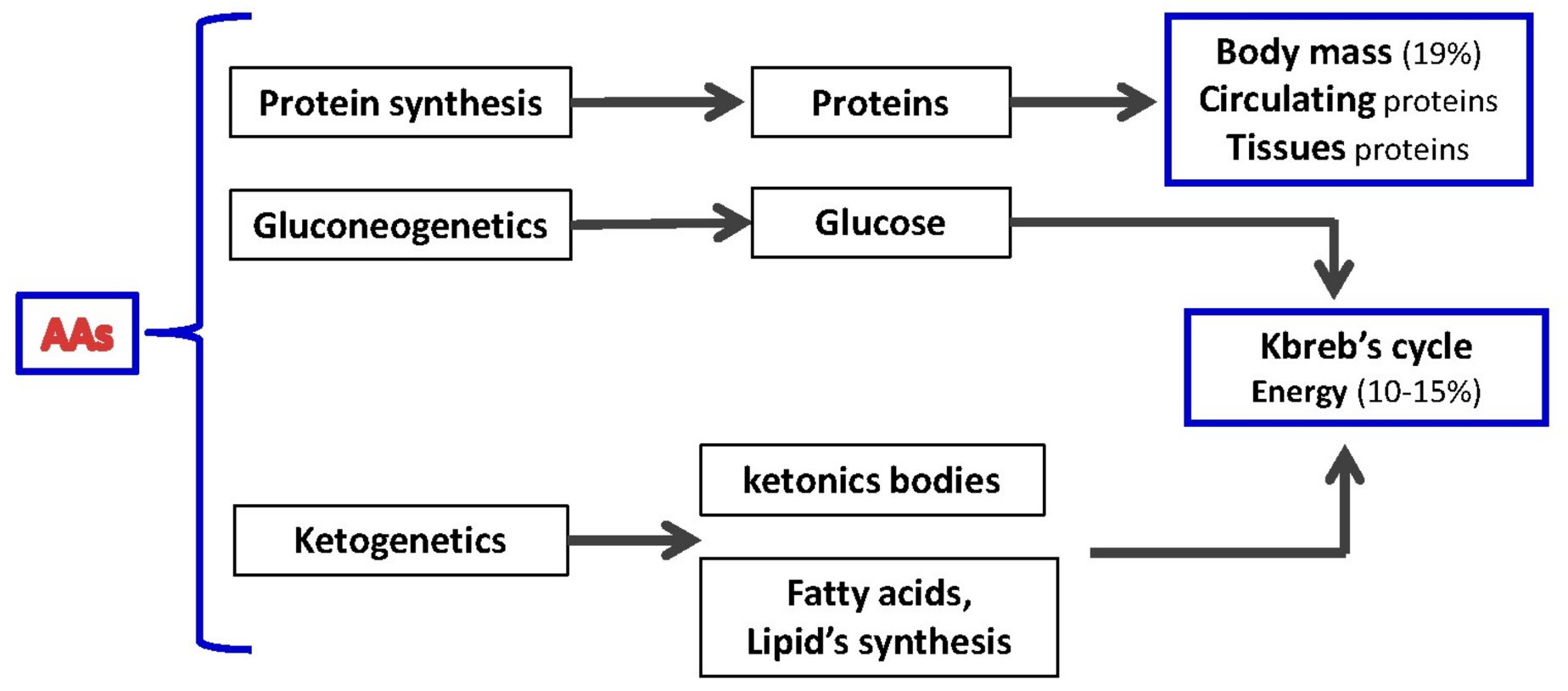
:1. Metabolism of Proteins and Amino Acids: Essential Cellular Blocks
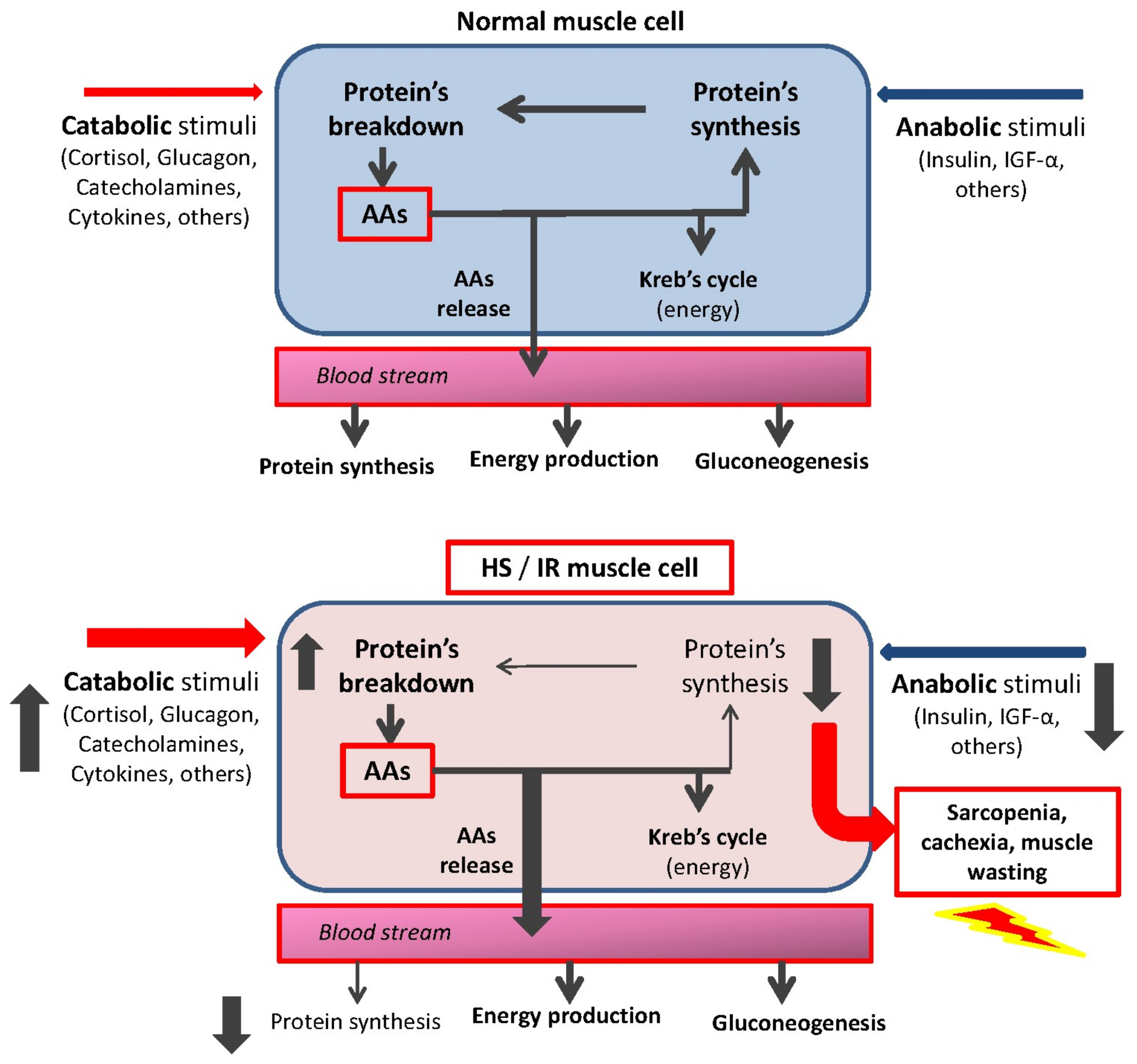
2. The Pathogenesis of Protein Disarrangement: The Hypermetabolic Syndrome
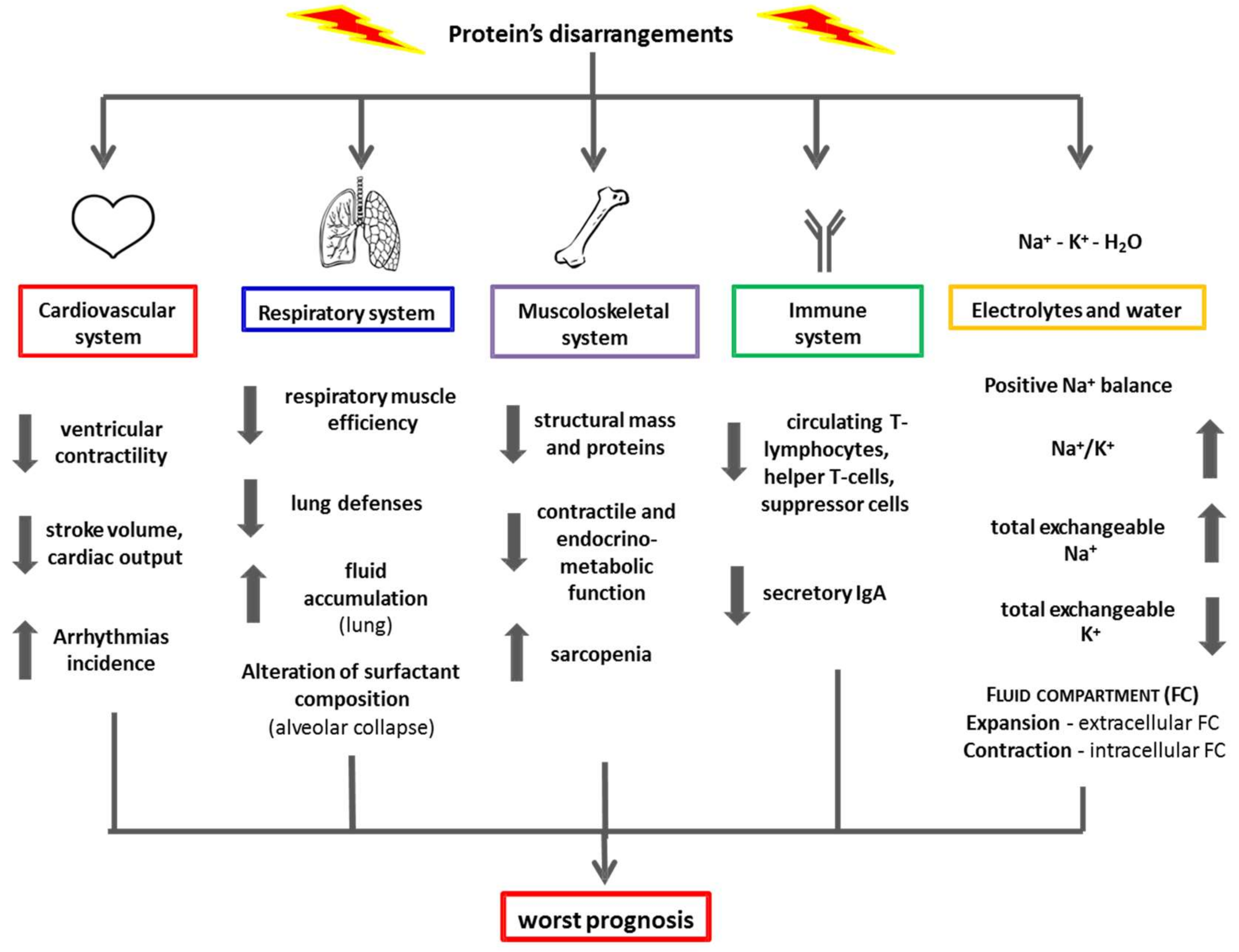
3. Clinical Impact of Protein Disarrangements
4. Clinical Steps to Evaluate Protein Disarrangement
4.1. Circulating Visceral Proteins
4.2. Nitrogen Balance
4.3. 3-Methylistidine
4.4. Blood Lymphocyte Count
5. Possible Therapeutic Interventions
6. Conclusions
- Proteins are building blocks for living organisms consisting of amino acids (AAs).
- AAs are totipotent biochemical molecules essential for cellular activity.
- Pathological conditions increase protein/AA demand.
- Hypercatabolic syndromes, due to increased inflammation and catabolic hormones, cause significant changes in body metabolism and lead to an imbalance between nutritional input and synthetic/energy needs.
- Both circulating (i.e., albumin) and muscle proteins are a reservoir of AAs (above all, essential AAs) within the body.
- Altered protein metabolism in patients (especially those with chronic diseases and/or older), can increase the risk of developing life-threatening complications (e.g., infection, cardiac and/or renal dysfunction).
- The pathophysiology of conditions characterized by protein disarrangements (i.e., muscle wasting and/or hypoalbuminemia) need to be fully clarified to develop adequate therapeutic (nutritional) strategies.
- Clinicians need to consider protein metabolism as a critical aspect for the management of patients with chronic diseases.
Author Contributions
Conflicts of Interest
References
- Lehninger, A.L. Principles of Biochemistry; Worth Publishers Inc.: New York, NY, USA, 1982. [Google Scholar]
- Bischoffa, R.; Hartmut, S. Amino acids: Chemistry, functionality and selected non-post-translational modifications. J. Proteom. 2012, 75, 2275–2296. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.H.; Block, R.J. Some relationships between the amino acid contents of proteins and their nutritive values for the rat. J. Biol. Chem. 1946, 163, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Jefferson, L.S. Signalling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J. Nutr. 2001, 131, 856S–860S. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, A.M.J. Amino acids-their role as an energy source. Proc. Nutr. Soc. 1983, 42, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, F.S. Wasting and the substrate to energy controller pathway: A role for insulin resistance and amino acids. Am. J. Cardiol. 2004, 93, 6A–12A. [Google Scholar] [CrossRef] [PubMed]
- Taegtmayer, H. Energy metabolism of the heart: From basic concepts to clinical applications. Curr. Prob. Cardiol. 1994, 19, 59–113. [Google Scholar] [CrossRef]
- Aquilani, R.; Opasich, C.; Verri, M.; Boschi, F.; Febo, O.; Pasini, E.; Pastoris, O. Is nutritional intake adequate in chronic heart failure patients? J. Am. Coll. Cardiol. 2003, 42, 1218–1223. [Google Scholar] [CrossRef]
- Anker, S.D.; Chaua, T.P.; Ponikowski, P.; Harrington, D.; Swan, J.W.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 1997, 96, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S.; D’Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatbolic syndrome: Molecular basis and effects of nutritional supplementation with amino acids. Am. J. Cardiol. 2008, 101, 11E–15E. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Opasic, C.; Dossena, M.; Iadarola, P.; Gualco, A.; Arcidiaco, P.; Viglio, S.; Boschi, F.; Verri, M.; Pasini, E. Increased skeletal muscle amino acid release with light exercise in deconditioned patients with heart failure. J. Am. Coll. Cardiol. 2005, 45, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Di Giorgio, A.; Nesci, A.; Sisto, A.; Santoliquido, A.; Landi, F. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. Biomed. Res. Int. 2017, 2672435. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, K.C.H.; Ferrucci, L.; Hellerstein, M.K.; et al. Society for Sarcopenia, Cachexia, and Wasting Disease. Nutritional recommendations for the management of Sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Short, K.R.; Campbell, W.W.; Volpi, E.; Wolfe, R.R. Role of dietary protein in the Sarcopenia of aging. Am. J. Clin. Nutr. 2008, 87, 1562S–1566S. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of Dietary Proteins and Amino Acids in the Pathogenesis of Insulin Resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y. The mini nutritional assessment review of the literature: What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485. [Google Scholar] [PubMed]
- Liu, M.; Chan, C.P.; Yan, B.P.; Zhang, Q.; Lam, Y.Y.; Li, R.J.; Sanderson, J.E.; Coats, A.J.; Sun, J.P.; Yip, G.W.; Yu, C.M. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2012, 14, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef]
- Wolfe, E.W. The underappreciated role of muscle in health and diseases. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; von Haehling, S.; Doehner, W.; Anker, S.D. The obesity paradox in chronic disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2012, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Opasich, C.; Viglio, S.; Iadarola, P.; Pasini, E. Nutrition in acute decompensation of patients with acute heart failure syndrome. In Acute Heart Failure; Springer: London, UK, 2008; pp. 876–882. [Google Scholar]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S. The enemy within. How to identify chronic diseases induced-protein metabolism impairment and its possible pharmacological treatment. Pharmacol. Res. 2013, 76, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Magnani, R. Sampling guide. In Food and Nutrition Technical Assistance (FANTA) Project; Academy for Educational Development: Washington, DC, USA, 1997. [Google Scholar]
- Cogil, B. Anthropometric indicators measurement guide. In Food and Nutrition Technical Assistance (FANTA) Project; Academy for Educational Development: Washington, DC, USA, 2003. [Google Scholar]
- Frisancho, R. Anthropometric Standards for the Assessment of Growth and Nutritional Status; The University of Michigan Press: Ann Arbor, MI, USA, 1990. [Google Scholar]
- Watson, R.R. Nutritional stresses: Levels of complement proteins and their functions. In Nutrition, Disease Resistance and Immune Function; Watson, R.R., Ed.; Marcel Dekker: New York, NY, USA, 1984; pp. 175–188. [Google Scholar]
- Ferrari, F.; Fumagalli, M.; Viglio, S.; Aquilani, R.; Pasini, E.; Iadarola, P. A rapid method for simultaneous determination of creatine, 1-and 3 methyhistidine in human urine. Electrophoresis 2009, 30, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Opasic, C.; Gualco, A.; Bairdi, P.; Pasini, E.; Testa, A.; Viglio, S.; Iadarola, P.; Verri, M.; D’Agostino, L.; Boschi, F. A practical method to diagnose muscle degradation in normo-nourished patient with chronic heart failure. Int. J. Med. 2009, 2, 226–230. [Google Scholar]
- Acanfora, D.; Gheorghiade, M.; Trojano, L.; Furgi, G.; Pasini, E.; Picone, C.; Papa, A.; Iannuzzi, G.L.; Bonow, R.O.; Rengo, F. Relative lymphocyte count: A prognostic indicator of mortality in elderly patients with congestive heart failure. Am. Heart J. 2001, 142, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F.S. Pathogenic Gut Flora in Patients with Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Aquilani, R.; Verri, M.; Boschi, F.; Pasini, E.; Perna, S.; Faliva, A.; Condino, A.M. Plasma kinetics of essential amino acids following their ingestion as free formula or as dietary protein components. Aging Clin. Exp. Res. 2017, 29, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Cozzi, V.; Carruba, M. Amino Acids and mitochondrial biogenesis. Am. J. Cardiol. 2008, 101, 22E–25E. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Cadenas, J.G.; Yoshizawa, F.; Volpi, E.; Rasmussen, B.B. Nutrient signalling in the regulation of human muscle protein synthesis. J. Physiol. 2007, 582, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Viglio, S.; Idarola, P.; Opasich, C.; Testa, A.; Dioguardi, F.S.; Pasini, E. Oral amino acid supplementation improve exercise capacities in elderly patients with heart failure. Am. J. Cardiol. 2008, 101, 104E–110E. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, R.; Testa, A.; Aquilani, R.; Dioguardi, F.S.; Pasini, E. Impairment in walking capacity and myocardial function in the elderly: It is a role for non-pharmacologic therapy with nutritional amino acid supplementation? Am. J. Cardiol. 2008, 101, 78E–81E. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Opasich, C.; Gualco, C.; Veiir, M.; Testa, A.; Pasini, E.; Viglio, C.; Iadarola, P.; Pastoris, O.; Dossena, M.; et al. Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur. J. Heart Fail. 2008, 10, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Gazzaruso, C.; Bonacasa, R.; Rondanelli, M.; Zamboni, M.; Basso, C.; Locatelli, E.; Schifino, N.; Giustina, A.; Fioravanti, M. Nutritional supplements with oral amino acid misture increases whole body lean mass and insulin sensitivity in elderly subjects with Sarcopenia. Am. J. Cardiol. 2008, 101, 69E–77E. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Pasini, E.; D’Antona, G.; Nisoli, E.; Flati, V.; Assanelli, D.; Dioguardi, S.F.; Bianchi, R. Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am. J. Cardiol. 2008, 101, 26E–34E. [Google Scholar] [CrossRef] [PubMed]
- Broqvist, M.; Dahlstrom, U.; Larsson, J.; Larsson, J.; Nylander, E.; Permert, J. Nutritional assessment and muscle energy metabolism in severe chronic congestive heart failure: Effects of long-term dietary supplementation. Eur. Heart J. 1994, 15, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr. Metab. 2009, 6, 12. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F.S. Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions. Nutrients 2018, 10, 391. https://doi.org/10.3390/nu10040391
Pasini E, Corsetti G, Aquilani R, Romano C, Picca A, Calvani R, Dioguardi FS. Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions. Nutrients. 2018; 10(4):391. https://doi.org/10.3390/nu10040391
Chicago/Turabian StylePasini, Evasio, Giovanni Corsetti, Roberto Aquilani, Claudia Romano, Anna Picca, Riccardo Calvani, and Francesco Saverio Dioguardi. 2018. "Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions" Nutrients 10, no. 4: 391. https://doi.org/10.3390/nu10040391
APA StylePasini, E., Corsetti, G., Aquilani, R., Romano, C., Picca, A., Calvani, R., & Dioguardi, F. S. (2018). Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions. Nutrients, 10(4), 391. https://doi.org/10.3390/nu10040391









