The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
1.1. Description of the Intervention
1.2. How the Intervention Might Work
1.3. Why It Is Important to Do This Review
1.4. Objectives
2. Methods
2.1. Types of Studies
2.2. Types of Participants
2.3. Types of Interventions
2.4. Types of Outcome Measures
Search Methods for Identification of Studies
2.5. Electronic Searches
2.6. Searching Other Resources
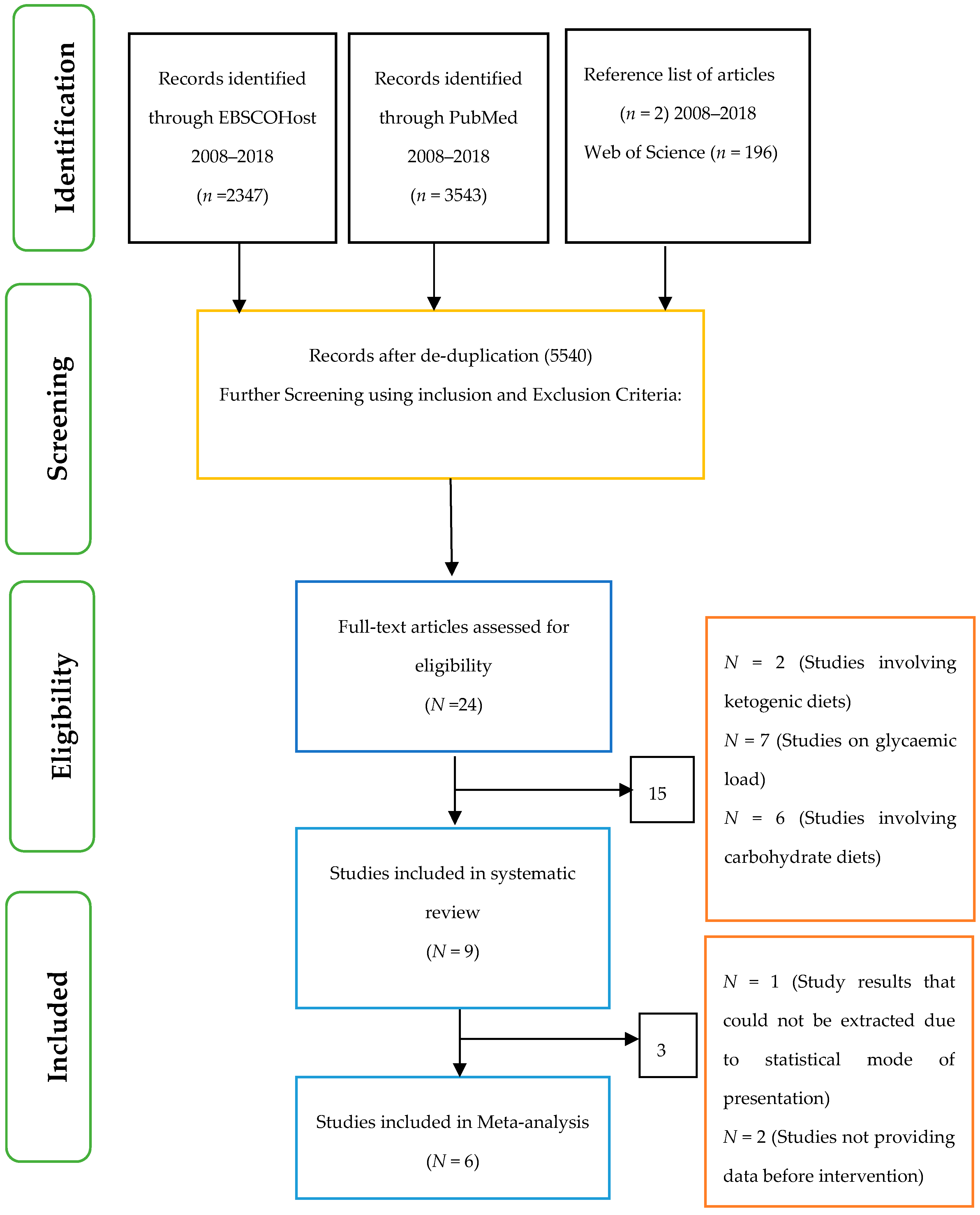
2.7. Selection of Studies
2.8. Evaluation of Quality
2.9. Data Extraction and Management
2.10. Meta-Analysis Methods
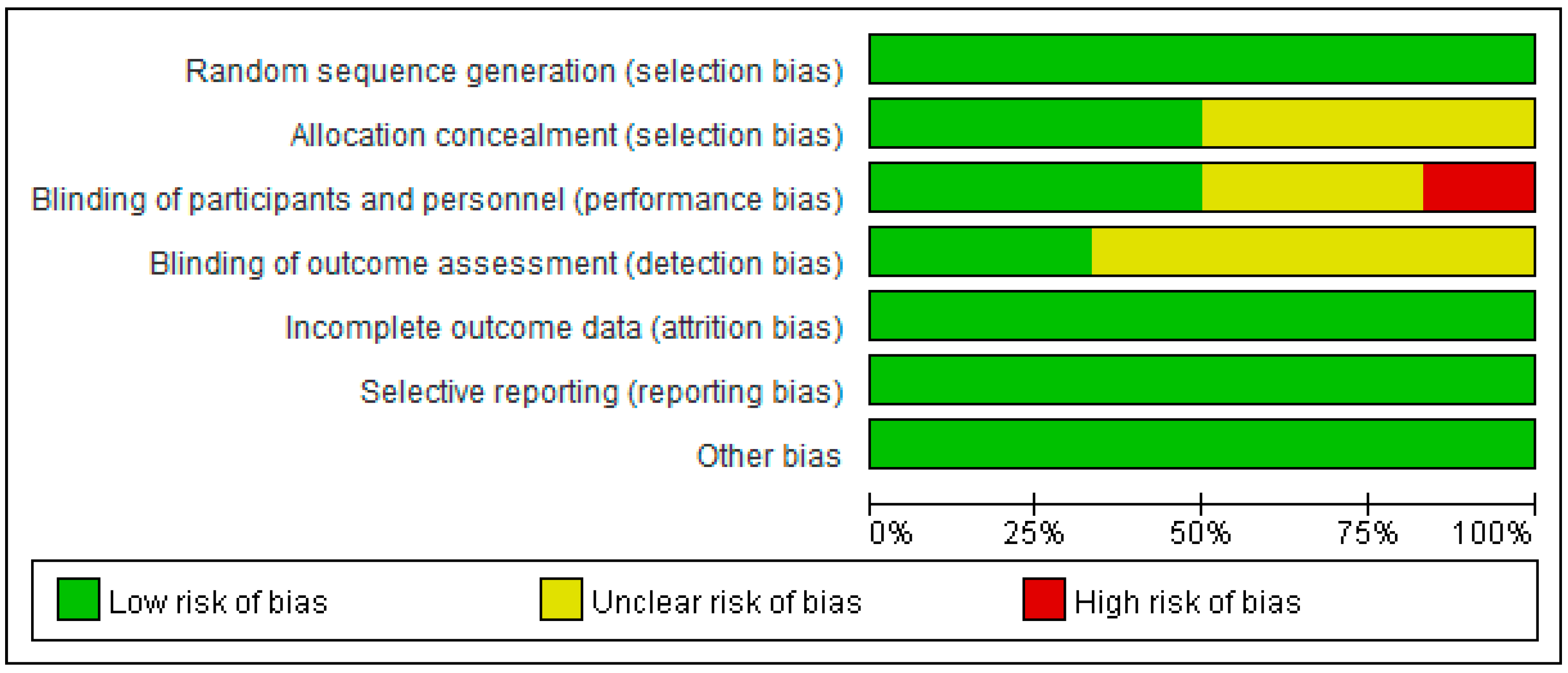
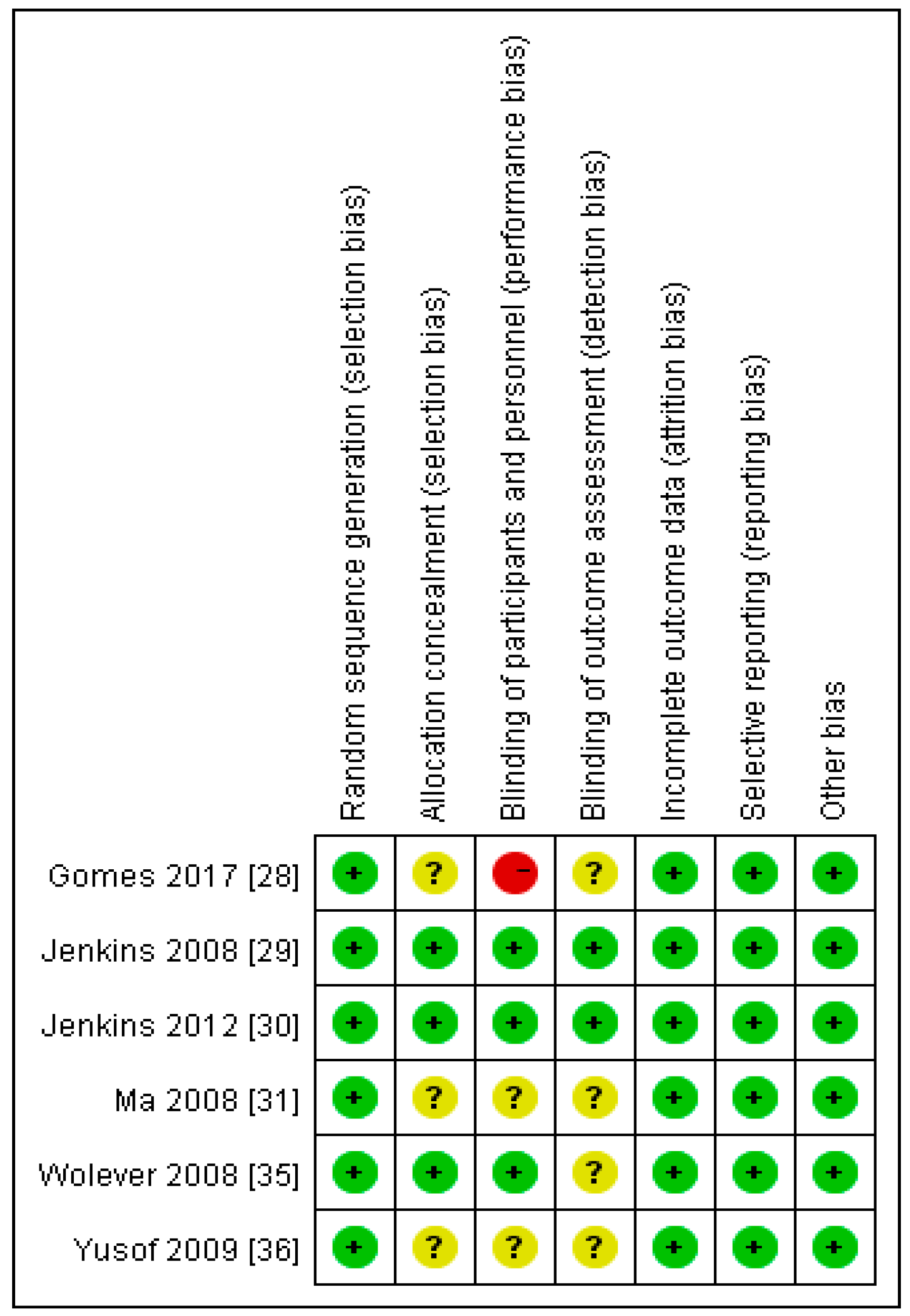
2.11. Assessment of Risk of Bias in Included Studies
3. Results
3.1. Assessment of Risk of Bias in Included Studies
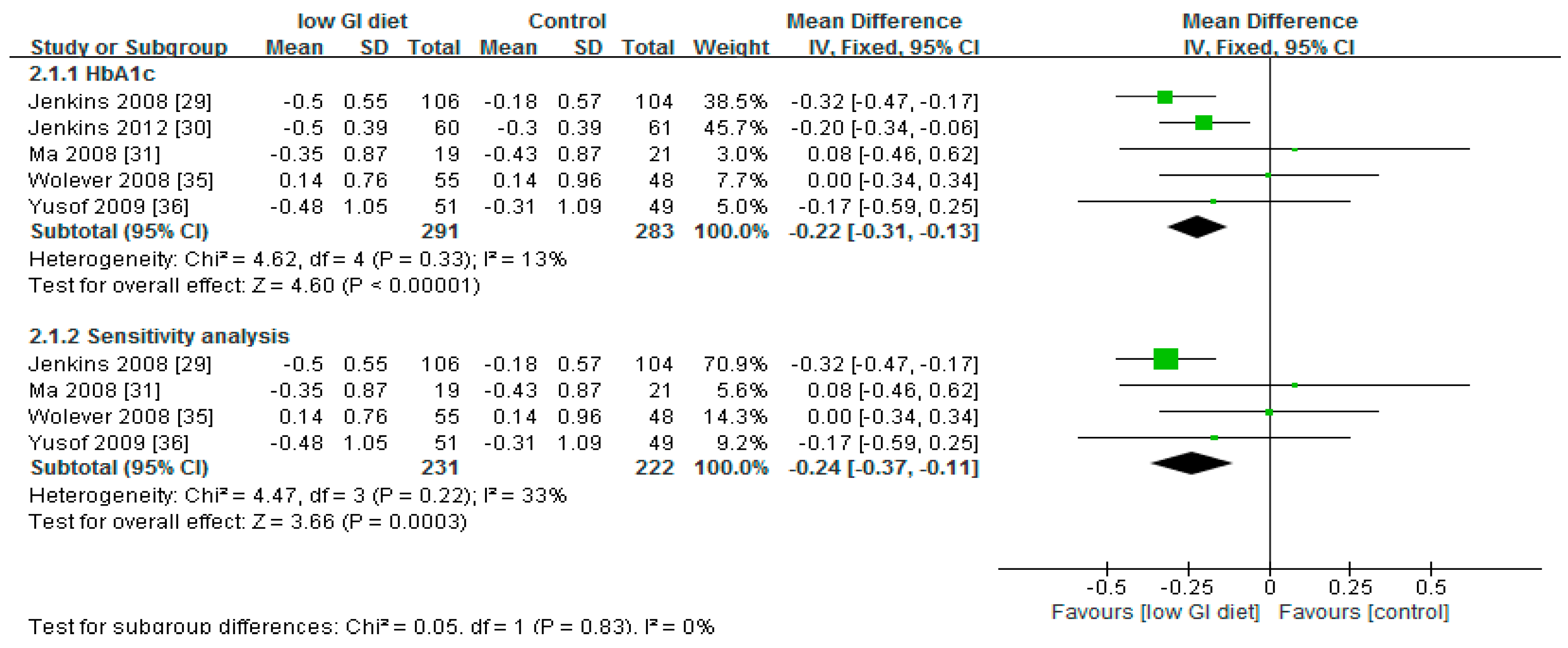
3.2. Effect of Low Glycaemic Index on Glycated Haemoglobin
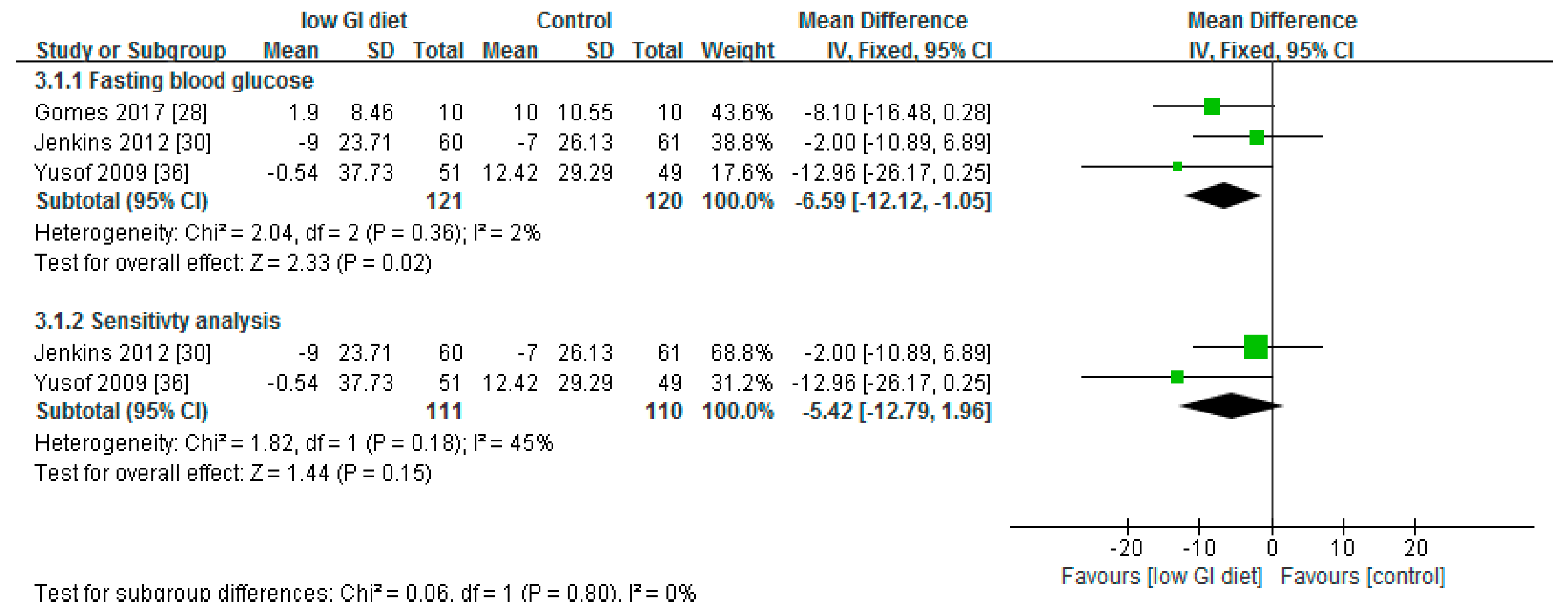
3.3. Effect of Low Glycaemic Index on Fasting Blood Glucose
4. Discussion
5. Strengths and Limitation of the Study
6. Conclusions
7. Perspectives for Future Research
Author Contributions
Conflicts of Interest
References
- Zghebi, S.S.; Steinke, D.T.; Carr, M.J.; Rutter, M.K.; Emsley, R.A.; Ashcroft, D.M. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes Obes. Metab. 2017, 19, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Nazareth, I.; Petersen, I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: A retrospective cohort study. BMJ Open 2016, 6, e010210. [Google Scholar] [CrossRef] [PubMed]
- Haynes-Maslow, L.; Leone, L.A. Examining the relationship between the food environment and adult diabetes prevalence by county economic and racial composition: An ecological study. BMC Public Health 2017, 17, 648. [Google Scholar] [CrossRef] [PubMed]
- Hill, J. Management of diabetes in South Asian communities in the UK. Prim. Health Care 2007, 17, 49–55. [Google Scholar] [CrossRef]
- National Collaborating Centre for Chronic Conditions (NCCCC). Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update); Royal College of Physicians: London, UK, 2008. [Google Scholar]
- Russell, W.R.; Baka, A.; Björck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Weickert, M.O. Impact of Diet Composition on Blood Glucose Regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Taylor, A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog. Retin. Eye Res. 2011, 30, 18–53. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.E.; Elliott, E.J. The use of low-glycaemic index diets in diabetes control. Br. J. Nutr. 2010, 104, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organisation (FAO). Carbohydrates in Human Nutrition. Report of a Joint FAO/WHO Expert Consultation; FAO (Food and Nutrition paper–66); FAO: Rome, Italy, 1998; Available online: http://www.fao.org/docrep/w8079e/w8079e00.htm (accessed on 16 January 2018).
- Chang, K.T.; Lampe, J.W.; Schwarz, Y.; Breymeyer, K.L.; Noar, K.A.; Song, X.; Neuhouser, M.L. Low Glycemic Load Experimental Diet More Satiating Than High Glycemic Load Diet. Nutr. Cancer 2012, 64, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Anjana, R.M.; Gayathri, R.; Ramya Bai, M.; Lakshmipriya, N.; Ruchi, V.; Sudha, V. Glycemic Index of a Novel High-Fiber White Rice Variety Developed in India—A Randomized Control Trial Study. Diabetes Technol. Ther. 2016, 18, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Similä, M.E.; Valsta, L.M.; Kontto, J.P.; Albanes, D.; Virtamo, J. Low-, medium- and high-glycaemic index carbohydrates and risk of type 2 diabetes in men. Br. J. Nutr. 2011, 105, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Choi, K.M. Impact of High-Carbohydrate Diet on Metabolic Parameters in Patients with Type 2 Diabetes. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Ikpotokin, O.S.; Adeleye, O.S.; Aliu, E.D.; Osayande, A.B. Ehiabhi, S. Dietary factors in fasting blood glucose levels and weight gain in female Sprague Dawley in rats. J. Clin. Nutr. Diet. 2017, 3, 2. [Google Scholar] [CrossRef]
- Esfahani, A.; Wong, J.W.; Mirrahimi, A.; Villa, C.R.; Kendall, C.C. The application of the glycemic index and glycemic load in weight loss: A review of the clinical evidence. IUBMB Life 2011, 63, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fabricatore, A.N.; Ebbeling, C.B.; Wadden, T.A.; Ludwig, D.S. Continuous glucose monitoring to assess the ecologic validity of dietary glycemic index and glycemic load. Am. J. Clin. Nutr. 2011, 94, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T. Care of People with Diabetes a Manual of Nursing Practice, 4th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Villegas, R.; Liu, S.; Gao, Y.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch. Intern. Med. 2007, 167, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Rosenberg, L.; Singer, M.; Hu, F.B.; Djoussé, L.; Cupples, L.A.; Palmer, J.R. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch. Intern. Med. 2007, 167, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Carey, V.J.; Anderson, C.M.; Miller, E.; Copeland, T.; Charleston, J.; Appel, L.J. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: The OmniCarb randomized clinical trial. JAMA 2014, 312, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic Index, Glycemic Load, Carbohydrates, and Type 2 Diabetes. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef] [PubMed]
- Fabricatore, A.N.; Wadden, T.A.; Ebbeling, C.B.; Thomas, J.G.; Stallings, V.A.; Schwartz, S.; Ludwig, D.S. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: A randomized controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 37–45. [Google Scholar] [CrossRef] [PubMed]
- The Nordic Cochrane Centre. Review Manager (RevMan) [Computer Program]; Version 5.3.; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Critical Appraisal Skills Programme (CASP). Randomised Controlled Trial Checklist. 2017. Available online: http://docs.wixstatic.com/ugd/dded87_4239299b39f647ca9961f30510f52920.pdf (accessed on 18 January 2018).
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Gomes, J.G.; Fabrini, S.P.; Alfenas, R.G. Low glycemic index diet reduces body fat and attenuates inflammatory and metabolic responses in patients with type 2 diabetes. Arch. Endocrinol. Metab. 2017, 61, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.A.; Kendall, C.C.; McKeown-Eyssen, G.; Josse, R.G.; Silverberg, J.; Booth, G.L.; Leiter, L.A. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: A randomized trial. JAMA 2008, 300, 2742–2753. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.A.; Kendall, C.C.; Augustin, L.A.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Josse, R.G. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Olendzki, B.C.; Merriam, P.A.; Chiriboga, D.E.; Culver, A.L.; Li, W.; Pagoto, S.L. A randomized clinical trial comparing low-glycemic index versus ADA dietary education among individuals with type 2 diabetes. Nutrition 2008, 24, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Reis, C.E.; Dullius, J. Glycemic acute changes in type 2 diabetics caused by low and high glycemic index diets. Nutr. Hosp. 2011, 26, 546–552. [Google Scholar] [PubMed]
- Stenvers, D.J.; Schouten, L.J.; Jurgens, J.; Endert, E.; Kalsbeek, A.; Fliers, E.; Bisschop, P.H. Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2014, 112, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Visek, J.; Lacigova, S.; Cechurova, D.; Rusavy, Z. Comparison of a Low-Glycemic Index vs. Standard Diabetic Diet; Biomedical Papers of the Medical Faculty of The University Palacky; The University Palacky: Olomouc, Czechoslovakia, 2014; Volume 158, pp. 112–116. [Google Scholar]
- Wolever, T.; Gibbs, A.; Mehling, C.; Chiasson, J.; Connelly, P.; Josse, R.; Ryan, E. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: No effect on glycated hemoglobin but reduction in C-reactive protein. Am. J. Clin. Nutr. 2008, 87, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Yusof, B.M.; Talib, R.A.; Kamaruddin, N.A.; Karim, N.A.; Chinna, K.; Gilbertson, H. A low-GI diet is associated with a short-term improvement of glycaemic control in Asian patients with type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.K.; Headings, A.; Peyrot, M.; Nagaraja, H. A behavioural intervention incorporating specific glycaemic index goals improves dietary quality, weight control and glycaemic control in adults with type 2 diabetes. Public Health Nutr. 2011, 14, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Life style management: Standards of Medical Care in Diabetes–2018. Diabetes Care 2018, 41 (Suppl. 1), S38–S50. [Google Scholar]
- Ojo, O.; Brooke, J. Evaluation of the role of enteral nutrition in managing patients with diabetes: A systematic review. Nutrients 2014, 6, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Widanagamage, R.D.; Ekanayake, S.; Welihinda, J. Carbohydrate-rich foods: Glycaemic indices and the effect of constituent macronutrients. Int. J. Food Sci. Nutr. 2009, 60, 215–223. [Google Scholar] [CrossRef] [PubMed]
| Patient/Population | Intervention | Comparator | Study Designs | Combining Search Terms |
|---|---|---|---|---|
| Patients with diabetes | Low-glycaemic index diet | Higher-glycaemic index diet or control | Randomised controlled trial | |
| Patients with diabetes OR type 2 diabetes OR diabetes OR diabetes complications OR diabetes mellitus, type 2 OR diabetes mellitus | Glycaemic index OR glycemic index OR glycaemic load OR glycaemic indices or glycaemic index number or glycaemic index numbers | #1 Randomised controlled trial OR controlled clinical trial OR randomized OR placebo OR drug therapy OR randomly OR trial OR groups #2 “Animals” NOT “Humans” #3 #1 NOT #2 | Column 1 and Column 2 and Column 3 |
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Adult patients (≥18 years) with type 2 diabetes | Studies involving patients with type 1 diabetes or gestational diabetes and animal studies. Studies involving children with diabetes or healthy adults. |
| Intervention | Low-glycaemic index diet | Studies involving dietary supplements |
| Comparator | Higher-glycaemic index diet and/or control | Studies involving additional supplements |
| Outcomes | Blood glucose parameters: Glycated haemoglobin, fasting blood glucose | Qualitative outcomes |
| Types of study: quantitative | Randomised controlled trials | Observational studies Letters Comments Reviews |
| Citation | Length of Study | Study Type | Sample Size | Age (Years) | Diabetes Duration (Years) | Interventions | Glycated Haemoglobin (HbA1c) % | Blood Glucose | Dietary Glycaemic Index |
|---|---|---|---|---|---|---|---|---|---|
| Gomes et al. [28] | 1 month | Parallel Design | 20 | # 42.4 ± 5.1 | # Low-GI (Glycaemic Index) diet (4.8 ± 1.5) higher-GI diet (4.9 ± 1.6) | Low-GI diet versus higher-GI diet | No data | *# Baseline Low-GI diet = 148.9 ± 8.2 vs. higher-GI diet 147.8 ± 10.7 30 days Low-GI diet = 150.8 ± 8.7 vs. higher-GI diet = 157.8 ± 10.4 p = 0.43 | ## Baseline Low-GI diet = 63 ± 6 vs. higher-GI diet = 66 ± 4 30 days Low-GI diet = 54 ± 4 vs. higher-GI diet = 72 ± 3 p = 0.005 |
| Jenkins et al. [29] | 6 months | Parallel Design | 210 | # Low-GI diet = 60 (10) High-cereal fibre diet = 61 (9) | # Low-GI diet = 8.3 (6.5) High-cereal fibre diet = 7.2 (5.9) | Low-GI diet versus high-cereal fibre diet | Low-GI diet Δ = −0.5% (95% CI, −0.61% to −0.39%) vs. high-cereal fibre diet Δ = −0.18% (95% CI, −0.29% to −0.07%) p < 0.001 | * (Mean) Week 0 Low-GI diet = 138.8 vs. high-cereal fibre diet = 141.2 Week 24 Low-GI diet = 127.7 vs. high-cereal fibre diet = 136.8 p = 0.02 | #### Week 0 Low-GI diet = 80.8 (79.6–82.0) vs. high-cereal fibre diet = 81.5 (80.4–82.7) Week 24 Low-GI diet = 69.6 (67.7–71.4) vs. high-cereal fibre diet = 83.5 (82.4–84.7) |
| Jenkins et al. [30] | 3 months | Parallel Design | 121 | ## Low-GI legume diet = 58 (1.3) High-wheat fibre diet = 61 (1.0) | ## Low-GI legume diet = 9.2 (8.0) High-wheat fibre diet = 8.6 (0.8) | Low-GI legume diet vs. high-wheat fibre diet | Low GI legume diet Δ = −0.5% (95%, −0.6% to −0.4%) vs. high-wheat fibre diet Δ = −0.3% (95% Cl, −0.4 to −0.2%) p < 0.001 | *#### Baseline Low-GI legume diet = 141 (135–147) (95% CI) vs. high-wheat fibre diet = 134 (127–141) (95% CI) End of study Low-GI legume diet = 132(126–138) (95% CI) vs. high-wheat fibre diet = 127 (121–133) (95% CI) p = 0.001 | #### Baseline Low-GI legume diet = 80 (79–82) (95% CI) vs. high-wheat fibre diet = 78 (77–80) (95% CI) End of study Low-GI legume diet = 66 (64–67) (95% CI) vs. high-wheat fibre diet = 82 (81–83) (95% CI) p < 0.001 |
| Ma et al. [31] | 12 months | Parallel Design | 40 | # 53.53 ± 8.40 | # 9.32 ± 9.66 | Low-GI diet vs. American Diabetes Association diet (ADA) | ## Baseline Low-GI diet = 8.74 ± 0.29% vs. baseline ADA diet = 8.1 ± 0.28% 12 months Low-GI diet = 8.39 ± 0.30% vs. 12-month ADA diet = 7.67 ± 0.28% p = 0.08 | No data | ## Baseline Low-GI diet = 79.35 ± 1.36 vs. ADA diet = 82.03 ± 1.31 12 months Low-GI diet = 76.64 ± 1.46 vs. ADA diet = 80.36 ± 1.40 p = 0.07 |
| Gonçalves Reis and Dullius [32] | 2 weeks | Cross-over study | 12 | # 60 ± 8 | # 12 ± 7 | Low-GI diet vs. higher-GI diet | No data | *# Low-GI diet first day (127 ± 30) vs. higher-GI diet (148 ± 62) (p < 0.05) By the second day FBG levels had the same average value (132 mg/dL) (p = 0.78) | No data |
| Stenvers et al. [33] | 22 months | Cross-over study | 20 | # 60 ± 7 | ### 5 (1–9) | Low-GR (Glycaemic Response) liquid formula versus free choice (control) | ### Baseline Low-GR = 6.5% (6.1–6.9) Control = 6.5% (6.2–6.9) 12 weeks Low-GR = 6.5% (6.3–7.1) Control = 6.6% (6.3–7.0) | **### Baseline Low-GR = 7.3 (6.4–8.1) Control = 6.8 (6.1–7.4) 12 weeks Low-GR = 7.2 (6.5–7.7) Control = 7.0 (6.7–7.8) | No data |
| Visek et al. [34] | 3 months | Cross–over study | 20 (12 men + 8 women) | # 62.7 ± 5.8 | # 7 ± 4.1 | Low-GI diet versus standard diabetic diet | ### Low-GI diet = 6.63 (6.08–7.0)% Standard diabetic diet = 6.45 (6.18–6.91)% (p > 0.05) | **### Low-GI diet = 6.5 (5.6–8.4) Standard diabetic diet = 6.7 (6.1–7.5) (p > 0.05) | ### Low-GI diet = 49 (48–51) Standard diabetic diet = 68 (61–72) (p < 0.01) |
| Wolever et al. [35] | 12 months | Parallel Design | 162 | Low-GI diet = 60.6 ± 1.0 Higher-GI diet = 60.4 ± 1.1 | No data | Low-GI diet vs. higher-GI diet | ## Baseline Low-GI diet = 6.2 ± 0.8% Higher-GI diet = 6.2 ± 1% Outcomes Low-GI diet = 6.34 ± 0.05% Higher-GI diet = 6.34 ± 0.05% p > 0.05 | No data | ## Baseline Low-GI diet = 60.3 ± 0.4 Higher-GI diet = 61.5 ± 0.4 Study Low-GI diet = 55.1 ± 0.4 Higher-GI diet = 63.2 ± 0.4 p < 0.001 |
| Yusof et al. [36] | 12 weeks | Parallel Design | 100 | 53.5 | No data | Low-GI diet vs. conventional carbohydrate exchange (CCE) | ## Baseline Low-GI diet = 7.68 ± 1.13% CCE = 7.51 ± 1.24% Week 12 Low-GI diet = 7.2 ± 0.1% CCE = 7.2 ± 0.2% p > 0.05 | **## Baseline Low-GI diet = 7.33 ± 2.23 CCE = 7.01 ± 1.79 Week 12 Low-GI diet = 7.3 ± 0.3 CCE = 7.7 ± 0.4 p < 0.05 | # Week 12 Low-GI diet = 57 ± 6 Week 12 CCE = 64 ± 5 p < 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.-H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. https://doi.org/10.3390/nu10030373
Ojo O, Ojo OO, Adebowale F, Wang X-H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2018; 10(3):373. https://doi.org/10.3390/nu10030373
Chicago/Turabian StyleOjo, Omorogieva, Osarhumwese Osaretin Ojo, Fajemisin Adebowale, and Xiao-Hua Wang. 2018. "The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 10, no. 3: 373. https://doi.org/10.3390/nu10030373
APA StyleOjo, O., Ojo, O. O., Adebowale, F., & Wang, X.-H. (2018). The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 10(3), 373. https://doi.org/10.3390/nu10030373





