Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption
Abstract
1. Introduction
2. Intestinal Absorption
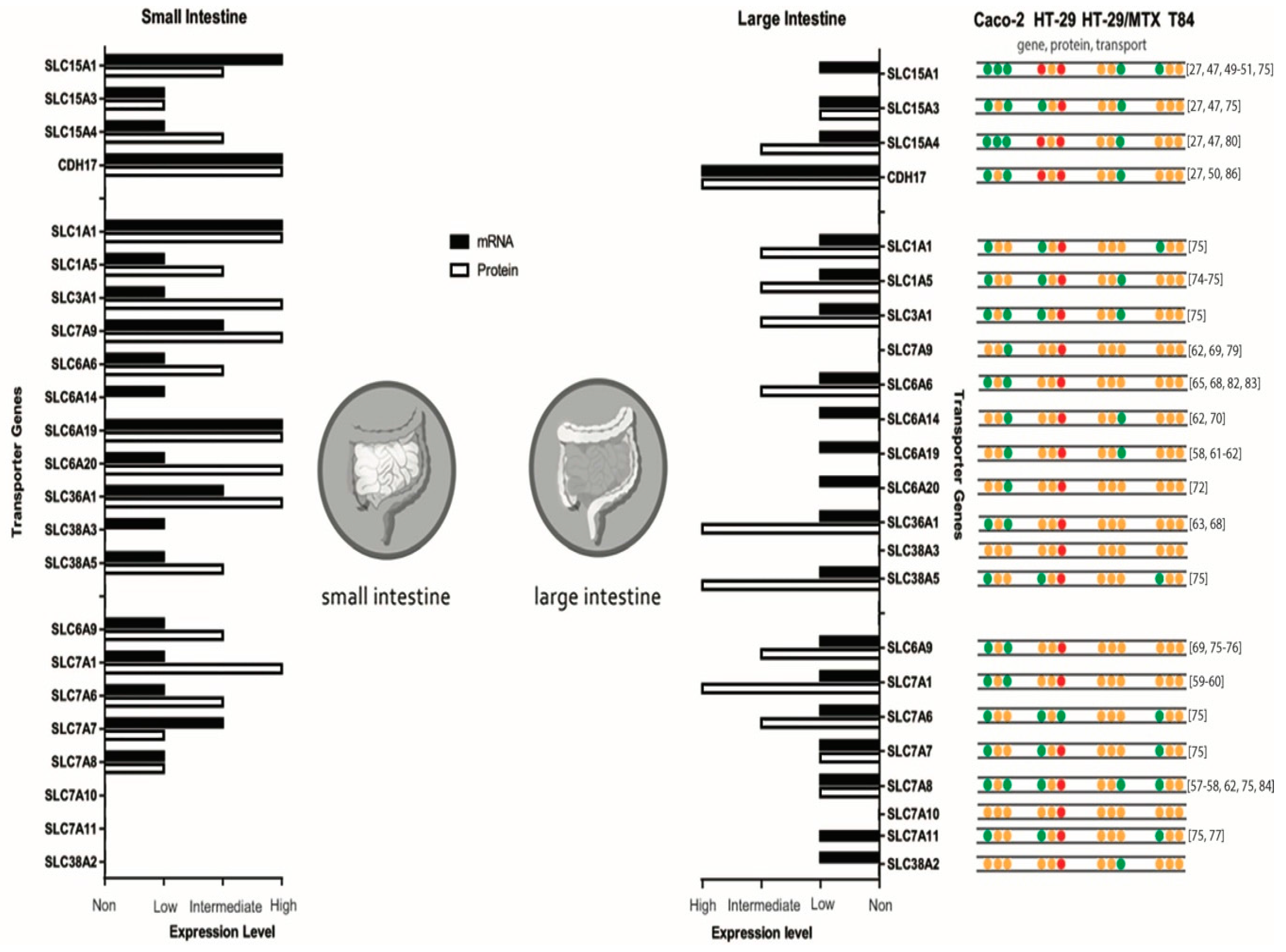
3. Intestinal Di- and Tripeptide and Amino Acid Transporters
4. In Vitro Models for Intestinal Peptide and Amino Acid Absorption
5. Caco-2
5.1. Caco-2: Peptide Transporter Expression
5.2. Caco-2: Amino Acid Transporter Expression
6. HT-29
7. T84
8. Contradictive Results Due to Inter-Laboratory Differences
9. Advanced Culturing Conditions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Metabolic Regulation: A Human Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nishizuka, Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 1984, 308, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Proteins Are Degraded to Amino Acids. In Biochemistry; W. H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Raufman, J.-P. Gastric chief cells: Receptors and signal-transduction mechanisms. Gastroenterology 1992, 102, 699–710. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Kim, K.-J. Drug Absorption Studies: In Situ, In Vitro and In Silico Models; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Li, H.; Limenitakis, J.P.; Fuhrer, T.; Geuking, M.B.; Lawson, M.A.; Wyss, M.; Brugiroux, S.; Keller, I.; Macpherson, J.A.; Rupp, S.; et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat. Commun. 2015, 6, 8292. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Lin, H.C.; McSweeney, C.S.; Mackie, R.I.; Gaskins, H.R. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annu. Rev. Food Sci. Technol. 2010, 1, 363–395. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Epithelia and Gastrointestinal Function. In Yamada’s Textbook of Gastroenterology; Wiley Blackwell: Hoboken, NJ, USA, 2016; pp. 317–329. [Google Scholar]
- Bornhorst, G.M.; Paul Singh, R. Gastric digestion in vivo and in vitro: How the structural aspects of food influence the digestion process. Annu. Rev. Food Sci. Technol. 2014, 5, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Rinderknecht, H. Activation of pancreatic zymogens. Dig. Dis. Sci. 1986, 31, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Erickson, R.H.; Kim, Y.S. Digestion and absorption of dietary protein. Annu. Rev. Med. 1990, 41, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; Van Den Bogert, B.; Kleerebezem, M. The small intestine microbiota, nutritional modulation and relevance for health. Curr. Opin. Biotechnol. 2015, 32, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.-L.; Wu, G.; Zhu, W.-Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. WJG 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Regazzo, D.; Mollé, D.; Gabai, G.; Tomé, D.; Dupont, D.; Leonil, J.; Boutrou, R. The (193–209) 17-residues peptide of bovine β-casein is transported through Caco-2 monolayer. Mol. Nutr. Food Res. 2010, 54, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Sawada, K.; Saito, H.; Hashimoto, Y.; Inui, K.-I. Functional characteristics of basolateral peptide transporter in the human intestinal cell line Caco-2. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 276, G1435–G1441. [Google Scholar] [CrossRef]
- Shimizu, M. Modulation of intestinal functions by food substances. Mol. Nutr. Food Res. 1999, 43, 154–158. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Alpers, D.H.; Taylor, B.; Klein, S. General nutritional principles. In Principles of Clinical Gastroenterology; Tadataka, Y., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 557–587. [Google Scholar]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, M.; Knütter, I.; Leibach, F.H. The intestinal H+/peptide symporter PEPT1: Structure–affinity relationships. Eur. J. Pharm. Sci. 2004, 21, 53–60. [Google Scholar] [CrossRef]
- Dantzig, A.H.; Hoskins, J.; Tabas, L.B.; Bright, S.; Shepard, R.L.; Jenkins, I.L.; Duckworth, D.C.; Sportsman, J.R.; Mackensen, D.; Rosteck, P.R., Jr. Association of intestinal peptide transport with a protein related to the cadherin superfamily. Science 1994, 264, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, D.; Wang, Q.; Cook, T.J.; Knipp, G.T.; Gudmundsson, O.S.; Smith, R.L.; Faria, T.N. Spatial expression patterns of peptide transporters in the human and rat gastrointestinal tracts, Caco-2 in vitro cell culture model, and multiple human tissues. AAPS Pharmsci. 2001, 3, 100–111. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Daniel, H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica 2008, 38, 1022–1042. [Google Scholar] [CrossRef] [PubMed]
- Adibi, S.A.; Morse, E.L. Intestinal transport of dipeptides in man: Relative importance of hydrolysis and intact absorption. J. Clin. Investig. 1971, 50, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.E.; Keely, S.J. Electrolyte Secretion and Absorption in the Small Intestine and Colon. In Yamada's Textbook of Gastroenterology; Wiley Blackwell: Hoboken, NJ, USA, 2016; pp. 420–449. [Google Scholar]
- Palacín, M.; Kanai, Y. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflüg. Arch. 2004, 447, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Fort, J.; Laura, R.; Burghardt, H.E.; Ferrer-Costa, C.; Turnay, J.; Ferrer-Orta, C.; Usón, I.; Zorzano, A.; Fernández-Recio, J.; Orozco, M.; et al. The structure of human 4F2hc ectodomain provides a model for homodimerization and electrostatic interaction with plasma membrane. J. Biol. Chem. 2007, 282, 31444–31452. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Terada, T.; Okuda, M.; Inui, K.-I. Efflux properties of basolateral peptide transporter in human intestinal cell line Caco-2. Pflüg. Arch. 2004, 449, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Clémençon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 2013, 34, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Pramod, A.B.; Foster, J.; Carvelli, L.; Henry, L.K. SLC6 transporters: Structure, function, regulation, disease association and therapeutics. Mol. Asp. Med. 2013, 34, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Schiöth, H.B.; Roshanbin, S.; Hägglund, M.G.A.; Fredriksson, R. Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol. Asp. Med. 2013, 34, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Sawada, K.; Irie, M.; Saito, H.; Hashimoto, Y.; Inui, K. Structural requirements for determining the substrate affinity of peptide transporters PEPT1 and PEPT2. Pflüg. Arch. 2000, 440, 679–684. [Google Scholar] [CrossRef]
- Geiss-Friedlander, R.; Parmentier, N.; Moller, U.; Urlaub, H.; Van den Eynde, B.J.; Melchior, F. The cytoplasmic peptidase DPP9 is rate-limiting for degradation of proline-containing peptides. J. Biol. Chem. 2009, 284, 27211–27219. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Russell, R.C.; Guan, K.-L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014, 99, 223S–230S. [Google Scholar] [CrossRef] [PubMed]
- Meunier, V.; Bourrie, M.; Berger, Y.; Fabre, G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 1995, 11, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zweibaum, A.; Pinto, M.; Chevalier, G.; Dussaulx, E.; Triadou, N.; Lacroix, B.; Haffen, K.; Brun, J.-L.; Rousset, M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J. Cell. Physiol. 1985, 122, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Rousset, M. The human colon carcinoma cell lines HT-29 and Caco-2: Two in vitro models for the study of intestinal differentiation. Biochimie 1986, 68, 1035–1040. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Barbat, A.; Dussaulx, E.; Zweibaum, A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990, 50, 6334–6343. [Google Scholar] [PubMed]
- Madara, J.L.; Stafford, J.; Dharmsathaphorn, K.; Carlson, S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology 1987, 92, 1133–1145. [Google Scholar] [CrossRef]
- Tai, W.; Chen, Z.; Cheng, K. Expression profile and functional activity of peptide transporters in prostate cancer cells. Mol. Pharm. 2012, 10, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Groneberg, D.A.; Doring, F.; Theis, S.; Nickolaus, M.; Fischer, A.; Daniel, H. Peptide transport in the mammary gland: Expression and distribution of PEPT2 mRNA and protein. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1172–E1179. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Kissel, T. Do cell culture conditions influence the carrier-mediated transport of peptides in Caco-2 cell monolayers? Eur. J. Pharm. Sci. 2003, 19, 433–442. [Google Scholar] [CrossRef]
- Behrens, I.; Kamm, W.; Dantzig, A.H.; Kissel, T. Variation of peptide transporter (PepT1 and HPT1) expression in Caco-2 cells as a function of cell origin. J. Pharm. Sci. 2004, 93, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cook, T.J.; Sinko, P.J. Evidence for diminished functional expression of intestinal transporters in Caco-2 cell monolayers at high passages. Pharm. Res. 1997, 14, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.M.; Levine, S.A.; Yun, C.H.; Brant, S.R.; Pouyssegur, J.; Montrose, M.H.; Donowitz, M. Functional characteristics of a cloned epithelial Na+/H+ exchanger (NHE3): Resistance to amiloride and inhibition by protein kinase C. Proc. Natl. Acad. Sci. USA 1993, 90, 9110–9114. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Inui, K. Dipeptide transporters in apical and basolateral membranes of the human intestinal cell line Caco-2. Am. J. Physiol. 1993, 265, G289–G294. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Enjoh, M.; Nakamura, Y.; Takano, T.; Kawamura, Y.; Arai, S.; Shimizu, M. Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2002, 66, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; Brown, C.D.A.; Hirst, B.H.; Simmons, N.L. H+-coupled dipeptide (glycylsarcosine) transport across apical and basal borders of human intestinal Caco-2 cell monolayers display distinctive characteristics. Biochim. Biophys. Acta (BBA)-Biomembr. 1993, 1151, 237–245. [Google Scholar] [CrossRef]
- Pan, M.; Souba, W.W.; Karinch, A.M.; Lin, C.-M.; Stevens, B.R. Epidermal growth factor regulation of system L alanine transport in undifferentiated and differentiated intestinal Caco-2 cells. J. Gastrointest. Surg. 2002, 6, 410–417. [Google Scholar] [CrossRef]
- Pan, M.; Souba, W.W.; Wolfgang, C.L.; Karinch, A.M.; Stevens, B.R. Posttranslational alanine trans-stimulation of zwitterionic amino acid transport systems in human intestinal Caco-2 cells. J. Surg. Res. 2002, 104, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Stevens, B.R. Protein kinase C-dependent regulation of L-arginine transport activity in Caco-2 intestinal cells. Biochim. Biophys. Acta (BBA)-Biomembr. 1995, 1239, 27–32. [Google Scholar] [CrossRef]
- Pan, M.; Malandro, M.; Stevens, B.R. Regulation of system y+ arginine transport capacity in differentiating human intestinal Caco-2 cells. Am. J. Physiol. 1995, 268, G578–G585. [Google Scholar] [CrossRef] [PubMed]
- Souba, W.W.; Copeland, E.M. Cytokine modulation of Na(+)-dependent glutamine transport across the brush border membrane of monolayers of human intestinal Caco-2 cells. Ann. Surg. 1992, 215, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Huneau, J.-F.; Tomé, D. Characteristics of L-glutamine transport during Caco-2 cell differentiation. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1509, 95–102. [Google Scholar] [CrossRef]
- Chen, Z.; Fei, Y.J.; Anderson, C.M.; Wake, K.A.; Miyauchi, S.; Huang, W.; Thwaites, D.T.; Ganapathy, V. Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J. Physiol. 2003, 546, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, P.L.; Irwin, W.J.; Hassan, I.F.; Mackay, M. Proline uptake by monolayers of human intestinal absorptive (Caco-2) cells in vitro. Biochim. Biophys. Acta (BBA)-Biomembr. 1992, 1104, 283–292. [Google Scholar] [CrossRef]
- Satsu, H.; Watanabe, H.; Arai, S.; Shimizu, M. Characterization and regulation of taurine transport in Caco-2, human intestinal cells. J. Biochem. 1997, 121, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; McEwan, G.T.; Brown, C.D.; Hirst, B.H.; Simmons, N.L. Na(+)-independent, H(+)-coupled transepithelial beta-alanine absorption by human intestinal Caco-2 cell monolayers. J. Biol. Chem. 1993, 268, 18438–18441. [Google Scholar] [PubMed]
- Thwaites, D.T.; McEwan, G.T.A.; Brown, C.D.A.; Hirst, B.H.; Simmons, N.L. L-Alanine absorption in human intestinal Caco-2 cells driven by the proton electrochemical gradient. J. Membr. Biol. 1994, 140, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.H.; Howard, A.; Walters, J.R.F.; Ganapathy, V.; Thwaites, D.T. Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+-and Cl−-dependent TauT (SLC6A6). J. Physiol. 2009, 587, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; Markovich, D.; Murer, H.; Simmons, N.L. Na+-independent lysine transport in human intestinal Caco-2 cells. J. Membr. Biol. 1996, 151, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Borchardt, R.T. Transport of a large neutral amino acid (phenylalanine) in a human intestinal epithelial cell line: Caco-2. Biochim. Biophys. Acta (BBA)-Biomembr. 1990, 1028, 25–30. [Google Scholar] [CrossRef]
- Hu, M.; Borchardt, R.T. Transport of a large neutral amino acid in a human intestinal epithelial cell line (Caco-2): Uptake and efflux of phenylalanine. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1992, 1135, 233–244. [Google Scholar] [CrossRef]
- Thwaites, D.T.; McEwan, G.T.A.; Cook, M.J.; Hirst, B.H.; Simmons, N.L. H+-coupled (Na+-independent) proline transport in human intestinal (Caco-2) epithelial cell monolayers. FEBS Lett. 1993, 333, 78–82. [Google Scholar] [CrossRef]
- Nicklin, P.L.; Irwin, W.J.; Hassan, I.F.; Mackay, M.; Dixon, H.B.F. The transport of acidic amino acids and their analogues across monolayers of human intestinal absorptive (Caco-2) cells in vitro. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1995, 1269, 176–186. [Google Scholar] [CrossRef]
- Kekuda, R.; Torres-Zamorano, V.; Fei, Y.J.; Prasad, P.D.; Li, H.W.; Mader, L.D.; Leibach, F.H.; Ganapathy, V. Molecular and functional characterization of intestinal Na(+)-dependent neutral amino acid transporter B0. Am. J. Physiol. 1997, 272, G1463–G1472. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, J.; Billaut-Laden, I.; Happillon, M.; Lo-Guidice, J.M.; Maunoury, V.; Imbenotte, M.; Broly, F. Gene expression profiling of systems involved in the metabolism and the disposition of xenobiotics: Comparison between human intestinal biopsy samples and colon cell lines. Drug Metab. Dispos. 2012, 40, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Christie, G.R.; Ford, D.; Howard, A.; Clark, M.A.; Hirst, B.H. Glycine supply to human enterocytes mediated by high-affinity basolateral GLYT1. Gastroenterology 2001, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.; Gasol, E.; Manzoni, M.; Pineda, M.; Riboni, M.; Martín, R.; Zorzano, A.; Borsani, G.; Palacín, M. Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system xc–. Pflüg. Arch. 2001, 442, 286–296. [Google Scholar] [CrossRef]
- Sun, D.; Lennernas, H.; Welage, L.S.; Barnett, J.L.; Landowski, C.P.; Foster, D.; Fleisher, D.; Lee, K.-D.; Amidon, G.L. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm. Res. 2002, 19, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
- Berger, V.; Larondelle, Y.; Trouet, A.; Schneider, Y.J. Transport mechanisms of the large neutral amino acid l-phenylalanine in the human intestinal epithelial caco-2 cell line. J. Nutr. 2000, 130, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Fei, Y.J.; Prasad, P.D.; Ramamoorthy, S.; Han, H.; Yang-Feng, T.L.; Hediger, M.A.; Ganapathy, V.; Leibach, F.H. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J. Biol. Chem. 1995, 270, 6456–6463. [Google Scholar] [CrossRef] [PubMed]
- Lindley, D.J.; Roth, W.J.; Carl, S.M.; Knipp, G.T. The effects of media on pharmaceutically relevant transporters in the human HT-29 adenocarcinoma cell line: Does culture media need to be controlled? J. Pharm. Sci. 2012, 101, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Tiruppathi, C.; Brandsch, M.; Miyamoto, Y.; Ganapathy, V.; Leibach, F.H. Constitutive expression of the taurine transporter in a human colon carcinoma cell line. Am. J. Physiol. 1992, 263, G625–G631. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, M.; Miyamoto, Y.; Ganapathy, V.; Leibach, F.H. Regulation of taurine transport in human colon carcinoma cell lines (HT-29 and Caco-2) by protein kinase C. Am. J. Physiol. 1993, 264, G939–G946. [Google Scholar] [CrossRef] [PubMed]
- Kekuda, R.; Prasad, P.D.; Fei, Y.-J.; Torres-Zamorano, V.; Sinha, S.; Yang-Feng, T.L.; Leibach, F.H.; Ganapathy, V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J. Biol. Chem. 1996, 271, 18657–18661. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Hosoda, N.; Endo, H.; Saito, K.; Tsujihara, K.; Yamamura, M.; Sakata, T.; Anzai, N.; Wempe, M.F.; Kanai, Y.; et al. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010, 101, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus caco-2/HT29-MTX co-cultured cell lines: Permeabilities via diffusion, inside-and outside-directed carrier-mediated transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Merlin, D.; Steel, A.; Gewirtz, A.T.; Si-Tahar, M.; Hediger, M.A.; Madara, J.L. hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J. Clin. Investig. 1998, 102, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Zucco, F.; Batto, A.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.; de Angelis, I.; Fabre, G.; et al. An inter-laboratory study to evaluate the effects of medium composition on the differentiation and barrier function of Caco-2 cell lines. ATLA-NOTTINGHAM- 2005, 33, 603–618. [Google Scholar]
- Hayeshi, R.; Hilgendorf, C.; Artursson, P.; Augustijns, P.; Brodin, B.; Dehertogh, P.; Fisher, K.; Fossati, L.; Hovenkamp, E.; Korjamo, T.; et al. Comparison of drug transporter gene expression and functionality in Caco-2 cells from 10 different laboratories. Eur. J. Pharm. Sci. 2008, 35, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Gremmels, H.; Koelink, P.J.; Verheijden, K.A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014, 28, 2414–2429. [Google Scholar] [CrossRef] [PubMed]
- Bruhat, A.; Jousse, C.; Wang, X.-Z.; Ron, D.; Ferrara, M.; Fafournoux, P. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J. Biol. Chem. 1997, 272, 17588–17593. [Google Scholar] [CrossRef] [PubMed]
- Le Bacquer, O.; Laboisse, C.; Darmaun, D. Glutamine preserves protein synthesis and paracellular permeability in Caco-2 cells submitted to “luminal fasting”. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 285, G128–G136. [Google Scholar] [CrossRef] [PubMed]
- Chaumontet, C.; Azzout-Marniche, D.; Gaudichon, C.; Gausserès, N.; Vinoy, S.; Tomé, D. AMPK phosphorylation is decreased in response to amino acids and glucose in Caco-2 intestinal cells. FASEB J. 2007, 21, A1075. [Google Scholar]
- Vynnytska-Myronovska, B.O.; Kurlishchuk, Y.; Chen, O.; Bobak, Y.; Dittfeld, C.; Hüther, M.; Kunz-Schughart, L.A.; Stasyk, O.V. Arginine starvation in colorectal carcinoma cells: Sensing, impact on translation control and cell cycle distribution. Exp. Cell Res. 2016, 341, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bobak, Y.; Kurlishchuk, Y.; Vynnytska-Myronovska, B.; Grydzuk, O.; Shuvayeva, G.; Redowicz, M.J.; Kunz-Schughart, L.A.; Stasyk, O. Arginine deprivation induces endoplasmic reticulum stress in human solid cancer cells. Int. J. Biochem. Cell Biol. 2016, 70, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health; Springer: New York, NY, USA, 2015. [Google Scholar]
- Pan, F.; Han, L.; Zhang, Y.; Yu, Y.; Liu, J. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int. J. Food Sci. Nutr. 2015, 66, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Fritz, J.V.; Glaab, E.; Desai, M.S.; Greenhalgh, K.; Frachet, A.; Niegowska, M.; Estes, M.; Jäger, C.; Seguin-Devaux, C.; et al. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat. Commun. 2016, 7, 11535. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ingber, D.E. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 2013, 5, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Trietsch, S.J.; Naumovska, E.; Kurek, D.; Setyawati, M.C.; Vormann, M.K.; Wilschut, K.J.; Lanz, H.L.; Nicolas, A.; Ng, C.P.; Joore, J.; et al. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat. Commun. 2017, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012, 7, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhou, Y. Microfluidic Devices for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Rogal, J.; Probst, C.; Loskill, P. Integration concepts for multi-organ chips: How to maintain flexibility?! Future Sci. OA 2017, 3, FSO180. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S. Gut-liver axis. Int. J. Colorectal Dis. 2000, 15, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.; Ha, S.K.; Choi, I.; Choi, N.; Sung, J.H. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed. Microdevices 2017, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; Martin, G.R. (Eds.) Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar]
- Basson, M.D.; Turowski, G.; Emenaker, N.J. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp. Cell Res. 1996, 225, 301–305. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Howell, J.C.; Wells, J.M.; Spence, J.R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 2011, 6, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.L.; Mahe, M.M.; Múnera, J.; Howell, J.C.; Sundaram, N.; Poling, H.M.; Schweitzer, J.I.; Vallance, J.E.; Mayhew, C.N.; Sun, Y.; et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 2014, 20, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Fredlund, L.; Winiwarter, S.; Hilgendorf, C. In Vitro Intrinsic Permeability: A Transporter-Independent Measure of Caco-2 Cell Permeability in Drug Design and Development. Mol. Pharm. 2017, 14, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Lennernäs, H.; Palm, K.; Fagerholm, U.; Artursson, P. Comparison between active and passive drug transport in human intestinal epithelial (Caco-2) cells in vitro and human jejunum in vivo. Int. J. Pharm. 1996, 127, 103–107. [Google Scholar] [CrossRef]
- Bani-Jaber, A.; Alshawabkeh, I.; Abdullah, S.; Hamdan, I.; Ardakani, A.; Habash, M. In Vitro and In Vivo Evaluation of Casein as a Drug Carrier for Enzymatically Triggered Dissolution Enhancement from Solid Dispersions. AAPS PharmSciTech 2017, 18, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
| Encoding Gene | Transporter Protein | Transporter System | Mechanism | Ion Dependency | Dimer Formation | Substrate | |
|---|---|---|---|---|---|---|---|
| Di- and tripeptide transporters | |||||||
| Apical membrane | SLC15A1 | PEPT1 | S | H+ | Di- and tripeptide | ||
| SLC15A3 | PHT2 | S | H+ | Histidine, di- and tripeptide | |||
| SLC15A4 | PHT1 | S | H+ | Histidine, di- and tripeptide | |||
| CDH17 | HPT-1 | S | H+ | Di- and tripeptide | |||
| Basolateral membrane | Basolateral peptide transporter | U | Di- and tripeptide | ||||
| Amino acid transporters | |||||||
| Apical membrane | SLC1A1 | EAAT3/EAAC1 | X−AG | A | AA + 3Na+ + H+ ↔ K+ | Aspartic acid and glutamic acid | |
| SLC1A5 | ASCT2/AAAT | ASC | A | Na+ + AA ↔ Na+ + AA | Neutral amino acids primary substrates: alanine, asparagine, cysteine, glutamine, serine and threonine | ||
| SLC7A9 | b0,+AT | b0,+ | A | CAA/cystine ↔ NAA | rBAT | Cationic amino acids and cystine | |
| SLC6A6 | TauT | β | S | Cl− and 2 Na+ | β-alanine and taurine | ||
| SLC6A14 | ATB0,+ | B0,+ | S | 2 Cl− and Na+ | Cationic and neutral amino acids | ||
| SLC6A19 | B0AT1/HND | B0 | S | Na+ | Neutral amino acids | ||
| SLC6A20 | SIT1 | IMINO | S | Cl− and 2Na+ | Proline | ||
| SLC36A1 | PAT1/LYAAT1 | PAT | S | H+ | β-alanine, glycine and proline | ||
| SLC38A3 | SN1/SNAT3 | N | A | AA + Na+ ↔ H+ | Alanine, asparagine, glutamine and histidine | ||
| SLC38A5 | SN2/SNAT5 | N | A | AA + Na+ ↔ H+ | Asparagine, glutamine, histidine and serine | ||
| Basolateral membrane | SLC6A9 | GlyT1 | Gly+ | S | Cl− and 2Na+ | Glycine | |
| SLC7A1 | CAT1 | y+ | U | Arginine, histidine and lysine | |||
| SLC7A6 | y+LAT2 | y+L | A | CAA ↔ NAA + Na+ | 4F2hc | Cationic amino acids | |
| SLC7A7 | y+LAT1 | y+L | A | CAA ↔ NAA + Na+ | 4F2hc | Cationic amino acids | |
| SLC7A8 | LAT2 | L | A | NAA ↔ NAA | 4F2hc | Neutral amino acids | |
| SLC7A10 | asc-1 | Asc | A | NAA ↔ NAA | 4F2hc | Small neutral amino acids | |
| SLC7A11 | xCT | Xc | A | Cystine ↔ Glutamic acid | 4F2hc | Cystine | |
| SLC38A2 | SNAT2 | A | S | Na+ | Neutral amino acids and imino |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochems, P.G.M.; Garssen, J.; Van Keulen, A.M.; Masereeuw, R.; Jeurink, P.V. Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption. Nutrients 2018, 10, 322. https://doi.org/10.3390/nu10030322
Jochems PGM, Garssen J, Van Keulen AM, Masereeuw R, Jeurink PV. Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption. Nutrients. 2018; 10(3):322. https://doi.org/10.3390/nu10030322
Chicago/Turabian StyleJochems, Paulus G. M., Johan Garssen, Antonius M. Van Keulen, Rosalinde Masereeuw, and Prescilla V. Jeurink. 2018. "Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption" Nutrients 10, no. 3: 322. https://doi.org/10.3390/nu10030322
APA StyleJochems, P. G. M., Garssen, J., Van Keulen, A. M., Masereeuw, R., & Jeurink, P. V. (2018). Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption. Nutrients, 10(3), 322. https://doi.org/10.3390/nu10030322





