Iron Supplements Containing Lactobacillus plantarum 299v Increase Ferric Iron and Up-regulate the Ferric Reductase DCYTB in Human Caco-2/HT29 MTX Co-Cultures
Abstract
1. Introduction
1.1. Lactobacillus plantarum 299v and Iron Absorption
1.2. Intestinal Uptake Routes for Iron
1.3. The Present Study
2. Materials and Methods
2.1. Bacterial Strain and Capsule Formulation
2.2. Composition of Meals, Oat and Mango Drinks
2.3. Simulated Gastrointestinal Digestion of Capsule Meals
2.4. Simulated Gastrointestinal Digestion of Oat and Mango Drinks
2.5. Differential Pulse Anodic Stripping Voltammetry (DPASV) Measurements
2.6. Experiments in the Caco-2/HT29 Co-Culture Cell Model
2.7. Statistics
3. Results
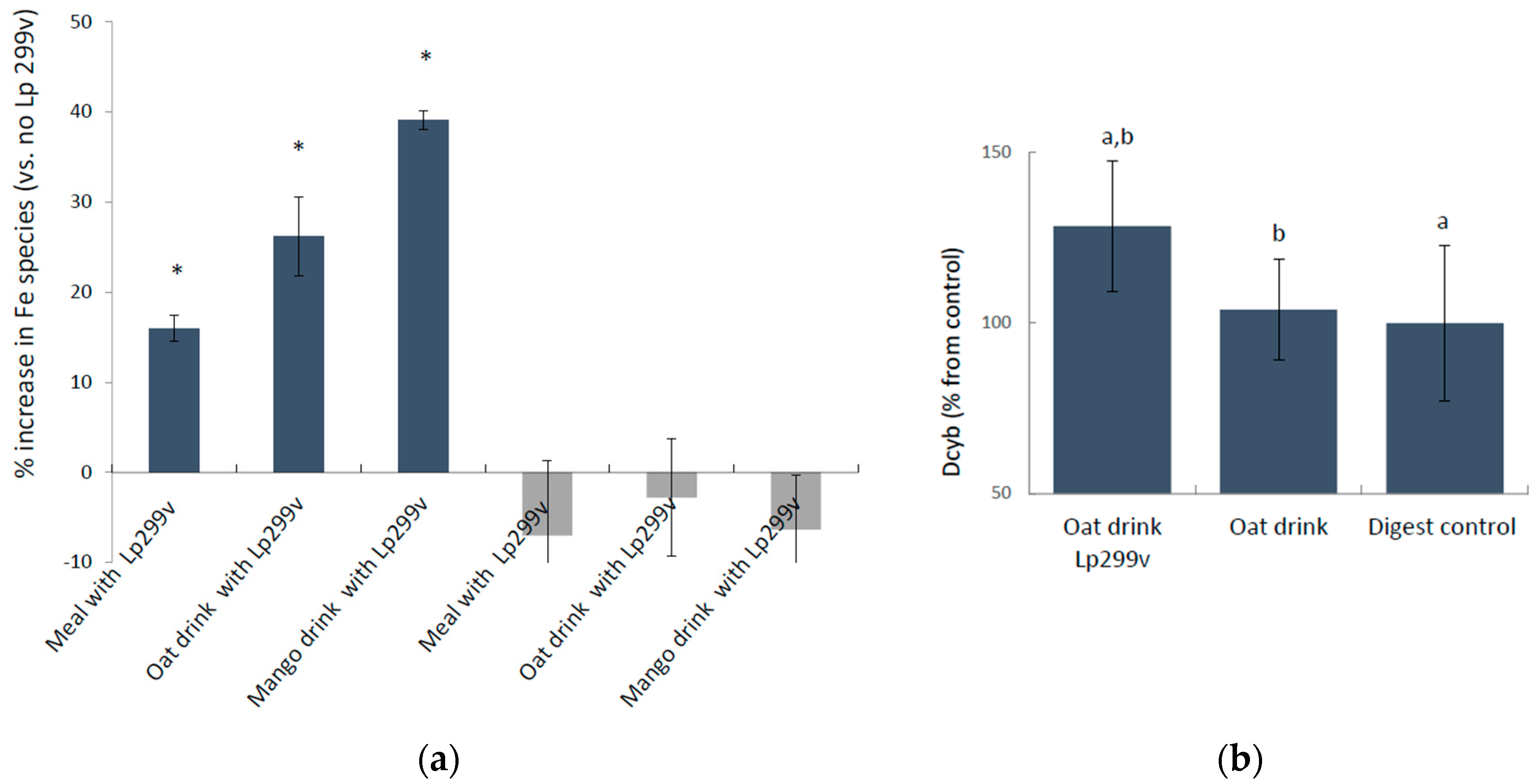
3.1. L. Plantarum 299v-Induced Increase in Ferric Iron in Simulated Gastrointestinal Digested Oat Drinks Was Associated with Elevated Expression of the Cellular Ferric Reductase DCYTB in the Caco-2/HT29 MTX Cell Model
3.2. Time-Response Experiments of Undigested Capsule Contents in Caco-2/HT29 Cells
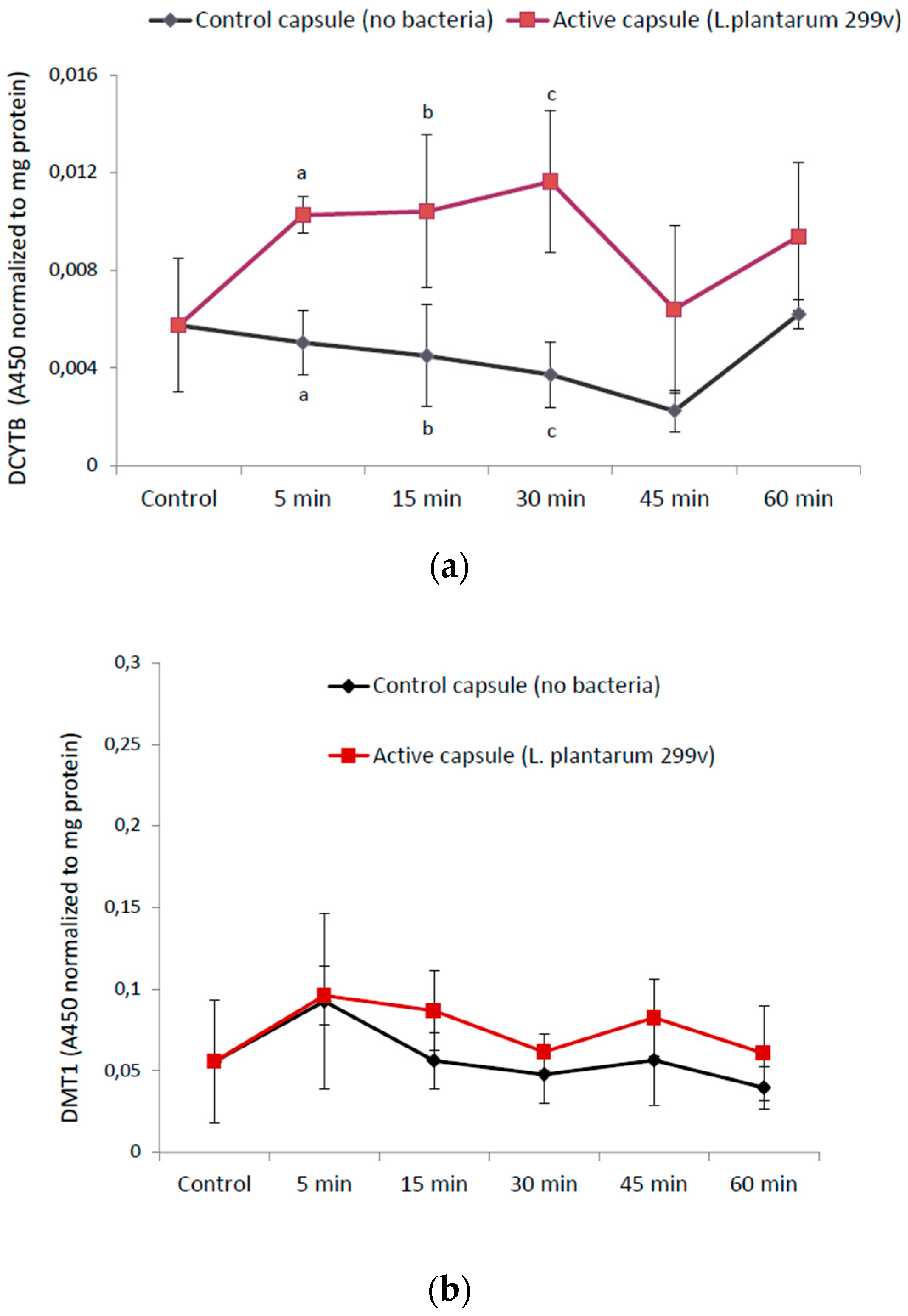
3.2.1. The Reductase DCYTB and the Ferrous Iron Importer DMT1
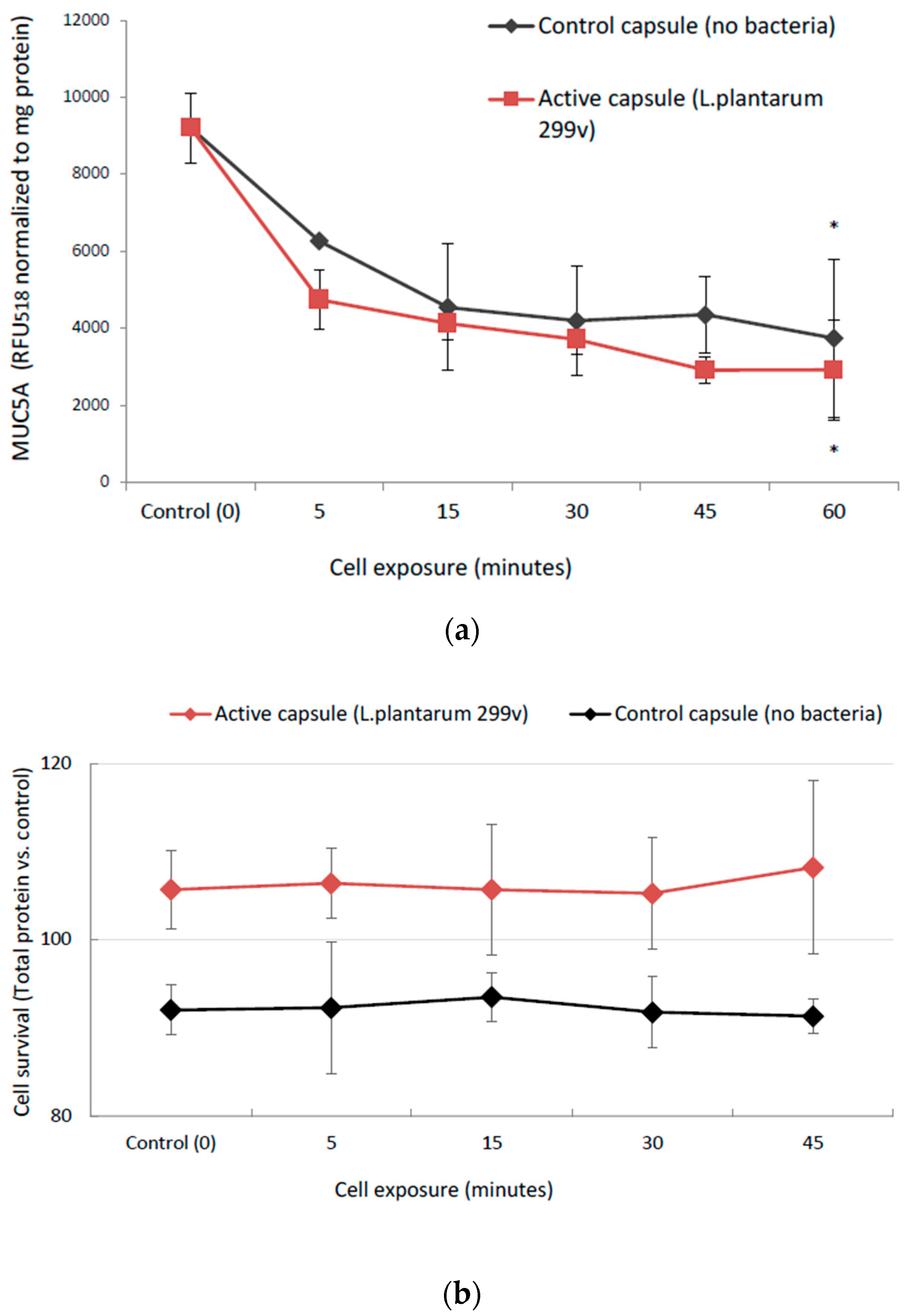
3.2.2. Cellular Mucin (MUC5AC) Production
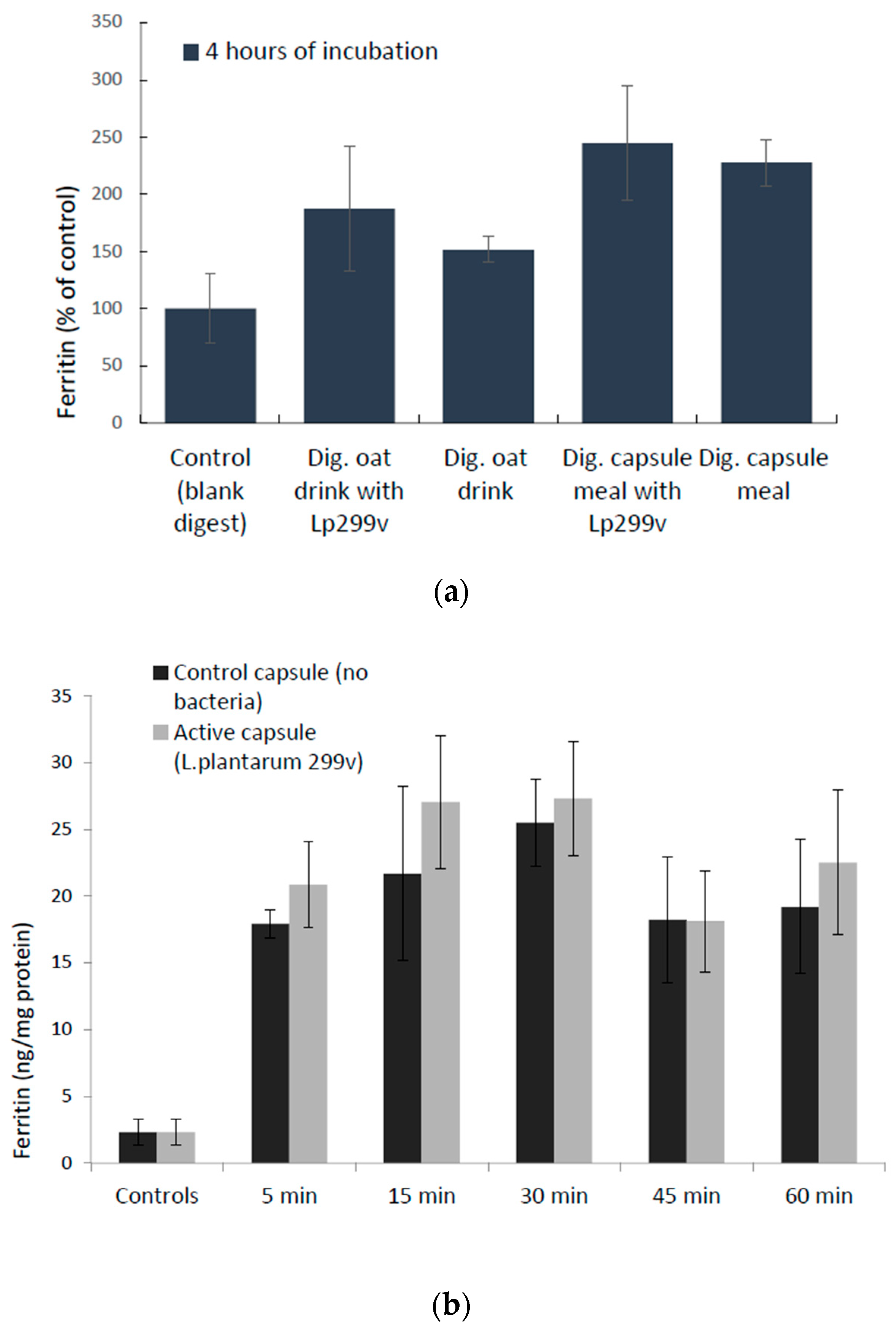
3.3. Cellular Uptake of Iron (Ferritin Expression) in Response to L. Plantarum 299v
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bering, S.; Suchdev, S.; Sjoltov, L.; Berggren, A.; Tetens, I.; Bukhave, K. A lactic acid-fermented oat gruel increases non-haem iron absorption from a phytate-rich meal in healthy women of childbearing age. Br. J. Nutr. 2006, 96, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Bering, S.; Sjoltov, L.; Wrisberg, S.S.; Berggren, A.; Alenfall, J.; Jensen, M.; Hojgaard, L.; Tetens, I.; Bukhave, K. Viable, lyophilized lactobacilli do not increase iron absorption from a lactic acid-fermented meal in healthy young women, and no iron absorption occurs in the distal intestine. Br. J. Nutr. 2007, 98, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Onning, G.; Hulthen, L. Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females-Double isotope sequential single-blind studies in menstruating women. PLoS ONE 2017, 12, e0189141. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Onning, G.; Berggren, A.; Hulthen, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Neuhauser-Berthold, M.; et al. Lactobacillus plantarum 299v and an increase of non-haem iron absorption: Evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924/2006. Efsa J. 2016, 14, 4450. [Google Scholar]
- Pereira, D.I.; Mergler, B.I.; Faria, N.; Bruggraber, S.F.; Aslam, M.F.; Poots, L.K.; Prassmayer, L.; Lonnerdal, B.; Brown, A.P.; Powell, J.J. Caco-2 cell acquisition of dietary iron (III) invokes a nanoparticulate endocytic pathway. PLoS ONE 2013, 8, E81250. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Hentze, M.W. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: Implications for regulation and cellular function. Proc. Natl. Acad. Sci. USA 2002, 99, 12345–12350. [Google Scholar] [CrossRef]
- Tandy, S.; Williams, M.; Leggett, A.; Lopez-Jimenez, M.; Dedes, M.; Ramesh, B.; Srai, S.K.; Sharp, P. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J. Biol. Chem. 2000, 275, 1023–1029. [Google Scholar] [CrossRef]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Scheers, N.; Rossander-Hulthen, L.; Torsdottir, I.; Sandberg, A.S. Increased iron bioavailability from lactic-fermented vegetables is likely an effect of promoting the formation of ferric iron (Fe(3+)). Eur. J. Nutr. 2016, 55, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Scheers, N.; Andlid, T.; Alminger, M.; Sandberg, A.S. Determination of Fe2+ and Fe3+ in aqueous solutions containing food chelators by differential pulse anodic stripping voltammetry. Electroanalysis 2010, 22, 1090–1096. [Google Scholar] [CrossRef]
- Conrad, M.E.; Umbreit, J.N.; Moore, E.G. A role for mucin in the absorption of inorganic iron and other metal-cations—A study in rats. Gastroenterology 1991, 100, 129–136. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E-coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Mahler, G.J.; Shuler, M.L.; Glahn, R.P. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J. Nutr. Biochem. 2009, 20, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Navabi, N.; McGuckin, M.A.; Linden, S.K. Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS ONE 2013, 8, e68761. [Google Scholar] [CrossRef] [PubMed]
- Scheers, N.M.; Sandberg, A.S. Ascorbic acid uptake affects ferritin, Dcytb and Nramp2 expression in Caco-2 cells. Eur. J. Nutr. 2008, 47, 401–408. [Google Scholar] [CrossRef]
- Barnett, A.M.; Roy, N.C.; Cookson, A.L.; McNabb, W.C. Metabolism of caprine milk carbohydrates by probiotic bacteria and Caco-2:HT29(-)MTX epithelial co-cultures and their impact on intestinal barrier integrity. Nutrients 2018, 10, 949. [Google Scholar] [CrossRef]
- Gonzalez, A.; Galvez, N.; Martin, J.; Reyes, F.; Perez-Victoria, I.; Dominguez-Vera, J.M. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chem. 2017, 228, 374–380. [Google Scholar] [CrossRef]
- Constante, M.; Fragoso, G.; Lupien-Meilleur, J.; Calve, A.; Santos, M.M. Iron supplements modulate colon microbiota composition and dextran sodium sulfate-induced colitis. Am. J. Hematol. 2017, 23, 753–766. [Google Scholar]
- Asard, H.; Barbaro, R.; Trost, P.; Berczi, A. Cytochromes b561: Ascorbate-mediated trans-membrane electron transport. Antioxid. Redox Signal. 2013, 19, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Starr, C.N.; DiRenzo, C.; Fleming, M.D.; Jin, J.; Greer, E.L.; Sellers, V.M.; Galica, S.M.; Andrews, N.C. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood 2005, 106, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
| Composition | Oat Drink Lp299v | Oat Drink Control | MangoLp299v | MangoControl | Capsule Lp299v | Capsule Control |
|---|---|---|---|---|---|---|
| L. plantarum 299v (CFU) a | 7 × 108 | nd | 1 × 109 | nd | 1 × 1010 | nd |
| Iron (mg) b | 4.1 | 4.0 | 4.0 | 4.1 | 4.2 | 4.2 |
| Ascorbic acid (mg) c | 12.1 | 15.8 | 7.5 | 7.8 | 12 | 12 |
| Folic acid (µg) | - | - | - | - | 30 | 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandberg, A.-S.; Önning, G.; Engström, N.; Scheers, N. Iron Supplements Containing Lactobacillus plantarum 299v Increase Ferric Iron and Up-regulate the Ferric Reductase DCYTB in Human Caco-2/HT29 MTX Co-Cultures. Nutrients 2018, 10, 1949. https://doi.org/10.3390/nu10121949
Sandberg A-S, Önning G, Engström N, Scheers N. Iron Supplements Containing Lactobacillus plantarum 299v Increase Ferric Iron and Up-regulate the Ferric Reductase DCYTB in Human Caco-2/HT29 MTX Co-Cultures. Nutrients. 2018; 10(12):1949. https://doi.org/10.3390/nu10121949
Chicago/Turabian StyleSandberg, Ann-Sofie, Gunilla Önning, Niklas Engström, and Nathalie Scheers. 2018. "Iron Supplements Containing Lactobacillus plantarum 299v Increase Ferric Iron and Up-regulate the Ferric Reductase DCYTB in Human Caco-2/HT29 MTX Co-Cultures" Nutrients 10, no. 12: 1949. https://doi.org/10.3390/nu10121949
APA StyleSandberg, A.-S., Önning, G., Engström, N., & Scheers, N. (2018). Iron Supplements Containing Lactobacillus plantarum 299v Increase Ferric Iron and Up-regulate the Ferric Reductase DCYTB in Human Caco-2/HT29 MTX Co-Cultures. Nutrients, 10(12), 1949. https://doi.org/10.3390/nu10121949





