Modulating Sterol Concentrations in Infant Formula Influences Cholesterol Absorption and Synthesis in the Neonatal Piglet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Experiments
2.2. Determination of Cholesterol and Other Sterols in Plasma, Ileal Digesta and Liver Tissues
2.3. Determining the Titanium Dioxide Concentration in Diets and Ileal Digesta
2.4. Determination of Cholesterol in Ileum
2.5. Quantitative Real-Time RT-PCR
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Food Intake and Body Weight of Piglets Receiving Different Infant Formulas
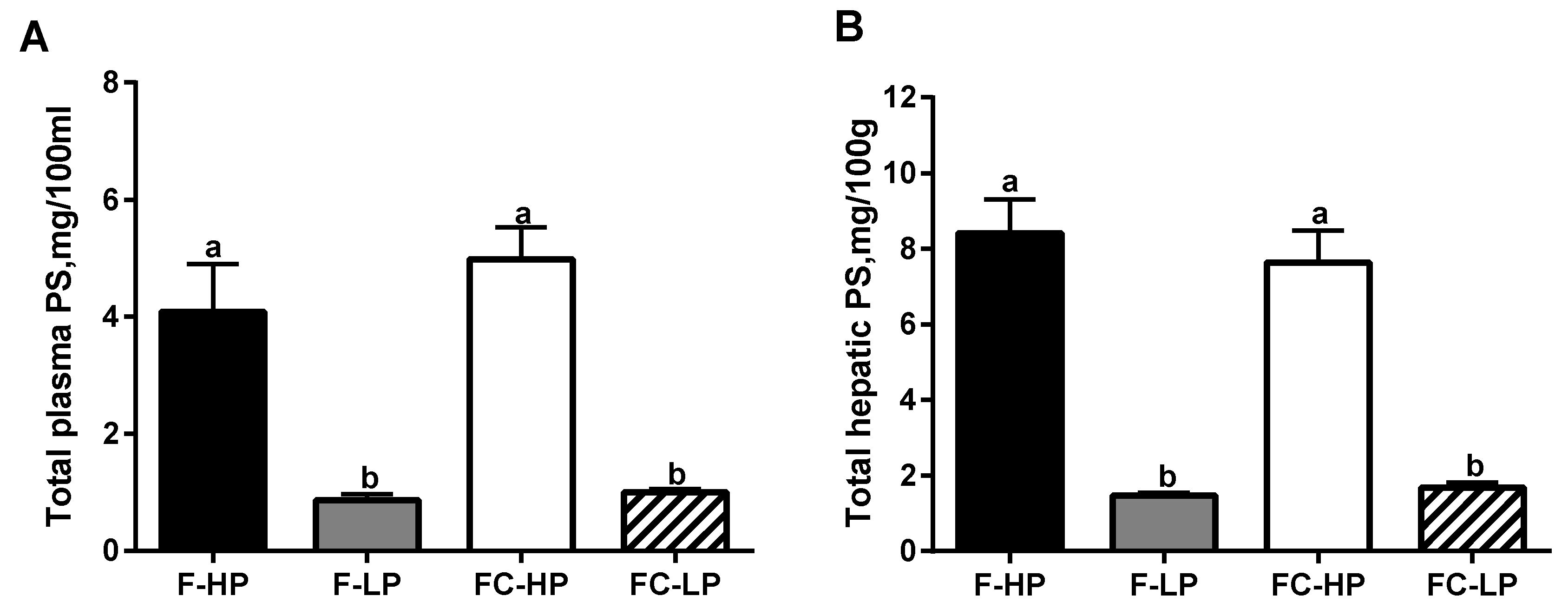
3.2. Plasma and Liver PS Levels
3.3. Plasma Total-Cholesterol and LDL-Cholesterol Levels
3.4. Apparent Ileal Cholesterol Digestibility and Apparent Ileal Digestible Cholesterol Content
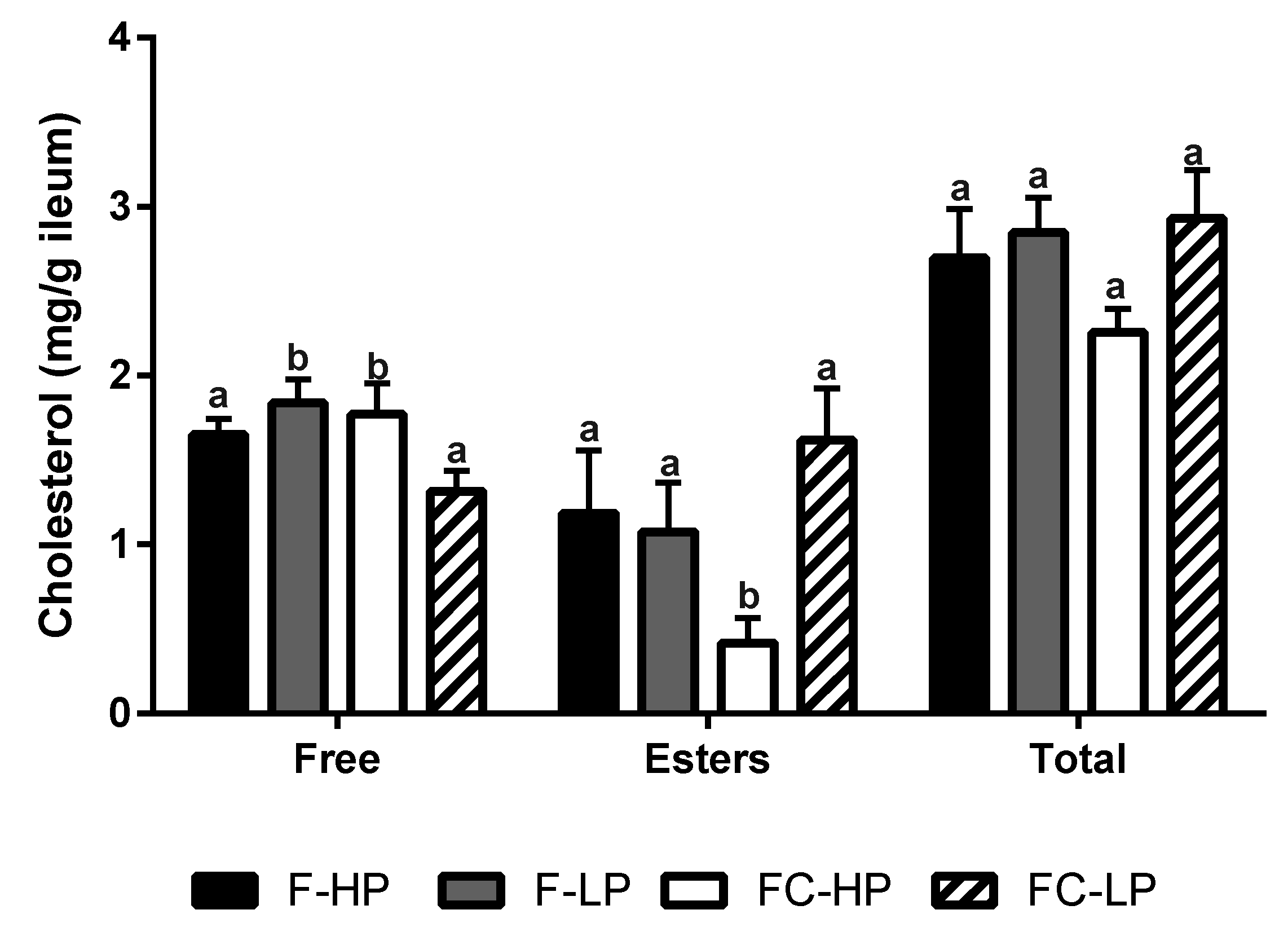
3.5. Cholesterol Synthesis and Transport in the Ileum
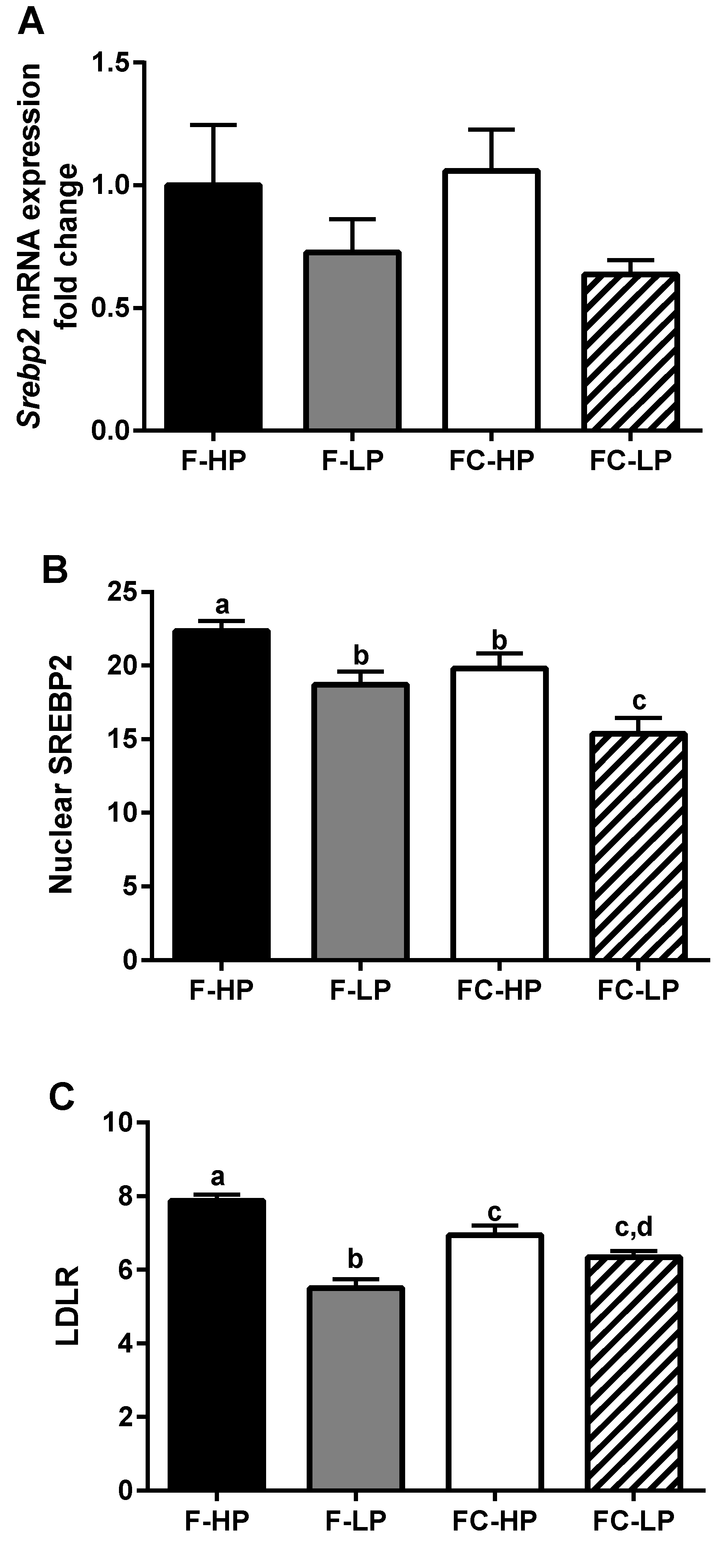
3.6. Hepatic Cholesterol Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maduko, C.O.; Park, Y.W. Modification of fatty acid and sterol composition of caprine milk for use as infant formula. Int. Dairy J. 2007, 17, 1434–1440. [Google Scholar] [CrossRef]
- Prosser, C.G.; Svetashev, V.I.; Vyssotski, M.V.; Lowry, D.J. Composition and distribution of fatty acids in triglycerides from goat infant formulas with milk fat. J. Dairy Sci. 2010, 93, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M.; Abbey, M.; Noakes, M.; Beltrame, S.; Rumbelow, N.; Nestel, P.J. Body fat distribution is a determinant of the high-density lipoprotein response to dietary fat and cholesterol in women. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M.; Nestel, P.J. Influence of gender, body mass index, and age on response of plasma lipids to dietary fat plus cholesterol. Arterioscler. Thromb. 1992, 12, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Mellies, M.; Glueck, C.J.; Sweeney, C.; Fallat, R.W.; Tsang, R.C.; Ishikawa, T.T. Plasma and dietary phytosterols in children. Pediatrics 1976, 57, 60–67. [Google Scholar] [PubMed]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Rodriguez-Palmero, M.; Demmelmair, H.; Fidler, N.; Jensen, R.; Sauerwald, T. Physiological aspects of human milk lipids. Early Hum. Dev. 2001, 65, S3–S8. [Google Scholar] [CrossRef]
- Wong, W.W.; Hachey, D.L.; Insull, W.; Opekun, A.R.; Klein, P.D. Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J. Lipid Res. 1993, 34, 1403–1411. [Google Scholar] [PubMed]
- Bayley, T.M.; Alasmi, M.; Thorkelson, T.; Krug-Wispe, S.; Jones, P.J.; Bulani, J.L.; Tsang, R.C. Influence of formula versus breast milk on cholesterol synthesis rates in four-month-old infants. Pediatr. Res. 1998, 44, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.L.; Wong, W.W.; Mimouni, F.; Hachey, D.L.; Setchell, K.D.; Klein, P.D.; Tsang, R.C. Effects of infant nutrition on cholesterol synthesis rates. Pediatr. Res. 1994, 35, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Demmers, T.A.; Jones, P.J.; Wang, J.; Krug, S.; Creutzinger, V.; Heubi, J.E. Effects of early cholesterol intake on cholesterol biosynthesis and plasma lipids among infants until 18 months of age. Pediatrics 2005, 115, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, J.L.; Gardner, J.M. Sterol content of foods of plant origin. J. Am. Diet. Assoc. 1978, 73, 39–47. [Google Scholar] [PubMed]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.; Lam, M.H.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1l1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Ostlund, J. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Takada, T.; Suzuki, H. Niemann-Pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and beta-sitosterol uptake in CaCo-2 cells. J. Pharmacol. Exp. Ther. 2007, 320, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Salen, G.; von Bergmann, K.; Lutjohann, D.; Kwiterovich, P.; Kane, J.; Patel, S.B.; Musliner, T.; Stein, P.; Musser, B. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation 2004, 109, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.E. Balancing cholesterol synthesis and absorption in the gastrointestinal tract. J. Clin. Lipidol. 2008, 2, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Harit, D.; Faridi, M.M.; Aggarwal, A.; Sharma, S.B. Lipid profile of term infants on exclusive breastfeeding and mixed feeding: A comparative study. Eur. J. Clin. Nutr. 2008, 62, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Darragh, A.J.; Moughan, P.J. The three-week-old piglet as a model animal for studying protein digestion in human infants. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Raeini-Sarjaz, M.; Ntanios, F.Y.; Vanstone, C.A.; Feng, J.Y.; Parsons, W.E. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J. Lipid Res. 2000, 41, 697–705. [Google Scholar] [PubMed]
- Short, F.J.; Gorton, P.; Wiseman, J.; Boorman, K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Mercer, K.E.; Bhattacharyya, S.; Diaz-Rubio, M.E.; Piccolo, B.D.; Pack, L.M.; Sharma, N.; Chaudhury, M.; Cleves, M.A.; Chintapalli, S.V.; Shankar, K.; et al. Infant Formula Feeding Increases Hepatic Cholesterol 7alpha Hydroxylase (CYP7A1) Expression and Fecal Bile Acid Loss in Neonatal Piglets. J. Nutr. 2018, 148, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Whincup, P.H.; Cook, D.G. Breast-feeding and cardiovascular risk factors and outcomes in later life: Evidence from epidemiological studies. Proc. Nutr. Soc. 2011, 70, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Bayley, T.M.; Alasmi, M.; Thorkelson, T.; Jones, P.J.; Corcoran, J.; Krug-Wispe, S.; Tsang, R.C. Longer term effects of early dietary cholesterol level on synthesis and circulating cholesterol concentrations in human infants. Metabolism 2002, 51, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Claumarchirant, L.; Matencio, E.; Sanchez-Siles, L.M.; Alegria, A.; Lagarda, M.J. Sterol Composition in Infant Formulas and Estimated Intake. J. Agric. Food Chem. 2015, 63, 7245–7251. [Google Scholar] [CrossRef] [PubMed]
- Le Huerou-Luron, I.; Blat, S.; Boudry, G. Breast- v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Whincup, P.H.; Kaye, S.J.; Martin, R.M.; Davey Smith, G.; Cook, D.G.; Bergstrom, E.; Black, S.; Wadsworth, M.E.; Fall, C.H.; et al. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am. J. Clin. Nutr. 2008, 88, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Reiser, R.; O’Brien, B.C.; Henderson, G.R.; Moore, R.W. Studies on a Possible Function for Cholesterol in Milk 1979. Available online: http://agris.fao.org/agris-search/search.do?recordID=US7939165 (accessed on 1 November 2018).
- Bosner, M.S.; Lange, L.G.; Stenson, W.F.; Ostlund, R.E., Jr. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 1999, 40, 302–308. [Google Scholar] [PubMed]
- Miettinen, T.A.; Vuoristo, M.; Nissinen, M.; Jarvinen, H.J.; Gylling, H. Serum, biliary, and fecal cholesterol and plant sterols in colectomized patients before and during consumption of stanol ester margarine. Am. J. Clin. Nutr. 2000, 71, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Sehayek, E.; Nath, C.; Heinemann, T.; McGee, M.; Seidman, C.E.; Samuel, P.; Breslow, J.L. U-shape relationship between change in dietary cholesterol absorption and plasma lipoprotein responsiveness and evidence for extreme interindividual variation in dietary cholesterol absorption in humans. J. Lipid Res. 1998, 39, 2415–2422. [Google Scholar] [PubMed]
- Sudhop, T.; Lutjohann, D.; Kodal, A.; Igel, M.; Tribble, D.L.; Shah, S.; Perevozskaya, I.; von Bergmann, K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 2002, 106, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Degirolamo, C.; Sabba, C.; Moschetta, A. Intestinal nuclear receptors in HDL cholesterol metabolism. J. Lipid Res. 2015, 56, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Rudel, L.L.; Lee, R.G.; Cockman, T.L. Acyl coenzyme A: Cholesterol acyltransferase types 1 and 2: Structure and function in atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Willner, E.L.; Tow, B.; Buhman, K.K.; Wilson, M.; Sanan, D.A.; Rudel, L.L.; Farese, R.V., Jr. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Buhman, K.K.; Accad, M.; Novak, S.; Choi, R.S.; Wong, J.S.; Hamilton, R.L.; Turley, S.; Farese, R.V., Jr. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 2000, 6, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Buhman, K.K.; Farese, R.V., Jr.; Dietschy, J.M.; Turley, S.D. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: Impact on hepatic cholesterol homeostasis. Hepatology 2004, 40, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Ikonen, E.; Olkkonen, V.M. Cholesterol precursors: More than mere markers of biosynthesis. Curr. Opin. Lipidol. 2014, 25, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Dimova, L.G.; de Boer, J.F.; Plantinga, J.; Plosch, T.; Hoekstra, M.; Verkade, H.J.; Tietge, U.J.F. Inhibiting Cholesterol Absorption During Lactation Programs Future Intestinal Absorption of Cholesterol in Adult Mice. Gastroenterology 2017, 153, 382–385.e3. [Google Scholar] [CrossRef] [PubMed]

| Formula | ||||
|---|---|---|---|---|
| F-HP | F-LP | FC-HP | FC-LP | |
| Energy, kCal | 513 | 512 | 514 | 512 |
| Protein, g | 12 | 12 | 11.9 | 11.9 |
| Fat, g | 26.6 | 26.5 | 26.7 | 26.5 |
| Carbohydrate, g | 56.5 | 56.3 | 56.4 | 56.4 |
| Sterols | ||||
| Cholesterol, mg | 22.5 | 24.2 | 85.6 | 84.2 |
| PS, mg | 79.2 | 9.5 | 79.1 | 9.9 |
| Minerals | ||||
| Calcium, mg | 381 | 395 | 375 | 380 |
| Phosphorus, mg | 284 | 302 | 296 | 297 |
| Potassium, mg | 491 | 515 | 489 | 488 |
| Sodium, mg | 147 | 152 | 152 | 144 |
| Chloride, mg | 360 | 360 | 360 | 360 |
| Iron, mg | 5.87 | 6.31 | 5.76 | 5.7 |
| Magnesium, mg | 43.7 | 46.1 | 44.8 | 46 |
| Manganese, µg | 316 | 321 | 309 | 310 |
| Zinc, mg | 5.49 | 5.54 | 5.45 | 5.55 |
| Copper, µg | 438 | 399 | 412 | 439 |
| Iodine, µg | 130 | 126 | 124 | 116 |
| Selenium, µg | 25.4 | 26 | 26.2 | 23.4 |
| Vitamins | ||||
| Vitamin A, µg | 699.4 | 708 | 743 | 685.5 |
| Vitamin D, µg | 8.1 | 8.8 | 8.3 | 8.4 |
| Vitamin E, mg | 12.4 | 17.2 | 13 | 16.5 |
| Vitamin K, µg | 38 | 33.4 | 39 | 41.3 |
| Thiamine, µg | 790 | 790 | 790 | 810 |
| Vitamin B2, µg | 1300 | 1300 | 1300 | 1300 |
| Pyridoxine, µg | 650 | 690 | 680 | 660 |
| Vitamin B12, µg | 1.9 | 1.9 | 1.9 | 1.9 |
| Niacin, µg | 5000 | 5000 | 5000 | 5000 |
| Folic acid, µg | 121 | 130 | 126 | 129 |
| Pantothenic acid, µg | 4800 | 4800 | 4000 | 4400 |
| Biotin, µg | 27.5 | 27.5 | 28.1 | 28.4 |
| Vitamin C, mg | 89.4 | 89.4 | 84.2 | 25.2 |
| Choline, mg | 145.6 | 147.6 | 144.9 | 143.1 |
| Taurine, mg | 49 | 49 | 49 | 50 |
| Formula | F-HP | F-LP | FC-HP | FC-LP | Overall | Overall | Overall |
|---|---|---|---|---|---|---|---|
| SE | p-Value | Significance | |||||
| Daily dry matter consumption (g) | |||||||
| Week 1 | 129 | 133 | 129 | 133 | 8.2 | 0.962 | NS |
| Week 2 | 155 | 162 | 152 | 161 | 9.6 | 0.864 | NS |
| Week 3 | 191 | 201 | 188 | 198 | 11.8 | 0.857 | NS |
| % consumed | 97 | 98 | 96 | 98 | 0.8 | 0.229 | NS |
| Daily body weight gain (g) | |||||||
| Week 1 | 85 | 96 | 78 | 91 | 5.3 | 0.141 | NS |
| Week 2 | 120 | 128 | 118 | 122 | 8.2 | 0.839 | NS |
| Week 3 | 145 | 150 | 128 | 149 | 9.2 | 0.371 | NS |
| Weeks 1–3 | 117 | 124 | 108 | 121 | 7.2 | 0.439 | NS |
| Formula | F-HP | F-LP | FC-HP | FC-LP | Overall | Overall | Overall |
|---|---|---|---|---|---|---|---|
| SE | p-Value | Significance | |||||
| Apparent ileal digestibility (% from formula) | 31.8 | 66.3 | 73.0 | 85.0 | 4.87 | <0.001 | S |
| Apparent ileal digestibility content (g/kg formula) | 69.8 | 160.4 | 625.0 | 710.0 | 26.2 | <0.001 | S |
| Formula | Plasma | Liver | ||
|---|---|---|---|---|
| D:C ratio | L:C ratio | D:C ratio | L:C ratio | |
| F-HP | 0.25 ± 0.03 a | 0.14 ± 0.02 a | 0.42 ± 0.03 a | 0.42 ± 0.03 a |
| F-LP | 0.19 ± 0.02 a | 0.12 ± 0.01 a | 0.18 ± 0.01 b | 0.37 ± 0.04 a,b |
| FC-HP | 0.22 ± 0.02 a | 0.10 ± 0.01 a | 0.37 ± 0.02 a | 0.24 ± 0.02 b |
| FC-LP | 0.14 ± 0.01 b | 0.07 ± 0.01 b | 0.19 ± 0.01 b | 0.20 ± 0.01 b,c |
| p Values | ||||
| PS | 0.010 | 0.199 | <0.001 | 0.057 |
| Cholesterol | 0.106 | 0.031 | 0.568 | <0.001 |
| Interaction | 0.682 | 0.241 | 0.434 | 0.499 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babawale, E.A.; Jones, P.J.; Mercer, K.E.; Lin, H.; Yeruva, L.; Bar Yoseph, F.; Rutherfurd, S.M. Modulating Sterol Concentrations in Infant Formula Influences Cholesterol Absorption and Synthesis in the Neonatal Piglet. Nutrients 2018, 10, 1848. https://doi.org/10.3390/nu10121848
Babawale EA, Jones PJ, Mercer KE, Lin H, Yeruva L, Bar Yoseph F, Rutherfurd SM. Modulating Sterol Concentrations in Infant Formula Influences Cholesterol Absorption and Synthesis in the Neonatal Piglet. Nutrients. 2018; 10(12):1848. https://doi.org/10.3390/nu10121848
Chicago/Turabian StyleBabawale, Elizabeth A, Peter JH Jones, Kelly E Mercer, Haixia Lin, Laxmi Yeruva, Fabiana Bar Yoseph, and Shane M Rutherfurd. 2018. "Modulating Sterol Concentrations in Infant Formula Influences Cholesterol Absorption and Synthesis in the Neonatal Piglet" Nutrients 10, no. 12: 1848. https://doi.org/10.3390/nu10121848
APA StyleBabawale, E. A., Jones, P. J., Mercer, K. E., Lin, H., Yeruva, L., Bar Yoseph, F., & Rutherfurd, S. M. (2018). Modulating Sterol Concentrations in Infant Formula Influences Cholesterol Absorption and Synthesis in the Neonatal Piglet. Nutrients, 10(12), 1848. https://doi.org/10.3390/nu10121848




