A Calcium-Deficient Diet in Dams during Gestation Increases Insulin Resistance in Male Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procedures
2.2. Serum Measurements
2.3. Serum Adipokine Levels
2.4. Serum Ionized Calcium and Magnesium Levels
2.5. Statistical Analyses
3. Results
3.1. HOMA-IR and Serum Adipokine Levels
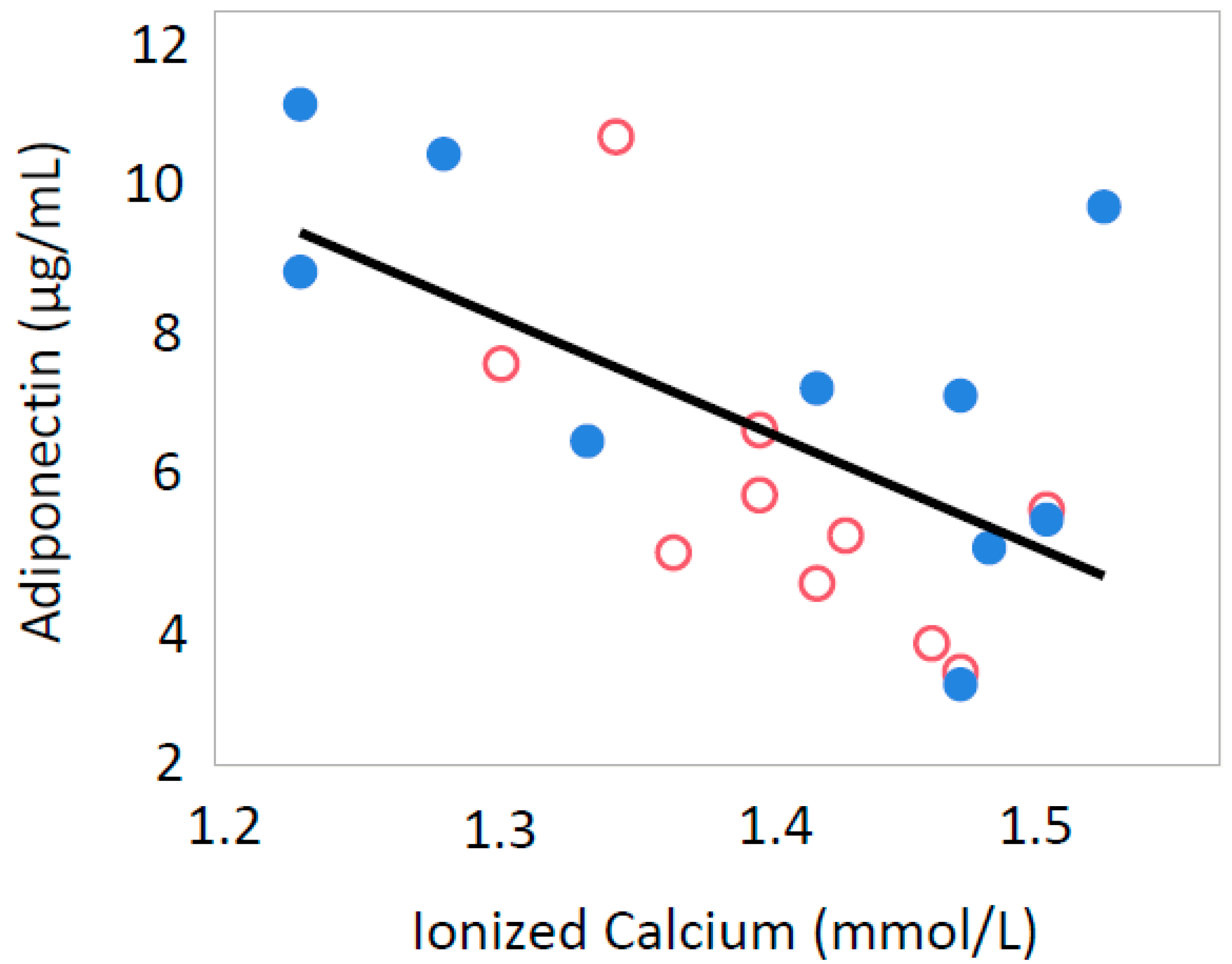
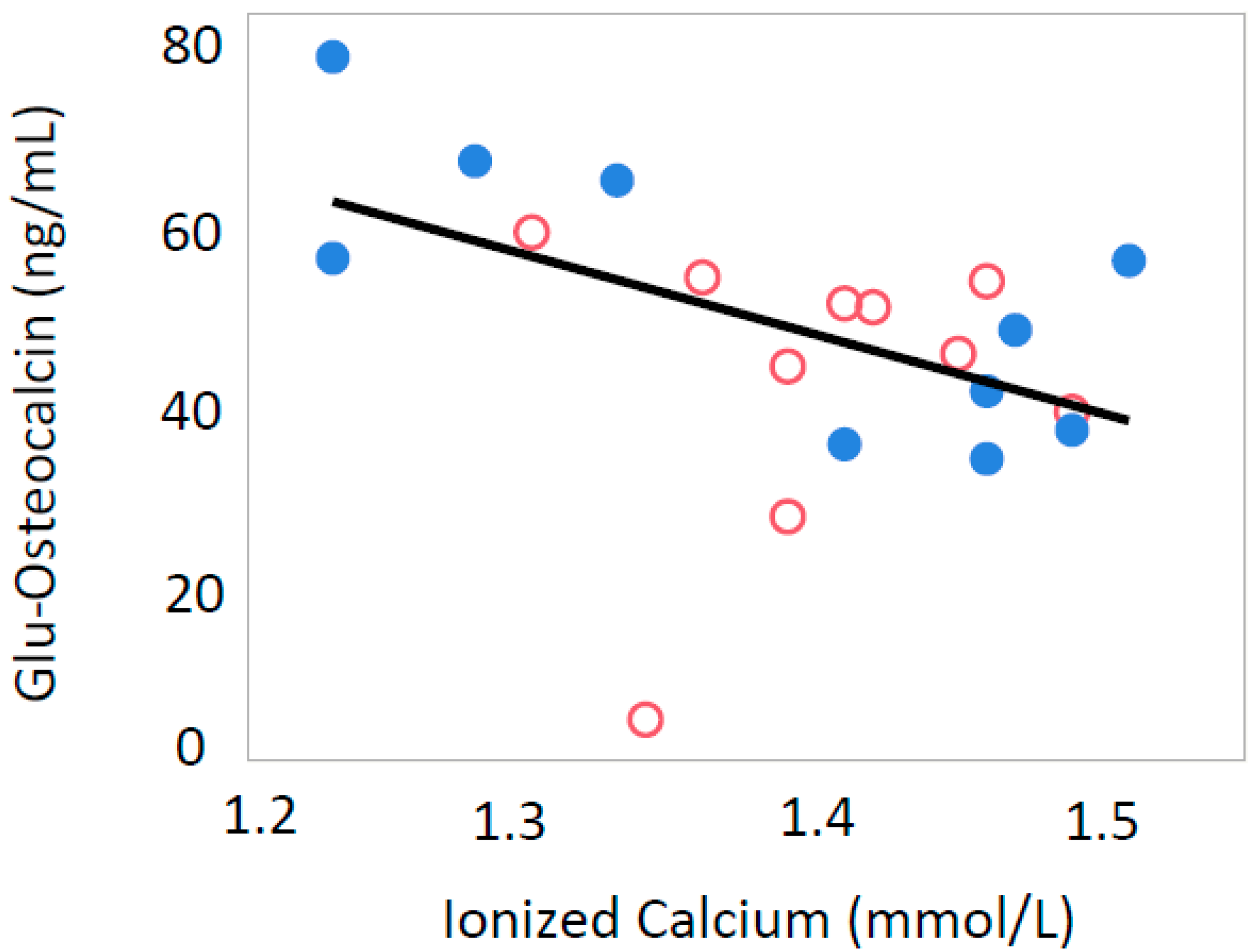
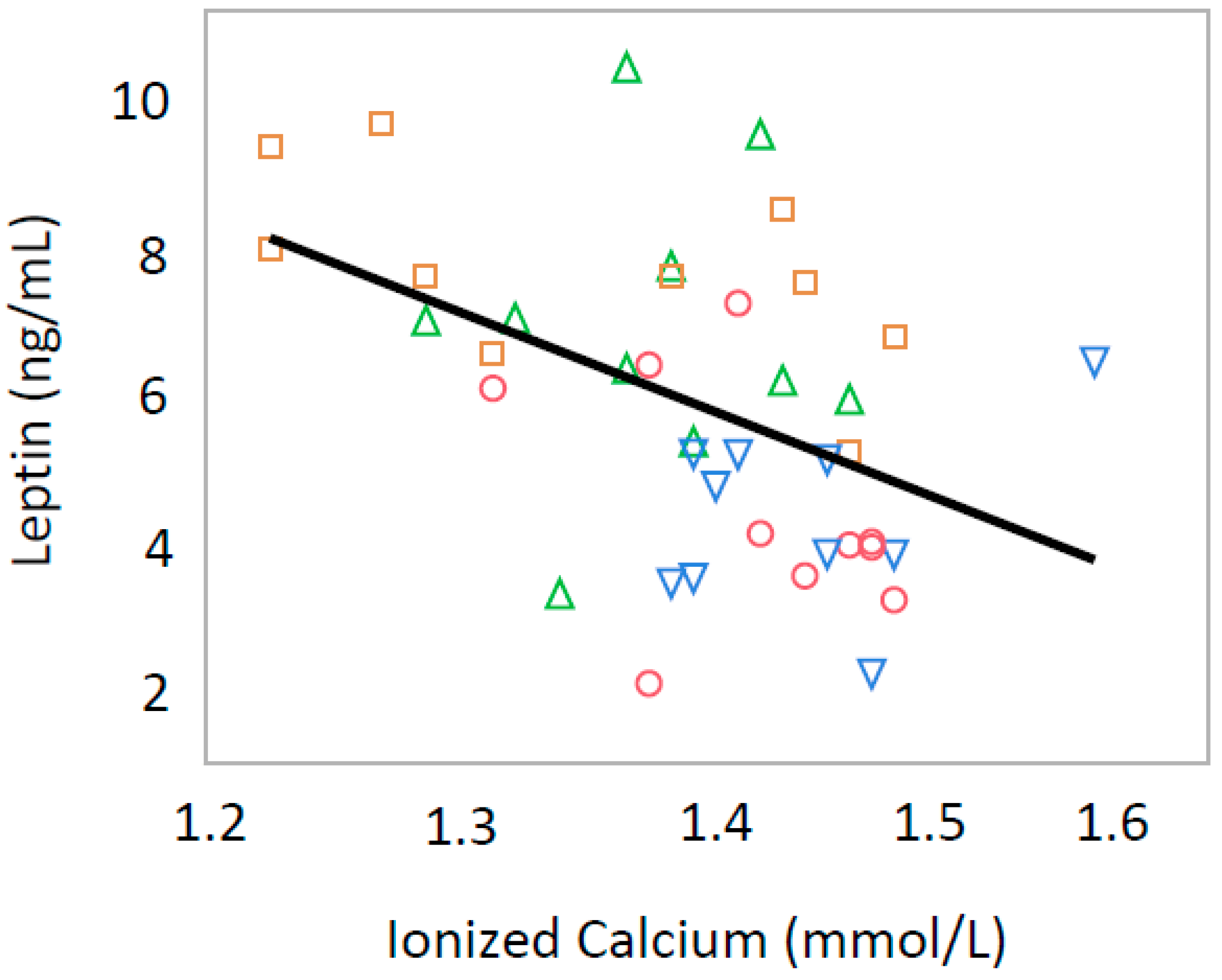
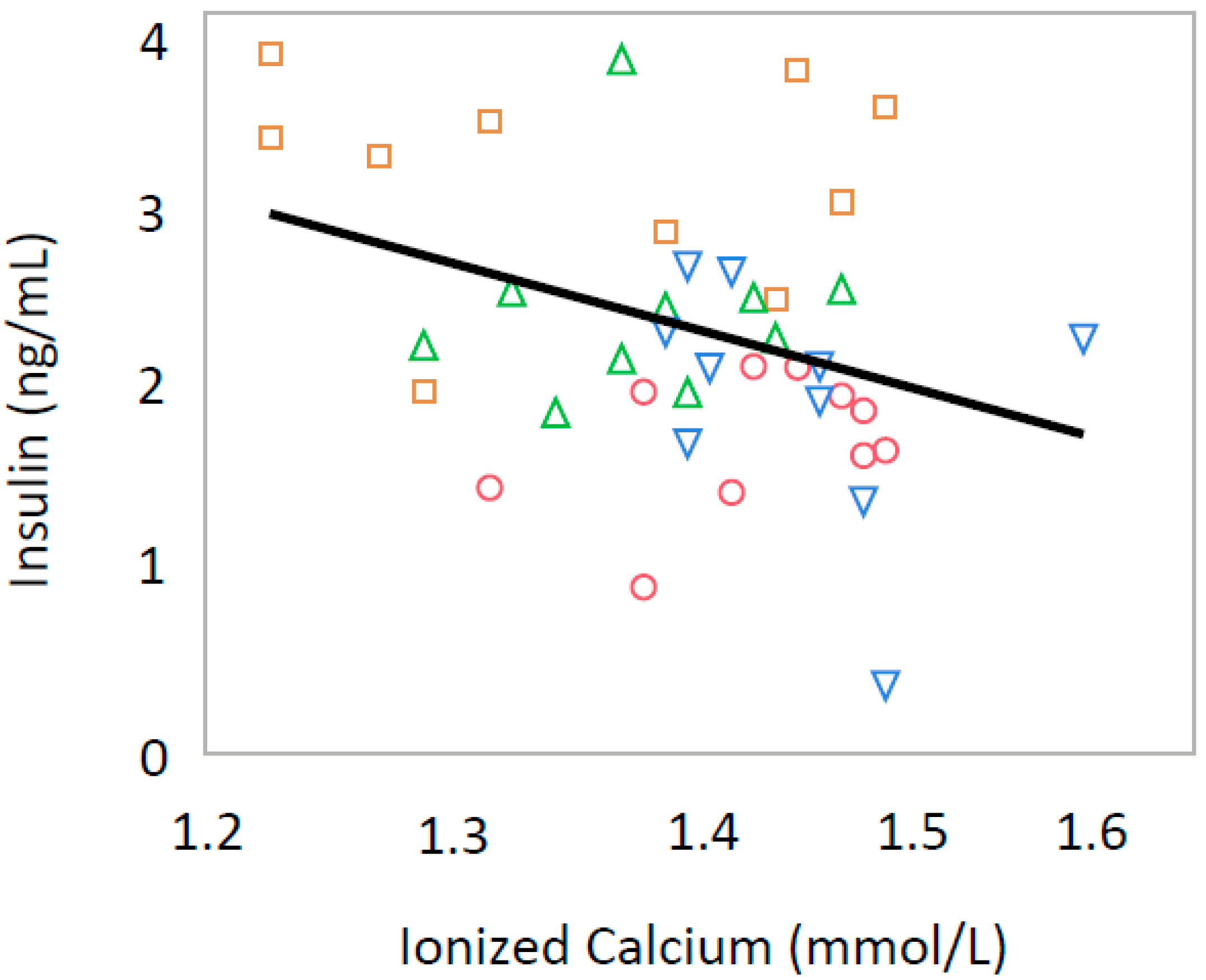
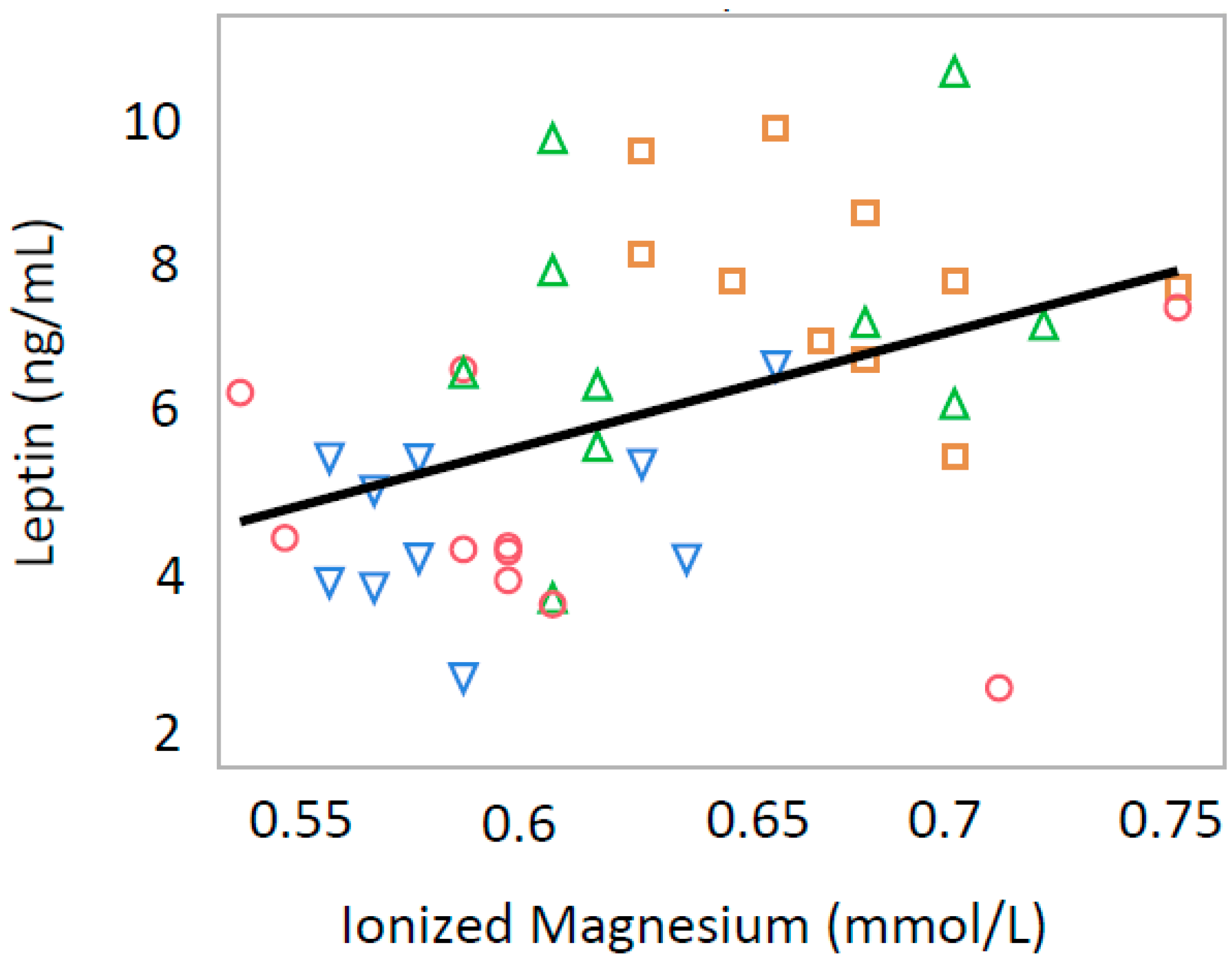
3.2. Ionized Calcium
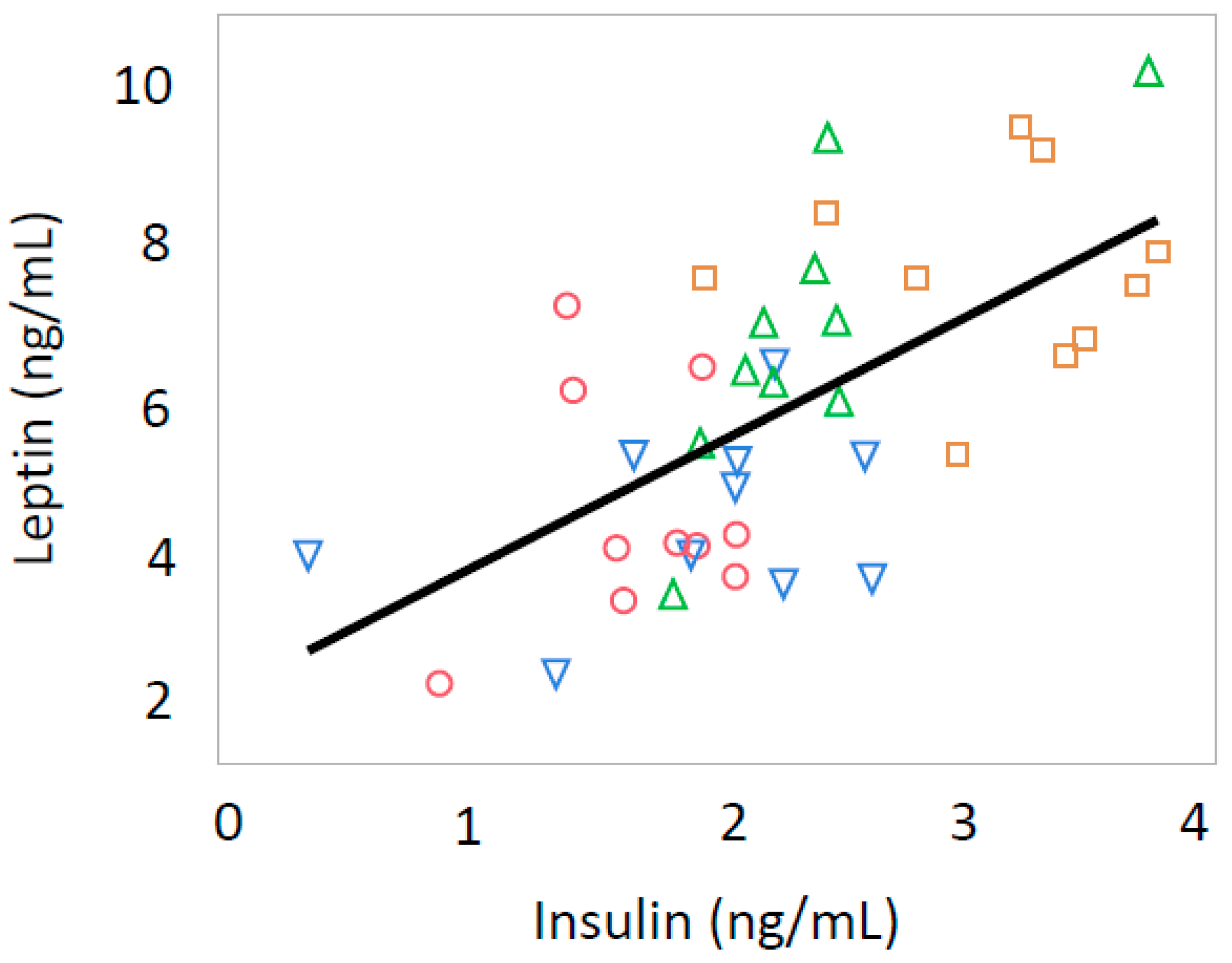
3.3. Leptin and Insulin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kiekwood, T.; Lahret, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Silva, N.; Frantz, E.D.; Mandarim-de-Lacerda, C.A.; Pinheiro-Mulder, A. Maternal protein restriction in mice causes adverse metabolic and hypothalamic effects in the F1 and F2 generations. Br. J. Nutr. 2011, 106, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Valdez, R.; Athens, M.A.; Thompson, G.H.; Bradshaw, B.S.; Stem, M.P. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia 1994, 37, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.J.; Ozanne, S.E. Mechanisms involved in the developmental programming of adulthood disease. Biochem. J. 2010, 427, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Byrne, C.D.; Zhang, J.; Petry, C.J.; Lucas, A.; Hales, C.N. Programming of hepatic insulin-sensitive enzymes in offspring of rat dams fed a protein-restricted diet. Am. J. Physiol. 1997, 272, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Drouillet, P.; Balkau, B.; Charies, M.A.; Vol, S.; Bedouel, M.; Ducimetiere, P. Calcium consumption and insulin resistance syndrome parameters. Data from the epidemiological study on the insulin resistance syndrome (DESIR). Nutr. Metab. Cardiovasc. Dis. 2007, 17, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.; Zemel, P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000, 14, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Iharada, A.; Okihana, H.; Kaneko, K. A calcium-deficient diet in pregnant, nursing rats induces hypomethylation of specific cytosines in the 11β-hydroxysteroid dehydrogenase-1 promoter in pup liver. Nutr. Res. 2013, 33, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Cheng, J. The role of calcium-dependent gene expression in autism spectrum disorders: Lessons from MeCP2, Ube3a and beyond. Neurosignals 2010, 18, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Volkov, P.; Dayeh, T.; Esguerra, J.L.; Salö, S.; Eliasson, L.; Rönn, T.; Bacos, K.; Ling, C. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome. Biol. 2014, 15, 522. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Dalla Man, C.; Campioni, M.; Basu, A.; Klee, G.; Toffolo, G.; Cobelli, C.; Rizza, R.A. Effects of age and sex on postprandial glucose metabolism: Differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 2006, 55, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Brazzaie, A.R.; Moro, E.; Vrbikova, J.; Bendlova, B.; Sbrugnadello, S.; Tura, A.; Pacini, G. Influence of increasing BMI on insulin sensitivity and secretion in normotolerant men and women of a wide age span. Obesity (Silver Spring) 2012, 20, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, M.; Shinar, D.; Rodan, G.A. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J. Bone Miner. Res. 1990, 5, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Hodges, S.; Eastell, R. Measurement of osteocalcin. Ann. Clin. Biochem. 2000, 37, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Hinoi, E.; Karsenty, G.; Ducy, P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA 2008, 105, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; McKee, M.D.; Levine, R.L.; Ducy, P.; Karsenty, G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 2012, 50, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; Mckee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.P.; Levy, J.C.; Morris, R.J.; Turner, R.C. Comparison of tests of beta-cell function across a range of glucose tolerance from normal to diabetes. Diabetes 1999, 48, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Iseri, L.T.; French, J.H. Magnesium: Nature’s physiologic calcium blocker. Am. Heart J. 1984, 108, 188–193. [Google Scholar] [CrossRef]
- Lillycrop, K.A.; Phillips, E.S.; Jackson, A.A.; Hanson, M.A.; Burdge, G.C. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J. Nutr. 2005, 135, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Yamanouchi, S.; Kaneko, K. A calcium-deficient diet in rat dams during gestation and nursing affects hepatic 11β-hydroxysteroid dehydrogenase-1 expression in the offspring. PLoS ONE 2014, 9, e84125. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Pickavance, L.C.; Brand, C.L.; Wassermann, K.; Wilding, J.P. The dual PPARalpha/gamma agonist, ragaglitazar, improves insulin sensitivity and metabolic profile equally with pioglitazone in diabetic and dietary obese ZDF rats. Br. J. Pharmacol. 2005, 144, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, Y.; Mizokami, A.; Kawakubo-Yasukochi, T.; Chishaki, S.; Takahashi, I.; Takeuchi, H.; Hirata, M. Long-term oral administration of osteocalcin induces insulin resistance in male mice fed a high-fat, high-sucrose diet. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E662–E675. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Lacombe, J. Regulation of energy metabolism by the skeleton: Osteocalcin and beyond. Arch. Biochem. Biophys. 2014, 561, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Yamaguchi, T.; Tada, Y.; Yamaguchi, M.; Yano, S.; Sugimoto, T. Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone 2011, 48, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Yamaguchi, T.; Yamamoto, M.; Yamauchi, M.; Kurioka, S.; Yano, S.; Sugimoto, T. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2009, 94, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Oosterwerff, M.M.; van Schoor, N.M.; Lips, P.; Eekhoff, E.M. Osteocalcin as a predictor of the metabolic syndrome in older persons: A population-based study. Clin. Endocrinol. 2013, 78, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lin, H.; Hu, Y.; Li, X.; He, W.; Jin, X.; Gao, J.; Zhao, N.; Gao, X. Serum levels of osteocalcin in relation to glucose metabolism and carotid atherosclerosis in Chinese middle-aged and elderly male adults: The Shanghai Changfeng Study. Eur. J. Intern. Med. 2014, 25, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Buday, B.; Pach, F.P.; Literati-Nagy, B.; Vitai, M.; Vecsei, Z.; Koranyi, L. Serum osteocalcin is associated with improved metabolic state via adiponectin in females versus testosterone in males. Gender specific nature of the bone-energy homeostasis axis. Bone 2013, 57, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Xu, B.; Su, J.; Pan, C.; Zhan, C.; Su, B.; Li, H.; Wang, J.; Sheng, H.; Qu, S. Differential pattern for regulating insulin secretion, insulin resistance, and lipid metabolism by osteocalcin in male and female T2DM patients. Med. Sci. Monit. 2014, 20, 711–719. [Google Scholar] [PubMed]
- Wang, Y.; Ali, Y.; Lim, C.Y.; Hong, W.; Pang, Z.P.; Han, W. Insulin-stimulated leptin secretion requires calcium and PI3K/Akt activation. Biochem. J. 2014, 458, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, A.; Parhar, R.; Saleh, S.; Al-Hijailan, R.; Inglis, A.; Al-Jufan, M.; Bazzi, M.; Hashmi, S.; Conca, W.; Collison, K.; et al. Intracellular calcium and NF-κB regulate hypoxia-induced leptin, VEGF, IL-6 and adiponectin secretion in human adipocytes. Life Sci. 2018, 212, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Havel, P.J.; Utzshneider, K.M.; Carr, D.B.; Sinha, M.K.; Boyko, E.J.; Retzlaff, B.M.; Knopp, R.H.; Brunzell, J.D.; Kahn, S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia 2003, 46, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Yamaguchi, T.; Yano, S.; Yamaguchi, M.; Yamamoto, M.; Sugimoto, T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kaskoao, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Sabek, O.M.; Fraga, D.; Minze, L.J.; Nishimoto, S.K.; Liu, J.Z.; Afshar, S.; Gaber, L.; Lyon, C.J.; Gaber, A.O.; et al. Osteocalcin protects against nonalcoholic steatohepatitis in a mouse model of metabolic syndrome. Endocrinology 2014, 155, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, L.; Luo, L.; Wu, P.; Yan, S. Osteocalcin Improves Metabolic Profiles, Body Composition and Arterial Stiffening in an Induced Diabetic Rat Model. Exp. Clin. Endocrinol. Diabetes 2017, 125, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Ditzel, N.; Laborda, J.; Karsenty, G.; Kassem, M. DLK1 Regulates Whole-Body Glucose Metabolism: A Negative Feedback Regulation of the Osteocalcin-Insulin Loop. Diabetes 2015, 64, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (%) | Control | Calcium Deficient |
|---|---|---|
| Milk casein | 24.50 | 24.50 |
| Corn starch | 45.50 | 45.50 |
| Granulated sugar | 10.00 | 10.00 |
| Corn oil | 6.00 | 6.00 |
| Cellulose powder | 5.00 | 5.00 |
| α-Starch | 1.00 | 1.00 |
| Vitamin mix | 1.00 | 1.00 |
| Mineral mix * | 6.10 | 7.00 |
| Calcium | 0.90 | 0.008 |
| Female | Male | |||
|---|---|---|---|---|
| Control | Low Calcium | Control | Low Calcium | |
| Body weight (g) | 258 ± 19 | 268 ± 39 | 418 ± 19 | 440 ± 44 |
| Heart rate (bpm) | 452 ± 29 | 417 ± 24 * | 435 ± 34 | 391 ± 35 ** |
| Systolic blood pressure (mmHg) | 115 ± 13 | 98 ± 11 ** | 119 ± 12 | 110 ± 15 |
| Glucose (mg/dL) | 144 ± 39 | 174 ± 55 | 166 ± 42 | 246 ± 120 ** |
| Insulin (ng/mL) | 1.69 ± 0.39 | 1.99 ± 0.69 | 2.21 ± 0.26 | 2.96 ± 0.65 ** |
| HOMA-IR | 0.62 ± 0.27 | 0.87 ± 0.39 | 0.91 ± 0.26 | 1.73 ± 0.96 ** |
| HOMA-β | 8.84 ± 3.91 | 7.50 ± 3.55 | 8.92 ± 3.50 | 7.52 ± 4.99 |
| Leptin (ng/mL) | 4.42 ± 1.65 | 4.31 ± 1.11 | 6.36 ± 1.90 | 7.45 ± 1.45 |
| Adiponectin (μg/mL) | 7.06 ± 0.92 | 7.83 ± 1.16 | 5.62 ± 1.90 | 7.03 ± 1.70 * |
| Gla-osteocalcin (ng/mL) | 72.9 ± 45.7 | 182 ± 105 ** | 127 ± 52 | 147 ± 66 |
| Glu-osteocalcin (ng/mL) | 19.6 ± 9.9 | 76.7 ± 48.4 ** | 41.4 ± 16.8 | 66.6 ± 28.3 |
| Ionized Calcium (mmol/L) | 1.44 ± 0.06 | 1.47 ± 0.06 | 1.40 ± 0.04 | 1.38 ± 0.10 |
| Ionized Magnesium (mmol/L) | 0.61 ± 0.07 | 0.59 ± 0.03 | 0.61 ± 0.03 | 0.67 ± 0.03 ** |
| Calcium/Magnesium | 2.41 ± 0.26 | 2.51 ± 0.08 | 2.21 ± 0.15 | 2.05 ± 0.13 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaya, J.; Yamanouchi, S.; Kino, J.; Tanabe, Y.; Kaneko, K. A Calcium-Deficient Diet in Dams during Gestation Increases Insulin Resistance in Male Offspring. Nutrients 2018, 10, 1745. https://doi.org/10.3390/nu10111745
Takaya J, Yamanouchi S, Kino J, Tanabe Y, Kaneko K. A Calcium-Deficient Diet in Dams during Gestation Increases Insulin Resistance in Male Offspring. Nutrients. 2018; 10(11):1745. https://doi.org/10.3390/nu10111745
Chicago/Turabian StyleTakaya, Junji, Sohsaku Yamanouchi, Jiro Kino, Yuko Tanabe, and Kazunari Kaneko. 2018. "A Calcium-Deficient Diet in Dams during Gestation Increases Insulin Resistance in Male Offspring" Nutrients 10, no. 11: 1745. https://doi.org/10.3390/nu10111745
APA StyleTakaya, J., Yamanouchi, S., Kino, J., Tanabe, Y., & Kaneko, K. (2018). A Calcium-Deficient Diet in Dams during Gestation Increases Insulin Resistance in Male Offspring. Nutrients, 10(11), 1745. https://doi.org/10.3390/nu10111745




