Bioavailability Study of Maqui Berry Extract in Healthy Subjects
Abstract
1. Introduction
2. Study Design and Methods
2.1. Participants
2.2. Study Design
2.3. Intervention
2.4. Sample Collection and Processing
2.5. Methods for Safety (Adverse Events, Concomitant and Tolerability)
2.6. Methods for Assessing Efficacy Variables–Analysis of DG, CS, GA and PCA
2.7. Analysis Software and Statistical Analysis
3. Results
3.1. Participant Characteristics
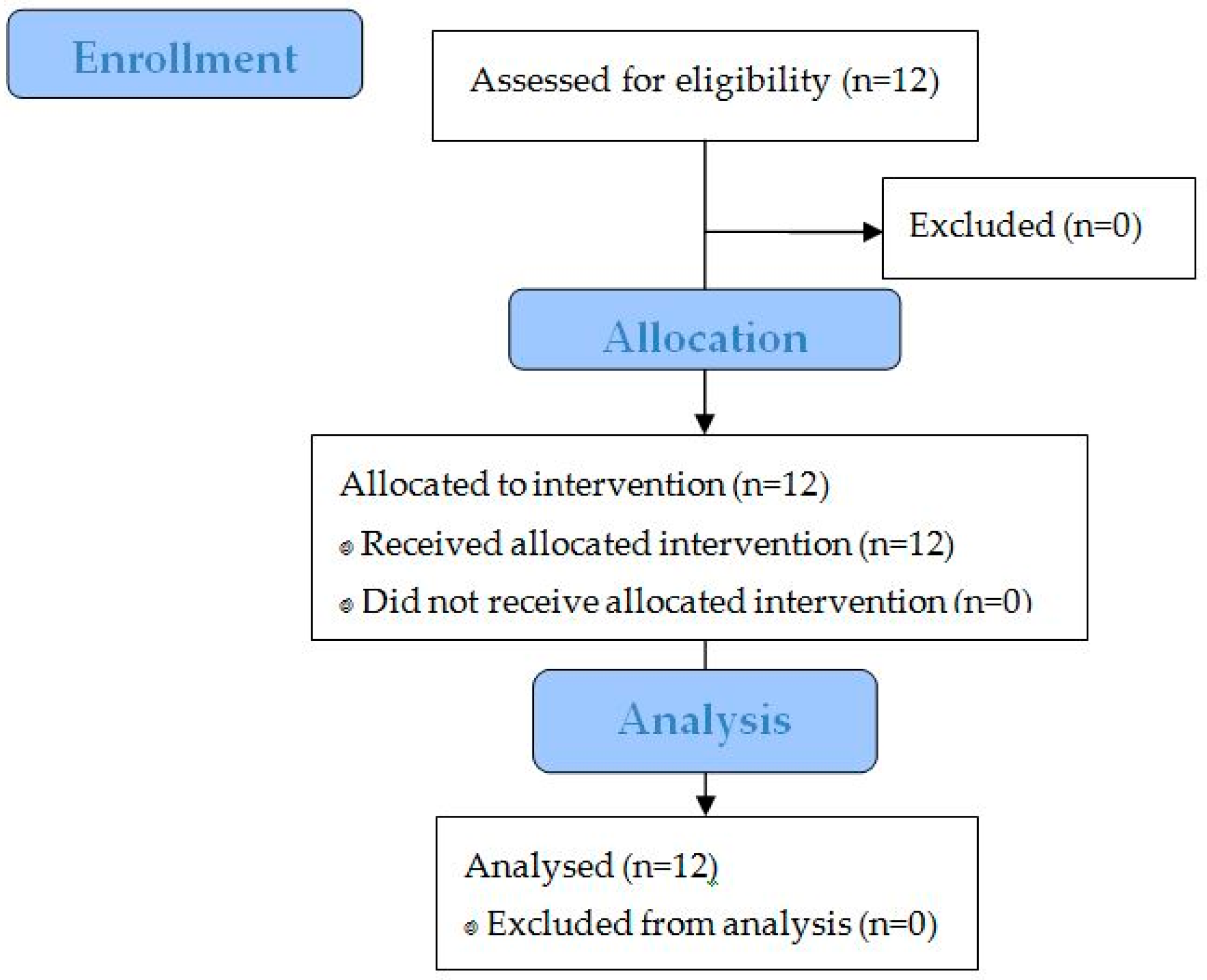
3.2. Study Performance
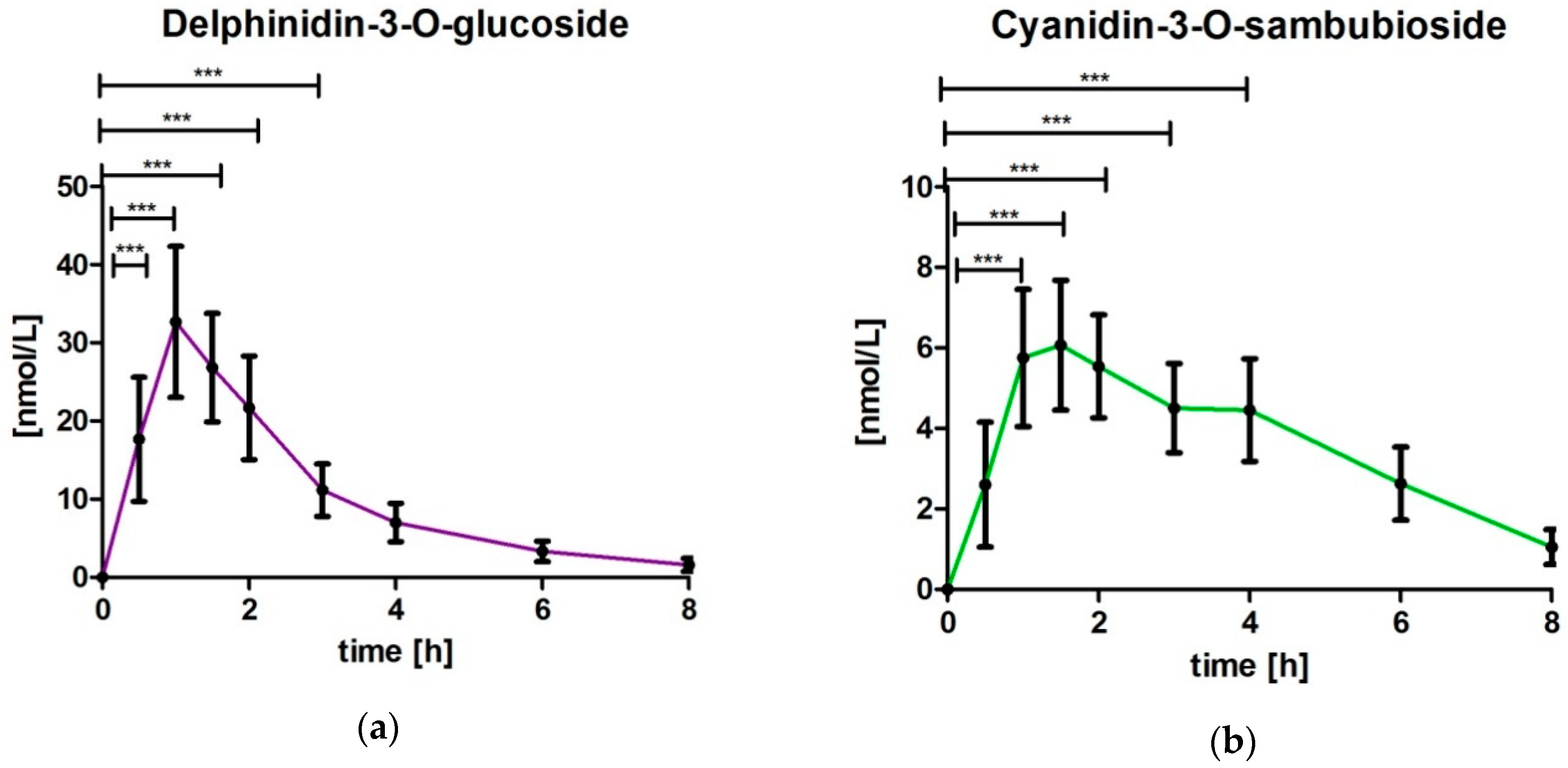
3.3. Analysis of Delphinidin-3-O-glucoside (DG) and Cyanidin-3-O-sambubioside (CS)
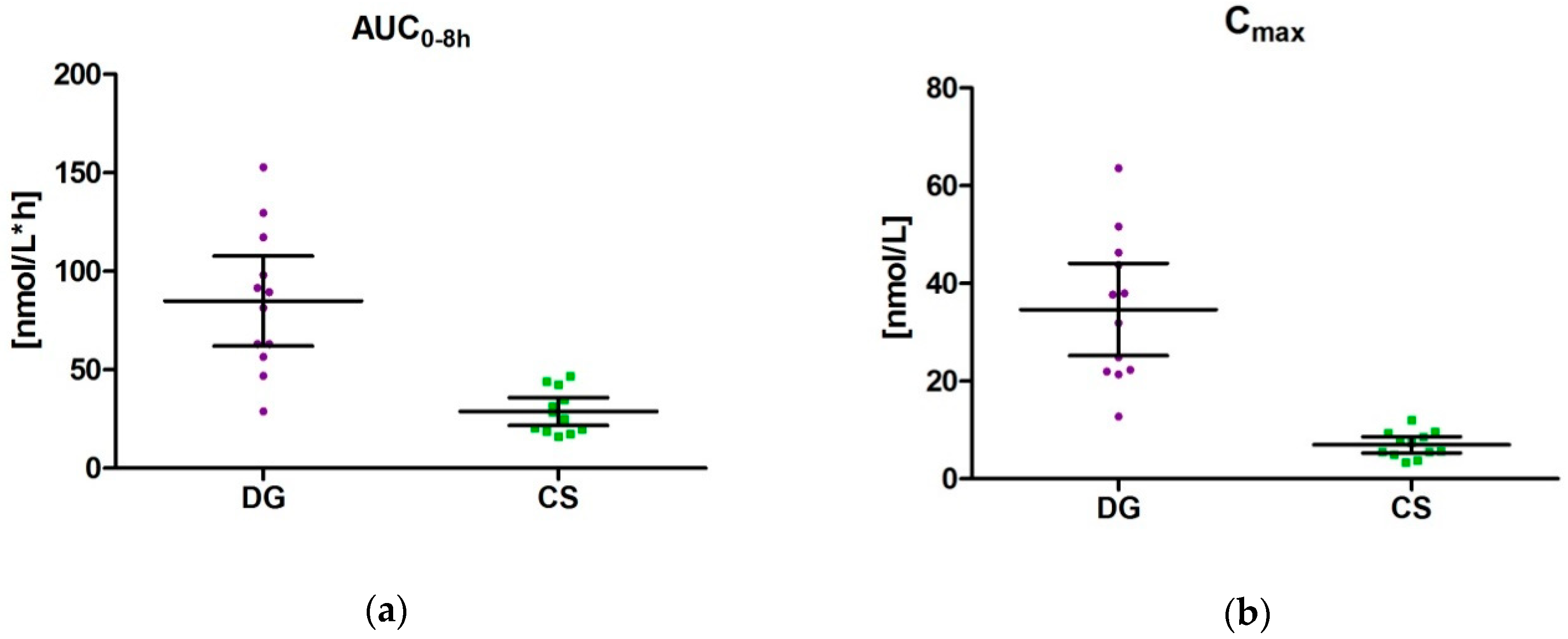
3.4. Pharmacokinetic Parameters (AUC0–8 h, Tmax und Cmax) for DG and CS
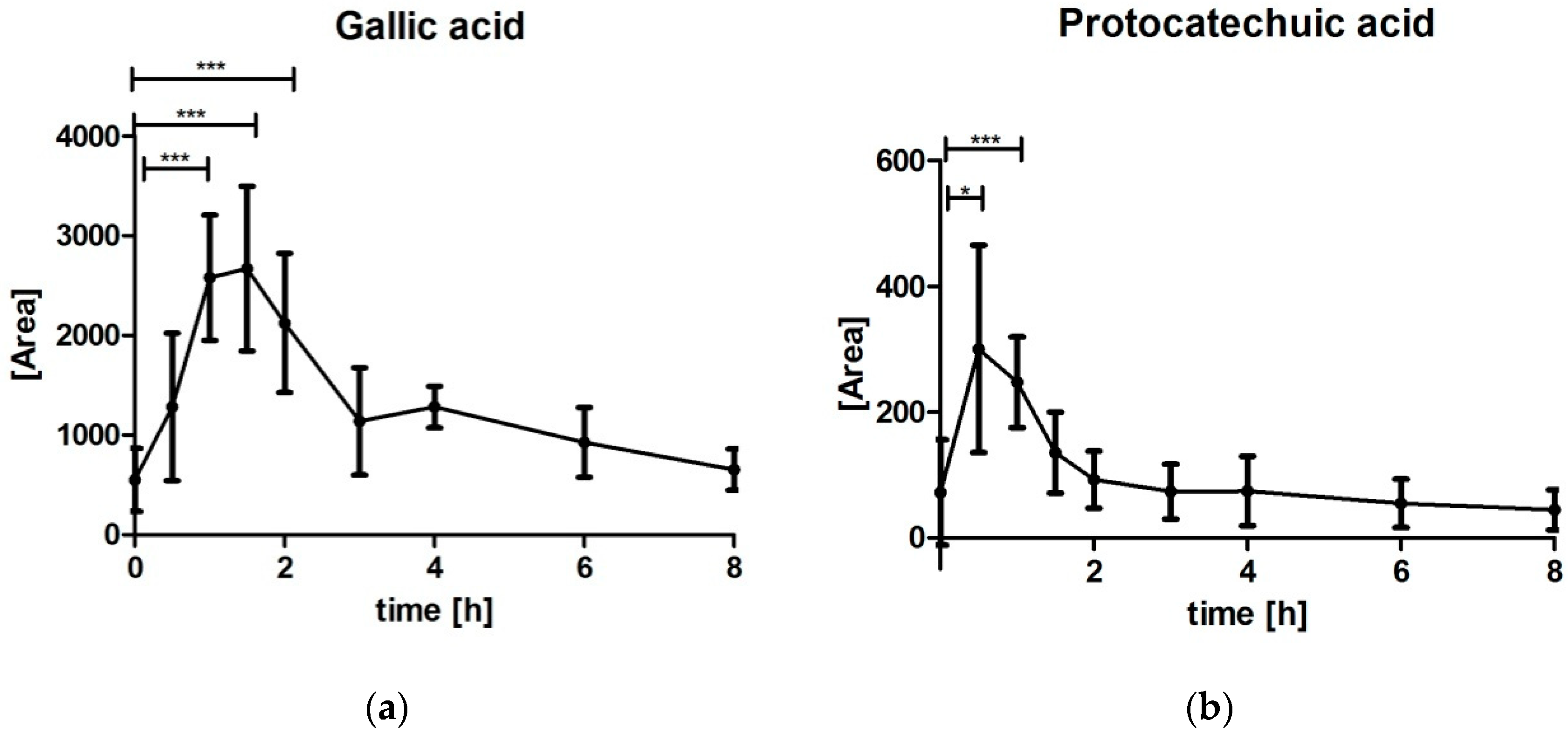
3.5. Analysis of Gallic Acid (GA) and Protocatechuic Acid (PCA)
3.6. Safety Assessments
4. Discussion
4.1. Delphinidin-3-O-glucoside (DG) and Cyanidin-3-O-sambubioside (CS)
4.2. Gallic Acid (GA) and Protocatechuic Acid (PCA)
4.3. Reporting Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- WHO Technical Staff. Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. E-Library of Evidence for Nutrition Actions; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Perez, D.D.; Vasquez, L.; Martinez, A.L.; Leighton, F. Juice and Phenolic Fractions of the Berry Aristotelia chilensis Inhibit LDL Oxidation In Vitro and Protect Human Endothelial Cells against Oxidative Stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Schoenlau, F. Nutraceutical and Antioxidant Effects of a Delphinidin-Rich Maqui Berry Extract Delphinol®: A Review. Minerva Cardioangiol. 2015, 63, 1–12. [Google Scholar] [PubMed]
- Alvarado, J.; Schoenlau, F.; Leschot, A.; Salgad, A.M.; Vigil, P.P. Delphinol® Standardized Maqui Berry Extract Significantly Lowers Blood Glucose and Improves Blood Lipid Profile in Prediabetic Individuals in Three-Month Clinical Trial. Panminerva Med. 2016, 58, 1–6. [Google Scholar] [PubMed]
- Nakamura, S.; Tsubota, K.; Shimoda, H.; Imada, T.; Tanaka, J. Delphinidin 3,5-O-Diglucoside, a Constituent of the Maqui Berry (Aristotelia chilensis) Anthocyanin, Restores Tear Secretion in a Rat Dry Eye Model. J. Funct. Foods 2014, 10, 346–354. [Google Scholar] [CrossRef]
- Hitoe, S.; Tanaka, J.; Shimoda, H. MaquiBright® Standardized Maqui Berry Extract Significantly Increases Tear Fluid Production and Ameliorates Dry Eye-Related Symptoms in a Clinical Pilot Trial. Panminerva Med. 2014, 56, 1–6. [Google Scholar] [PubMed]
- Alvarado, J.L.; Leschot, A.; Olivera-Nappa, A.; Salgado, A.M.; Rioseco, H.; Lyon, C.; Vigil, P. Delphinidin-Rich Maqui Berry Extract (Delphinol®) Lowers Fasting and Postprandial Glycemia and Insulinemia in Prediabetic Individuals during Oral Glucose Tolerance Tests. Biomed. Res. Int. 2016, 2016, 9070537. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, J.W.; Li, C.; Wang, Y.; Andrews, M.C.; Chen, P.; Zhu, G.; Ling, W.; et al. Plant Food Delphinidin-3-O-Glucoside Significantly Inhibits Platelet Activation and Thrombosis: Novel Protective Roles against Cardiovascular Diseases. PLoS ONE 2012, 7, e37323. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of Anthocyanins and Derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Hester, S.N.; Mastaloudis, A.; Gray, R.; Antony, J.M.; Evans, M.; Wood, S.M. Efficacy of an Anthocyanin and Prebiotic Blend on Intestinal Environment in Obese Male and Female Subjects. J. Nutr. Metab. 2018, 2018, 7497260. [Google Scholar] [CrossRef] [PubMed]
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of Anthocyanin-Rich Foods in Obesity Control through Gut Microbiota Interactions. Biofactors 2017, 43, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Sandhu, A.; Edirisinghe, I. Chapter 35—Anthocyanins. In Nutraceuticals; Academic Press: Cambridge, MA, USA, 2016; Chapter 35. [Google Scholar]
- Wallace, T.C. Anthocyanins in Cardiovascular Disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Megson, I.L. Bioavailable Concentrations of Delphinidin and Its Metabolite, Gallic Acid, Induce Antioxidant Protection Associated with Increased Intracellular Glutathione in Cultured Endothelial Cells. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Frohling, B.; Unger, F.; Dold, S.; Spengler, B.; Rompp, A.; Kunz, C. Uptake and Bioavailability of Anthocyanins and Phenolic Acids from Grape/Blueberry Juice and Smoothie In Vitro and In Vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Tian, Y.; Mi, J.; Wang, J.; Xu, Y. Simultaneous Determination and Pharmacokinetic Study of Eight Components in Rat Plasma by UHPLC-MS/MS after Oral Administration of Hypericum Japonicum Thunb Extract. J. Pharm. Biomed. Anal. 2016, 118, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of Anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Remesy, C. Anthocyanins Are Efficiently Absorbed from the Small Intestine in Rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The Pharmacokinetics of Anthocyanins and Their Metabolites in Humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.; Vrhovsek, U.; Tramer, F.; Mattivi, F.; Passamonti, S. Exceptionally Fast Uptake and Metabolism of Cyanidin 3-O-Glucoside by Rat Kidneys and Liver. J. Nat. Prod. 2011, 74, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X. Anthocyanins: Structural Characteristics that Result in Unique Metabolic Patterns and Biological Activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic Acid Is the Major Human Metabolite of Cyanidin-Glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Pimpao, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic Sulfates as New and Highly Abundant Metabolites in Human Plasma after Ingestion of a Mixed Berry Fruit Puree. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.L.; Dragsted, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and Excretion of Black Currant Anthocyanins in Humans and Watanabe Heritable Hyperlipidemic Rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.C.; Hendriks, W.H.; Broomfield, A.M.; McGhie, T.K. Viscous Food Matrix Influences Absorption and Excretion but Not Metabolism of Blackcurrant Anthocyanins in Rats. J. Food Sci. 2009, 74, H22–H29. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.C.; McGhie, T.K.; Reynolds, G.W.; Hendriks, W.H. The Flavonol Quercetin-3-Glucoside Inhibits Cyanidin-3-O-Glucoside Absorption In Vitro. J. Agric. Food Chem. 2006, 54, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean | 95% CI |
|---|---|---|
| Age (years) | 29.5 | (23.4; 35.7) |
| BMI (kg/m2) | 23.6 | (21.6; 25.7) |
| Systolic BP | 122.5 | (111.7; 133.3) |
| Diastolic BP | 74.7 | (70.4; 79.0) |
| Fruit consumption/day | 1.5 | (1.1; 1.8) |
| Vegetable consumption | 1.7 | (1.2; 2.2) |
| Haemoglobin | 13.6 | (12.9; 14.3) |
| Total Cholesterol | 182.8 | (160.4; 205.2) |
| GOT | 23.2 | (14.7; 31.6) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schön, C.; Wacker, R.; Micka, A.; Steudle, J.; Lang, S.; Bonnländer, B. Bioavailability Study of Maqui Berry Extract in Healthy Subjects. Nutrients 2018, 10, 1720. https://doi.org/10.3390/nu10111720
Schön C, Wacker R, Micka A, Steudle J, Lang S, Bonnländer B. Bioavailability Study of Maqui Berry Extract in Healthy Subjects. Nutrients. 2018; 10(11):1720. https://doi.org/10.3390/nu10111720
Chicago/Turabian StyleSchön, Christiane, Roland Wacker, Antje Micka, Jasmin Steudle, Stefanie Lang, and Bernd Bonnländer. 2018. "Bioavailability Study of Maqui Berry Extract in Healthy Subjects" Nutrients 10, no. 11: 1720. https://doi.org/10.3390/nu10111720
APA StyleSchön, C., Wacker, R., Micka, A., Steudle, J., Lang, S., & Bonnländer, B. (2018). Bioavailability Study of Maqui Berry Extract in Healthy Subjects. Nutrients, 10(11), 1720. https://doi.org/10.3390/nu10111720





