Longitudinal Association between n-3 Long-Chain Polyunsaturated Fatty Acid Intake and Depressive Symptoms: A Population-Based Cohort Study in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
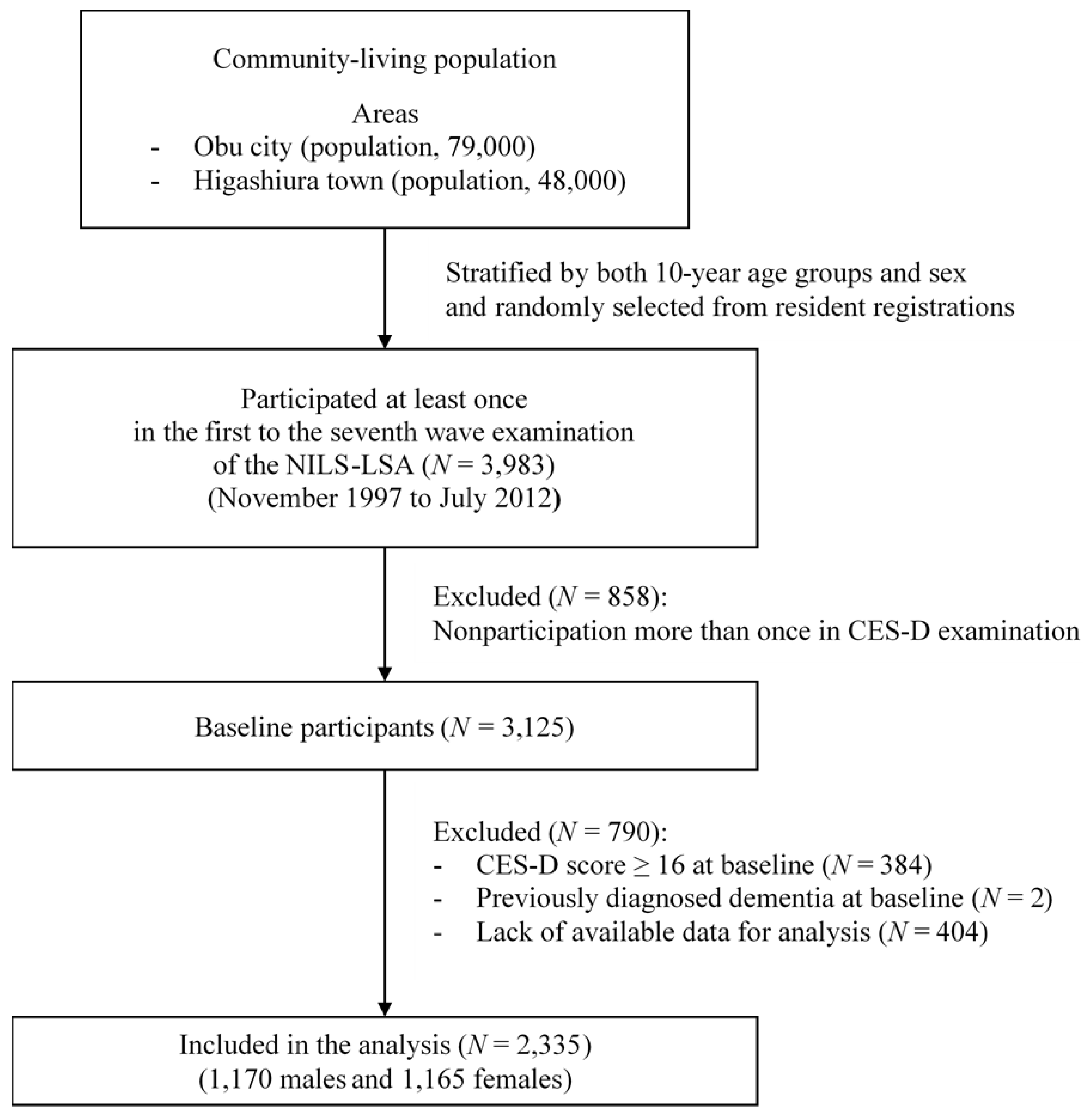
2.2. Study Population
2.3. Depressive Symptoms
2.4. Nutritional Assessment
2.5. Other Measurements
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Differences between Fatty Acid Intake with and without the Onset of Depressive Symptoms
3.3. Risk of Depressive Symptoms with Increased Intake of Fatty Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders. Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; Available online: http://www.who.int/mental_health/management/depression/en/ (accessed on 25 October 2017).
- World Health Organization. Health Topics/Depression. Available online: http://www.emro.who.int/health-topics/depression/index.html/ (accessed on 25 October 2017).
- Ministry of Health, Labour and Welfare. Patient Survey 2014, Statistical Tables 1–9, Statistical Table 2, Estimated Number of Patients (per Day), by Total/Inpatient/Outpatient, Type of Medical Institution, Year, and Classification of Diseases. Available online: http://www.mhlw.go.jp/english/database/db-hss/sps_2014.html (accessed on 25 October 2017).
- Blazer, D.G.; Kessler, R.C.; McGonagle, K.A.; Swartz, M.S. The prevalence and distribution of major depression in a national community sample: The National Comorbidity Survey. Am. J. Psychiatry 1994, 151, 979–986. [Google Scholar] [PubMed]
- Kawakami, N.; Takeshima, T.; Ono, Y.; Uda, H.; Hata, Y.; Nakane, Y.; Nakane, H.; Iwata, N.; Furukawa, T.A.; Kikkawa, T. Twelve-month prevalence, severity, and treatment of common mental disorders in communities in Japan: Preliminary findings from the World Mental Health Japan Survey 2002–2003. Psychiatry Clin. Neurosci. 2005, 59, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Touvier, M.; Andreeva, V.A.; Jeandel, C.; Ferry, M.; Hercberg, S.; Galan, P. Cross-sectional but not longitudinal association between n-3 fatty acid intake and depressive symptoms: Results from the SU.VI.MAX 2 study. Am. J. Epidemiol. 2012, 175, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Green, R.C.; Cupples, L.A.; Kurz, A.; Auerbach, S.; Go, R.; Sadovnick, D.; Duara, R.; Kukull, W.A.; Chui, H.; Edeki, T.; et al. Depression as a risk factor for Alzheimer disease: The MIRAGE Study. Arch. Neurol. 2003, 60, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ownby, R.L.; Crocco, E.; Acevedo, A.; John, V.; Loewenstein, D. Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 2006, 63, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.; Molero, P.; Ortuño Sánchez-Pedreño, F.; Van der Does, W.; Angel Martínez-González, M. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Galiot, A.; Goñi, I. Adherence to the Mediterranean diet pattern, cognitive status and depressive symptoms in an elderly non-institutionalized population. Nutr. Hosp. 2017, 34, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Je, Y. Fish consumption and depression in Korjean adults: The Korea National Health and Nutrition Examination Survey, 2013–2015. Eur. J. Clin. Nutr. 2018, 7, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Feng, L.; Gao, Q.; Li, J.L.; Rajendran, K.S.; Wong, J.C.; Kua, E.H.; Ng, T.P. Association between fish intake and depressive symptoms among community-living older Chinese adults in Singapore: A cross-sectional study. J. Nutr. Health Aging 2016, 20, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary fats and membrane function: Implications for metabolism and disease. Biol. Rev. Camb. Philos. Soc. 2005, 80, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L; De Kloet, E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 2015, 62, 389–391. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Molendijk, M.L. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural. Plast. 2016, 2016, 5098591. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, D.; Ni, N.; Ren, H.; Luo, C.; He, C.; Kang, J.X.; Wan, J.B.; Su, H. Omega-3 polyunsaturated fatty acids protect neural progenitor cells against oxidative injury. Mar. Drugs 2014, 12, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Belayev, L.; Khoutorova, L.; Obenaus, A.; Bazan, N.G. Docosahexaenoic acid confers enduring neuroprotection in experimental stroke. J. Neurol. Sci. 2014, 338, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Gleason, E.D. Omega-3 fatty acids and hippocampal neurogenesis in depression. CNS Neurol. Disord. Drug Targets 2013, 12, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.; Rand, K.L.; Muldoon, M.F.; Kamarck, T.W. A prospective evaluation of the directionality of the depression-inflammation association. Brain Behav. Immun. 2009, 23, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.; Harmsen, I.; Assies, J.; Koeter, M.W.; Ruhé, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Sallis, H.M.; Perry, R.; Ness, A.R.; Churchill, R. ω-3 Fatty acids for major depressive disorder in adults: An abridged Cochrane review. BMJ Open 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Marventano, S.; Castellano, S.; Mistretta, A.; Pajak, A.; Galvano, F. Dietary n-3 PUFA, fish consumption and depression: A systematic review and meta-analysis of observational studies. J. Affect Disord. 2016, 205, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.J.; Sawada, N.; Mimura, M.; Shikimoto, R.; Nozaki, S.; Hamazaki, K.; Uchitomi, Y.; Tsugane, S. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: A population-based prospective cohort study. Transl. Psychiatry 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Shimokata, H.; Ando, F.; Niino, N. A new comprehensive study on aging—The National Institute for Longevity Sciences, Longitudinal Study of Aging (NILS-LSA). J. Epidemiol. 2000, 10, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Shima, S. A new self-rating scale for depression. Clin. Psychiatry 1985, 27, 717–723. (In Japanese) [Google Scholar]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Berkman, L.F.; Berkman, C.S.; Kasl, S.; Freeman, D.H.; Leo, L.; Ostfeld, A.M.; Cornoni-Huntley, J.; Brody, J.A. Depressive symptoms in relation to physical health and functioning in the elderly. Am. J. Epidemiol. 1986, 124, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Sakai, S.; Mori, K.; Ando, F.; Niino, N.; Shimokata, H. Nutritional assessments of 3-day dietary records in National Institute for Longevity Sciences-Longitudinal Study of Aging (NILS-LSA). J. Epidemiol. 2000, 10, S70–S76. [Google Scholar] [CrossRef] [PubMed]
- Office for Resources, Policy Division Science and Technology Policy Bureau, Ministry of Education, Culture, Sports, Science and Technology. Standard Tables of Foods Composition in Japan 2010. Available online: http://www.mext.go.jp/b_menu/shingi/gijyutu/gijyutu3/houkoku/1298713.htm (accessed on 25 October 2017).
- Kozakai, R.; Ando, F.; Kim, H.Y.; Rantanen, T.; Shimokata, H. Regular exercise history as a predictor of exercise in community-dwelling older Japanese people. J. Phys. Fit. Sport Med. 2012, 1, 167–174. [Google Scholar] [CrossRef]
- Horikawa, C.; Otsuka, R.; Kato, Y.; Nishita, Y.; Tange, C.; Kakutani, S.; Rogi, T.; Kawashima, H.; Shibata, H.; Ando, F.; et al. Cross-sectional association between serum concentrations of n-3 long-chain PUFA and depressive symptoms: Results in Japanese community dwellers. Br. J. Nutr. 2016, 115, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Fanelli Kuczmarski, M.T.; Beydoun, H.A.; Rostant, O.S.; Evans, M.K.; Zonderman, A.B. Associations of the ratios of n-3 to n-6 dietary fatty acids with longitudinal changes in depressive symptoms among US women. Am. J. Epidemiol. 2015, 181, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, L.A.; He, K.; Whooley, M.A.; Daviglus, M.L.; Liu, K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition 2009, 25, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, N.; Miura, K.; Okuda, N. Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: A 24-year follow-up of NIPPON DATA80. Atherosclerosis 2014, 232, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Elmadfa, I.; Kornsteiner, M. Fats and fatty acid requirements for adults. Ann. Nutr. Metab. 2009, 55, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Pasco, J.A.; Henry, M.J.; Kotowicz, M.A.; Nicholson, G.C.; Berk, M. Dietary omega-3 fatty acids and depression in a community sample. Nutr. Neurosci. 2004, 7, 101–635. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Hirota, S.; Hirayama, T.; Adachi, N.; Hagiwara, C.; Iwama, N.; Kamachi, K.; Araki, E.; Kawashima, H.; Kiso, Y. Age-related changes of dietary intake and blood eicosapentaenoic acid, docosahexaenoic acid, and arachidonic acid levels in Japanese men and women. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, K.; Nagaya, T.; Imaeda, N.; Tokudome, Y.; Fujiwara, N.; Sato, J.; Ikeda, M.; Maki, S.; Tokudome, S. Discrepancies in dietary intakes and plasma concentrations of fatty acids according to age among Japanese female dieticians. Eur. J. Clin. Nutr. 2002, 56, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, R.; Partonen, T.; Haukka, J.; Virtamo, J.; Albanes, D.; Lönnqvist, J. Is low dietary intake of omega-3 fatty acids associated with depression? Am. J. Psychiatry 2004, 161, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Henríquez, P.; Figueiras, A.; Ortuño, F.; Lahortiga, F.; Martínez-González, M.A. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur. J. Nutr. 2007, 46, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Persons, J.E.; Robinson, J.G.; Ammann, E.M.; Coryell, W.H.; Espeland, M.A.; Harris, W.S.; Manson, J.E.; Fiedorowicz, J.G. Omega-3 fatty acid biomarkers and subsequent depressive symptoms. Int. J. Geriatr. Psychiatry 2014, 29, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Mirzaei, F.; O’Reilly, E.J.; Pan, A.; Willett, W.C.; Kawachi, I.; Koenen, K.; Ascherio, A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: A 10-y prospective follow-up study. Am. J. Clin. Nutr. 2011, 93, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Kipnis, V.; Subar, A.F.; Midthune, D.; Freedman, L.S.; Ballard-Barbash, R.; Troiano, R.P.; Bingham, S.; Schoeller, D.A.; Schatzkin, A.; Carroll, R.J. Structure of dietary measurement error: Results of the OPEN biomarker study. Am. J. Epidemiol. 2003, 158, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kipnis, V.; Troiano, R.P.; Midthune, D.; Schoeller, D.A.; Bingham, S.; Sharbaugh, C.O.; Trabulsi, J.; Runswick, S.; Ballard-Barbash, R.; et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: The OPEN study. Am. J. Epidemiol. 2003, 158, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fiske, A.; Wetherell, J.L.; Gatz, M. Depression in older adults. Annu. Rev. Clin. Psychol. 2009, 5, 363–589. [Google Scholar] [CrossRef] [PubMed]
- Blazer, D.G. Depression in late life: Review and commentary. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Lindén, T.; Blomstrand, C.; Skoog, I. Depressive disorders after 20 months in elderly stroke patients: A case-control study. Stroke 2007, 38, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, P.; Roy, J.F.; Saz, P.; Marcos, G.; Lobo, A. Prevalent and incident depression in community-dwelling elderly persons with diabetes mellitus: Results from the ZARADEMP project. Diabetologia 2006, 49, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, C.; Volpato, S.; Penninx, B.W.; Yaffe, K.; Simonsick, E.M.; Strotmeyer, E.S.; Cesari, M.; Kritchevsky, S.B.; Perry, S.; Ayonayon, H.N.; et al. Diabetes mellitus, glycemic control, and incident depressive symptoms among 70- to 79-year-old persons: The health, aging, and body composition study. Arch. Intern. Med. 2007, 167, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.G. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: Evidence from a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 2009, 28, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.H.; Hannestad, J. Omega-3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.E. Dietary essential fatty acids and brain function: A developmental perspective on mechanisms. Proc. Nutr. Soc. 2002, 61, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Begg, D.; Mathai, M.; Weisinger, R.S. Omega 3 fatty acids and the brain: Review of studies in depression. Asia Pac. J. Clin. Nutr. 2007, 1, 391–397. [Google Scholar]
- Meyer, B.J.; Grenyer, B.F.; Crowe, T.; Owen, A.J.; Grigonis-Deane, E.M.; Howe, P.R. Improvement of major depression is associated with increased erythrocyte DHA. Lipids 2013, 48, 863–886. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.F.; Mutch, D.M.; Leri, F. The relationship between fatty acids and different depression-related brain regions, and their potential role as biomarkers of response to antidepressants. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.R.; Ogbonna, E.M.; Lim, S.; Li, Y.; Zhang, J. Dietary linoleic and oleic fatty acids in relation to severe depressed mood: 10 years follow-up of a national cohort. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 2009 33, 972–977. [Google Scholar] [CrossRef]
| Characteristics | Total | CES-D ≥ 16 | CES-D < 16 | p-Value * | |||
|---|---|---|---|---|---|---|---|
| Subjects with New Onset of Depressive Symptoms | Subjects without New Onset of Depressive Symptoms | ||||||
| Mean | SD | Mean | SD | Mean | SD | ||
| No. of subjects (n) | 2335 | 515 | 1820 | ||||
| Sex (male/female) (n) | 1170/1165 (50.1/49.9%) | 227/288 (44.1/55.9%) | 943/877 (51.8/48.2%) | 0.002 | |||
| Age (years) | 56.1 | 11.9 | 56.4 | 11.6 | 56.1 | 12.0 | 0.62 |
| BMI (kg/m2) | 22.9 | 3.0 | 22.8 | 3.2 | 23.0 | 3.0 | 0.44 |
| CES-D | 5.4 | 4.2 | 8.0 | 4.2 | 4.7 | 3.9 | < 0.001 |
| Educational level (n) | |||||||
| ≤9 years | 581 (24.9%) | 150 (29.1%) | 431 (23.7%) | ||||
| 10–12 years | 950 (40.7%) | 204 (39.6%) | 746 (41.0%) | 0.032 | |||
| ≥13 years | 804 (34.4%) | 161 (31.3%) | 643 (35.3%) | ||||
| Unmarried (n) | 281 (13.7%) | 74 (14.4%) | 207 (11.4%) | 0.07 | |||
| Current smoker (n) | 481 (20.6%) | 108 (21.0%) | 373 (20.5%) | 0.81 | |||
| Energy intake (kcal/day) | 2136 | 428 | 2123 | 408 | 2140 | 433 | 0.44 |
| Alcohol consumption † (mL/day) | 9.7 | 16.1 | 8.9 | 16.5 | 10.0 | 16.0 | 0.18 |
| Total physical activity | 2.0 | 0.2 | 2.0 | 0.2 | 2.0 | 0.2 | 0.87 |
| (Mets * min/1000/day) | |||||||
| Employment (n) | |||||||
| Unemployed or household labor | 877 (37.6%) | 198 (38.5%) | 679 (37.3%) | ||||
| Nonregular employment | 407 (17.4%) | 102 (19.8%) | 305 (16.8%) | 0.15 | |||
| Regular employment | 1051 (45.0%) | 215 (41.8%) | 836 (45.9%) | ||||
| Medical history ‡ (n) | |||||||
| Stroke | 47 (2.0%) | 12 (2.3%) | 35 (1.9%) | 0.56 | |||
| Hypertension | 511 (21.9%) | 123 (23.9%) | 388 (21.3%) | 0.21 | |||
| Ischemic heart disease | 190 (8.1%) | 49 (9.5%) | 141 (7.8%) | 0.20 | |||
| Hyperlipidemia | 357 (15.3%) | 83 (16.1%) | 274 (15.1%) | 0.56 | |||
| Diabetes mellitus | 155 (6.6%) | 25 (4.9%) | 130 (7.1%) | 0.07 | |||
| Fatty Acid | Total | CES-D ≥ 16 | CES-D < 16 | p-Value * | ||||
|---|---|---|---|---|---|---|---|---|
| Subjects with New Onset of Depressive Symptoms | Subjects without New Onset of Depressive Symptoms | |||||||
| Mean | SD | Mean | SD | Mean | SD | |||
| SFA | (g/day) | 16.6 | 5.7 | 16.4 | 5.3 | 16.6 | 5.8 | 0.50 |
| MUFA | (g/day) | 20.7 | 7.0 | 20.6 | 6.6 | 20.7 | 7.1 | 0.67 |
| PUFA | (g/day) | 13.4 | 4.0 | 13.2 | 3.9 | 13.4 | 4.0 | 0.46 |
| n-3 PUFA | (g/day) | 2.5 | 1.0 | 2.4 | 0.9 | 2.5 | 1.0 | 0.040 |
| DHA | (mg/day) | 552.2 | 377.9 | 519.4 | 356.2 | 561.5 | 383.4 | 0.020 |
| EPA | (mg/day) | 301.0 | 240.2 | 282.6 | 229.0 | 306.2 | 243.1 | 0.049 |
| ALA | (mg/day) | 1438 | 532 | 1425 | 527.2 | 1442 | 533.4 | 0.53 |
| n-6 PUFA | (g/day) | 10.8 | 3.4 | 10.8 | 3.3 | 10.8 | 3.4 | 0.79 |
| ARA | (mg/day) | 176.8 | 65.5 | 174.7 | 61.6 | 177.5 | 66.6 | 0.38 |
| LA | (g/day) | 10.6 | 3.4 | 10.5 | 3.3 | 10.6 | 3.4 | 0.93 |
| n-6:n-3 | 4.7 | 1.6 | 4.8 | 1.5 | 4.7 | 4.6 | 0.048 | |
| T1 (Low) | T2 | T3 (High) | p for Trend * | |
|---|---|---|---|---|
| SFA | ||||
| Median (range) of intake (g/day) † | 11.1 (2.31–13.64) | 16.0 (13.64–18.52) | 21.8 (18.52–52.80) | |
| N with/without depressive symptoms ‡ | 173/604 | 167/611 | 175/605 | |
| Crude HR (95% CI) | 1.00 (reference) | 0.89 (0.72–1.10) | 0.91 (0.73–1.12) | 0.35 |
| Model I: Multivariate-adjusted HR (95% CI) § | 0.93 (0.75–1.16) | 1.00 (0.77–1.28) | 0.97 | |
| Model II: Multivariate-adjusted HR (95% CI) | 1.00 (0.79–1.25) | 1.12 (0.86–1.46) | 0.39 | |
| MUFA | ||||
| Median (range) of intake (g/day) † | 14.2 (4.15–17.14) | 20.0 (17.14–22.9) | 27.2 (22.9–55.19) | |
| N with/without depressive symptoms ‡ | 168/609 | 174/604 | 173/607 | |
| Crude HR (95% CI) | 1.00 (reference) | 0.94 (0.76–1.16) | 0.91 (0.74–1.13) | 0.38 |
| Model I: Multivariate-adjusted HR (95% CI) § | 1.04 (0.83–1.30) | 1.08 (0.83–1.41) | 0.58 | |
| Model II: Multivariate-adjusted HR (95% CI) | 1.05 (0.83–1.33) | 1.16 (0.88–1.53) | 0.29 | |
| PUFA | ||||
| Median (range) of intake (g/day) † | 9.6 (3.79–11.45) | 13.0 (11.45–14.70) | 16.9 (14.70–35.19) | |
| N with/without depressive symptoms ‡ | 166/611 | 178/600 | 171/609 | |
| Crude HR (95% CI) | 1.00 (reference) | 1.00 (0.81–1.23) | 0.91 (0.74–1.13) | 0.38 |
| Model I: Multivariate-adjusted HR (95% CI) § | 1.08 (0.86–1.35) | 1.04 (0.80–1.35) | 0.78 | |
| Model II: Multivariate-adjusted HR (95% CI) | 1.18 (0.94–1.47) | 1.14 (0.88–1.48) | 0.32 | |
| n-3 PUFA | ||||
| Median (range) of intake (g/day) † | 1.6 (0.498–1.977) | 2.3 (1.977–2.726) | 3.3 (2.727–9.664) | |
| N with/without depressive symptoms ‡ | 186/591 | 177/601 | 152/628 | |
| Crude HR (95% CI) | 1.00 (reference) | 0.92 (0.75–1.13) | 0.74 (059–0.91) | 0.005 |
| Model I: Multivariate-adjusted HR (95% CI) § | 0.94 (0.76–1.16) | 0.75 (0.59–0.95) | 0.018 | |
| Model II: Multivariate-adjusted HR (95% CI) | 1.01 (0.82–1.25) | 0.78 (0.61–0.99) | 0.009 | |
| n-6 PUFA | ||||
| Median (range) of intake (g/day) † | 7.7 (3.12–9.27) | 10.5 (9.28–11.96) | 13.8 (11.96–30.00) | |
| N with/without depressive symptoms ‡ | 171/606 | 165/613 | 179/601 | |
| Crude HR (95% CI) | 1.00 (reference) | 0.89 (0.72–1.10) | 0.93 (0.76–1.15) | 0.52 |
| Model I: Multivariate-adjusted HR (95% CI) § | 0.96 (0.77–1.20) | 1.08 (0.84–1.39) | 0.54 | |
| Model II: Multivariate-adjusted HR (95% CI) | 1.01 (0.81–1.27) | 1.16 (0.90–1.49) | 0.26 | |
| n-6:n-3 | ||||
| Median (range) of ratios † | 3.2 (0.805–3.961) | 4.6 (3.962–5.258) | 6.1 (5.262–16.849) | |
| N with/without depressive symptoms ‡ | 153/624 | 167/611 | 195/585 | |
| Crude HR (95% CI) | 1.00 (reference) | 1.08 (0.86–1.34) | 1.28 (1.03–1.58) | 0.024 |
| Model I: Multivariate-adjusted HR (95% CI) § | 1.10 (0.88–1.37) | 1.32 (1.07–1.64) | 0.011 | |
| Model II: Multivariate-adjusted HR (95% CI) | 1.11 (0.89–1.38) | 1.36 (1.10–1.69) | 0.005 |
| T1 (Low) | T2 | T3 (High) | p for Trend * | ||
|---|---|---|---|---|---|
| n-3 PUFA | |||||
| DHA | Median (range) of intake (mg/day) † | 220.7 (4.2–344.4) | 468.4 (344.6–627.3) | 865.0 (627.4–3872.5) | |
| N with/without depressive symptoms ‡ | 195/582 | 159/619 | 161/619 | ||
| Crude HR (95% CI) | 1.00 (reference) | 0.80 (0.65–0.99) | 0.79 (0.64–0.97) | 0.023 | |
| Model I: Multivariate-adjusted HR (95% CI) § | 0.80 (0.65–0.99) | 0.78 (0.63–0.97) | 0.027 | ||
| Model II: Multivariate-adjusted HR (95% CI) | 0.80 (0.65–0.99) | 0.79 (0.63–0.98) | 0.032 | ||
| EPA | Median (range) of intake (mg/day) † | 95.3 (2.1–164.8) | 242.3 (165.1–341.8) | 495.4 (341.9–2466.1) | |
| N with/without depressive symptoms ‡ | 195/582 | 167/611 | 153/627 | ||
| Crude HR (95% CI) | 1.00 (reference) | 0.87 (0.70–1.07) | 0.77 (0.62–0.95) | 0.016 | |
| Model I: Multivariate-adjusted HR (95% CI) § | 0.86 (0.70–1.06) | 0.76 (0.61–0.95) | 0.014 | ||
| Model II: Multivariate-adjusted HR (95% CI) | 0.85 (0.69–1.04) | 0.74 (0.60–0.93) | 0.009 | ||
| ALA | Median (range) of intake (mg/day) † | 929 (289.1–1176.9) | 1370.1 (1177.1–1604.8) | 1919.4 (1604.9–4295.7) | |
| N with/without depressive symptoms ‡ | 169/608 | 182/596 | 164/616 | ||
| Crude HR (95% CI) | 1.00 (reference) | 1.03 (0.84–1.28) | 0.90 (0.72–1.11) | 0.31 | |
| Model I: Multivariate-adjusted HR (95% CI) § | 1.08 (0.87–1.34) | 1.00 (0.78–1.27) | 0.99 | ||
| Model II: Multivariate-adjusted HR (95% CI) | 1.14 (0.92–1.41) | 1.13 (0.88–1.44) | 0.33 | ||
| n-6 PUFA | |||||
| ARA | Median (range) of intake (mg/day) † | 117 (13.1–144.6) | 170.4 (144.6–197.3) | 235.4 (197.4–612.5) | |
| N with/without depressive symptoms ‡ | 163/614 | 188/590 | 164/616 | ||
| Crude HR (95% CI) | 1.00 (reference) | 1.08 (0.88–1.33) | 0.91 (0.73–1.12) | 0.37 | |
| Model I: Multivariate-adjusted HR (95% CI) § | 1.14 (0.92–1.41) | 0.98 (0.77–1.25) | 0.87 | ||
| Model II: Multivariate-adjusted HR (95% CI) | 1.17 (0.94–1.45) | 1.04 (0.82–1.32) | 0.75 | ||
| LA | Median (range) of intake (g/day) † | 7.5 (3.0–9.0) | 10.2 (9.0–11.6) | 13.6 (11.6–29.9) | |
| N with/without depressive symptoms ‡ | 170/607 | 167/611 | 178/602 | ||
| Crude HR (95% CI) | 1.00 (reference) | 0.92 (0.74–1.14) | 0.94 (0.76–1.16) | 0.54 | |
| Model I: Multivariate-adjusted HR (95% CI) § | 0.99 (0.79–1.24) | 1.08 (0.84–1.39) | 0.54 | ||
| Model II: Multivariate-adjusted HR (95% CI) | 1.08 (0.86–1.35) | 1.18 (0.92–1.52) | 0.20 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horikawa, C.; Otsuka, R.; Kato, Y.; Nishita, Y.; Tange, C.; Rogi, T.; Kawashima, H.; Shibata, H.; Ando, F.; Shimokata, H. Longitudinal Association between n-3 Long-Chain Polyunsaturated Fatty Acid Intake and Depressive Symptoms: A Population-Based Cohort Study in Japan. Nutrients 2018, 10, 1655. https://doi.org/10.3390/nu10111655
Horikawa C, Otsuka R, Kato Y, Nishita Y, Tange C, Rogi T, Kawashima H, Shibata H, Ando F, Shimokata H. Longitudinal Association between n-3 Long-Chain Polyunsaturated Fatty Acid Intake and Depressive Symptoms: A Population-Based Cohort Study in Japan. Nutrients. 2018; 10(11):1655. https://doi.org/10.3390/nu10111655
Chicago/Turabian StyleHorikawa, Chika, Rei Otsuka, Yuki Kato, Yukiko Nishita, Chikako Tange, Tomohiro Rogi, Hiroshi Kawashima, Hiroshi Shibata, Fujiko Ando, and Hiroshi Shimokata. 2018. "Longitudinal Association between n-3 Long-Chain Polyunsaturated Fatty Acid Intake and Depressive Symptoms: A Population-Based Cohort Study in Japan" Nutrients 10, no. 11: 1655. https://doi.org/10.3390/nu10111655
APA StyleHorikawa, C., Otsuka, R., Kato, Y., Nishita, Y., Tange, C., Rogi, T., Kawashima, H., Shibata, H., Ando, F., & Shimokata, H. (2018). Longitudinal Association between n-3 Long-Chain Polyunsaturated Fatty Acid Intake and Depressive Symptoms: A Population-Based Cohort Study in Japan. Nutrients, 10(11), 1655. https://doi.org/10.3390/nu10111655





