Randomized, Double-Blind, Placebo-Controlled Parallel Clinical Trial Assessing the Effect of Fructooligosaccharides in Infants with Constipation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Intervention
2.3. Study Stages
2.4. Study Procedures
2.5. Outcomes
2.6. Ethical Issues
2.7. Statistical Analysis
3. Results
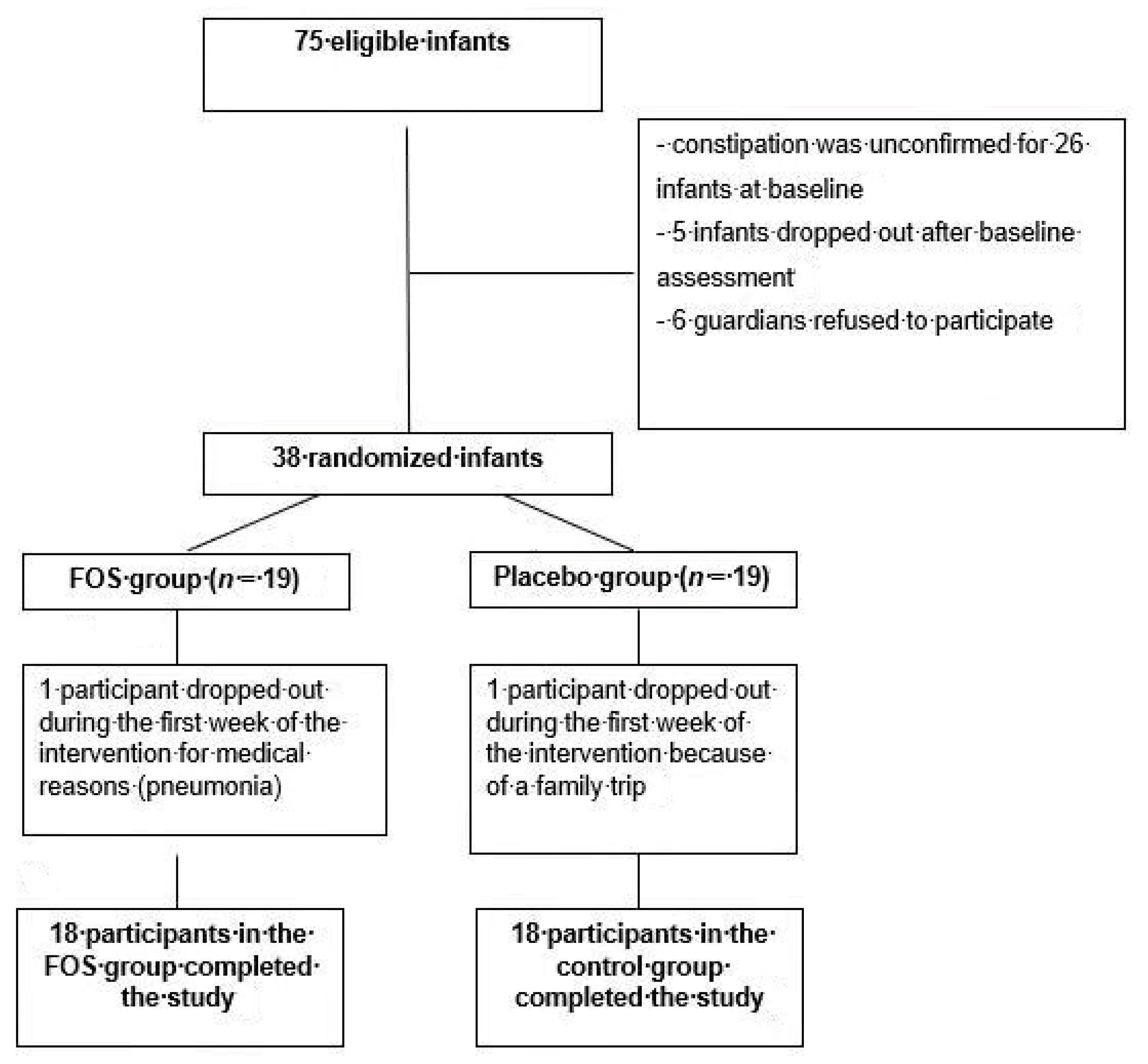
3.1. Patients
3.2. Primary Outcome
3.3. Secondary Outcome
3.4. Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mugie, S.M.; Benninga, M.A.; Di Lorenzo, C. Epidemiology of constipation in children and adults: A systematic review. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.A.; Faure, C.; Hyman, P.E.; St James Roberts, I.; Schechter, N.L.; Nurko, S. Childhood functional gastrointestinal disorders: Neonate/Toddler. Gastroenterology 2016, 150. [Google Scholar] [CrossRef] [PubMed]
- Tabbers, M.M.; DiLorenzo, C.; Berger, C.; Langendam, M.W.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, M.A. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Loening-Baucke, V. Constipation in early childhood: Patient characteristics, treatment, and long-term follow up. Gut 1993, 34, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.N.; Vitolo, M.R.; Puccini, R.F.; de Morais, M.B. Constipation in infants: Influence of type of feeding and dietary fiber intake. J. Pediatr. 2002, 78, 2002–2008. [Google Scholar] [CrossRef]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelson, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.T.; Ewing, G.; Taylor, L.C. The bowel habit of milk-fed infants. J. Pediatr. Gastroenterol. Nutr. 1998, 7, 568–571. [Google Scholar] [CrossRef]
- Tunc, V.T.; Camurdan, A.D.; Ilhan, M.N.; Sahin, F.; Beyazova, U. Factors associated with defecation patterns in 0–24 month-old children. Eur. J. Pediatr. 2008, 167, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Human milk oligosaccharides (HMOS): Structure, function, and enzyme-catalyzed synthesis. Adv. Carbohydr. Chem. Biochem. 2015, 72, 1013–1090. [Google Scholar] [CrossRef]
- Scholtens, P.A.M.J.; Goossens, D.A.M.; Staiano, A. Stool characteristics of infants receiving short-chain galacto-oligosaccharides and long-chain fructooligosaccharides: A review. World J. Gastroenterol. 2014, 20, 13446–13452. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Abkar, A.; Bellaiche, M.; Benninga, M.; Chouraqui, J.P.; Çokura, F.; Harb, T.; Hegar, B.; Lifschitz, C.; Ludwig, T.; et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Boehm, G.; Lidestri, M.; Casetta, P.; Jelinek, J.; Negretti, F.; Stahl, B.; Marini, A. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecalbifidobacteria in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 86, 178–181. [Google Scholar] [CrossRef]
- Moro, G.; Minoli, I.; Mosca, M.; Fanaro, S.; Jelinek, J.; Stahl, B.; Boehm, G. Dosagerelated bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 2006, 91, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Costalos, C.; Kapiki, A.; Apostolou, M.; Papathoma, E. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum. Dev. 2008, 84, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Bisceglia, M.; Indrio, F.; Riezzo, G.; Poerio, V.; Corapi, U.; Raimondi, F. The effect of prebiotics in the management of neonatal hyperbilirubinaemia. Acta Paediatr. 2009, 98, 1579–1581. [Google Scholar] [CrossRef] [PubMed]
- Veereman-Wauters, G.; Staelens, S.; Van de Broek, H.; Plaskie, K.; Wesling, F.; Roger, L.C.; McCartney, A.L.; Assam, P. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Beleli, C.A.V.; Antonio, M.A.R.G.; Santos, R.; Pastore, G.M.; Lomazi, E.A. Effect of 4′ galactooligosaccharide on constipation symptoms. J. Pediatr. 2015, 91, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.; Colleti, R.; Faure, C.; Gabriel-Martinez, E.; Maffei, H.V.; Morais, M.B.; Vandenplas, Y. Functional gastrointestinal disorders: Working group report of the first world congress of pediatric gastroenterology, hepatology and nutrition. J. Pediatr. Gastroenterol. Nutr. 2002, 3, 110–117. [Google Scholar] [CrossRef]
- Maffei, H.V.L.; Vicentini, A.P. Prospective evaluation of dietary treatment in childhood constipation: High dietary fiber and wheat bran intake are associated with constipation amelioration. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Dimson, S.B. Carmine as an index of transit time in children with simple constipation. Arch. Dis. Child. 1970, 45, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Byers, T. Dietary assessment resource manual. J. Nutr. 1994, 124, 2245–2301. [Google Scholar] [CrossRef]
- Núcleo de Estudos e Pesquisasem Alimentação (NEPA); UniversidadeEstadual de Campinas (UNICAMP). Tabela Brasileira de Composição de Alimentos (TACO), 2nd ed.; UNICAMP: Campinas, Brazil, 2006; Available online: http://www.cfn.org.br/wp-content/uploads/2017/03/taco_4_edicao_ampliada_e_revisada.pdf (accessed on 30 October 2018).
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- World Health Organization (WHO). Anthro Manual, Version 3.2.2: Software for Assessing Growth and Development of the World’s Children; 2011. Available online: http://www.who.int/childgrowth/software/en/ (accessed on 30 October 2018).
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, P.S.; Schut, F.; Jansen, G.J.; Raangs, G.C.; Kamphuis, G.R.; Wilkinson, M.H.; Welling, G.M. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 1995, 61, 3069–3075. [Google Scholar] [PubMed]
- De Araujo-Filho, H.B.; Carmo-Rodrigues, M.S.; Mello, C.S.; Melli, L.C.; Tahan, S.; Pignatari, A.C.; Morais, M.B. Children living near a sanitary landfill have increased breath methane and Methanobrevibactersmithii in their intestinal microbiota. Archaea 2014, 2014, 576249. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.S.; Rodrigues, M.S.D.C.; Araujo-Filho, H.B.A.; Melli, L.C.F.L.; Tahan, S.; Pignatari, A.C.C.; de Morais, M.B. Fecal microbiota analysis of children with small intestinal bacterial overgrowth among residents of an urban slum in Brazil. J. Pediatr. 2018, 94, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.S.; Carmo-Rodrigues, M.S.; Araujo-Filho, H.B.; Melli, L.C.; Tahan, S.; Pignatari, A.C.; de Morais, M.B. Gut microbiota differences in children from distinct socioeconomic levels living in the same urban area in Brazil. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Riva, E.; Giovannini, M. Dietary fiber in weaning foods of young children. Pediatrics 1995, 96, 1002–1005. [Google Scholar] [PubMed]
- Williams, C.L.; Bollella, M.; Wynder, E.L. A new recommendation for dietary fiber in childhood. Pediatrics 1995, 96, 985–988. [Google Scholar] [PubMed]
- National Research Council (NRC). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academy Press: Washington, DC, USA, 2002. [Google Scholar]
- Morais, T.B.; Sigulem, D.M.; Maranhão, H.S.; de Morais, M.B. Bacterial contamination and nutrient content of home-prepared milk feeding bottles of infants attending a public outpatient clinic. J. Trop. Pediatr. 2005, 51, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Penna de Carvalho, M.F.; Morais, T.B.; de Morais, M.B. Home-made feeding bottles have inadequacies in their nutritional composition regardless of socioeconomic class. J. Trop. Pediatr. 2013, 59, 286–291. [Google Scholar] [CrossRef] [PubMed]
| FOS Group (n = 19) | Control Group (n = 19) | p | |
|---|---|---|---|
| Age (months) | 12.77 ± 4.37 | 13.00 ± 5.19 | 0.890 † |
| Sex | |||
| Female | 10 (52.6%) | 10 (52.6%) | 1.000 * |
| Male | 9 (47.4%) | 9 (47.4%) | |
| Number of bowel movements per week | 6.27 ± 1.32 | 5.66 ± 1.87 | 0.133 † |
| Predominant stool shape and consistency | |||
| Cylindrical with cracks | 13 (68.4%) | 8 (42.1%) | 0.191 * |
| Scybalous | 6 (31.6%) | 11 (57.9%) | |
| Straining and/or difficulty in more than 50% of bowel movements | 16 (84.2%) | 16 (84.2%) | 1.000 * |
| Pain and/or crying in more than 50% of bowel movements | 11 (57.9%) | 12 (63.2%) | 1.000 * |
| FOS Group (n = 18) | Control Group (n = 18) | p * | |
|---|---|---|---|
| Number of bowel movements per week | |||
| Baseline week | 6.27 ± 1.32 | 5.66 ± 1.87 | 0.133 |
| Last week of the study | 6.33 ± 1.28 | 6.11 ± 1.53 | 0.320 |
| Pain/crying when passing stools (percent of bowel movements) | |||
| Baseline week | 55.13 ± 44.07 | 60.09 ± 44.44 | 0.369 |
| Last week of the study | 14.68 ± 29.15 | 28.39 ± 43.82 | 0.138 |
| Straining/difficulty when passing stools (percent of bowel movements) | |||
| Baseline week | 84.47 ± 29.39 | 79.47 ± 37.63 | 0.330 |
| Last week of the study | 29.65 ± 41.73 | 55.07 ± 43.44 | 0.041 |
| Soft stool consistency (percent of bowel movements) | |||
| Baseline week | 12.12 ± 15.91 | 16.92 ± 15.07 | 0.180 |
| Last week of the study | 73.38 ± 29.38 | 55.38 ± 36.32 | 0.035 |
| FOS Group (n = 18) | Control Group (n = 18) | p | |
|---|---|---|---|
| Bifidobacterium | |||
| Baseline | 6.39 (5.25–8.36) | 6.61 (4.48–7.99) | 0.301 |
| End of the study | 7.37 (5.86–8.43) | 5.60 (4.46–6.42) | 0.006 |
| Lactobacillus | |||
| Baseline | 6.27 (4.33–7.54) | 6.03 (2.95–7.23) | 0.248 |
| End of the study | 6.45 (4.83–7.61) | 5.39 (3.37–6.73) | 0.095 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, D.d.S.; Tahan, S.; Weber, T.K.; Araujo-Filho, H.B.d.; De Morais, M.B. Randomized, Double-Blind, Placebo-Controlled Parallel Clinical Trial Assessing the Effect of Fructooligosaccharides in Infants with Constipation. Nutrients 2018, 10, 1602. https://doi.org/10.3390/nu10111602
Souza DdS, Tahan S, Weber TK, Araujo-Filho HBd, De Morais MB. Randomized, Double-Blind, Placebo-Controlled Parallel Clinical Trial Assessing the Effect of Fructooligosaccharides in Infants with Constipation. Nutrients. 2018; 10(11):1602. https://doi.org/10.3390/nu10111602
Chicago/Turabian StyleSouza, Daniela da Silva, Soraia Tahan, Thabata Koester Weber, Humberto Bezerra de Araujo-Filho, and Mauro Batista De Morais. 2018. "Randomized, Double-Blind, Placebo-Controlled Parallel Clinical Trial Assessing the Effect of Fructooligosaccharides in Infants with Constipation" Nutrients 10, no. 11: 1602. https://doi.org/10.3390/nu10111602
APA StyleSouza, D. d. S., Tahan, S., Weber, T. K., Araujo-Filho, H. B. d., & De Morais, M. B. (2018). Randomized, Double-Blind, Placebo-Controlled Parallel Clinical Trial Assessing the Effect of Fructooligosaccharides in Infants with Constipation. Nutrients, 10(11), 1602. https://doi.org/10.3390/nu10111602






