Diet Quality for Sodium and Vegetables Mediate Effects of Whole Food Diets on 8-Week Changes in Stress Load
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Measurements
2.3.1. Dietary Measurements
2.3.2. Stress Measurements
2.3.3. Physical Measurements
2.4. Bio-Specimen Collection and Biochemical Assays
2.5. Statistical Analysis
3. Results
4. Discussion
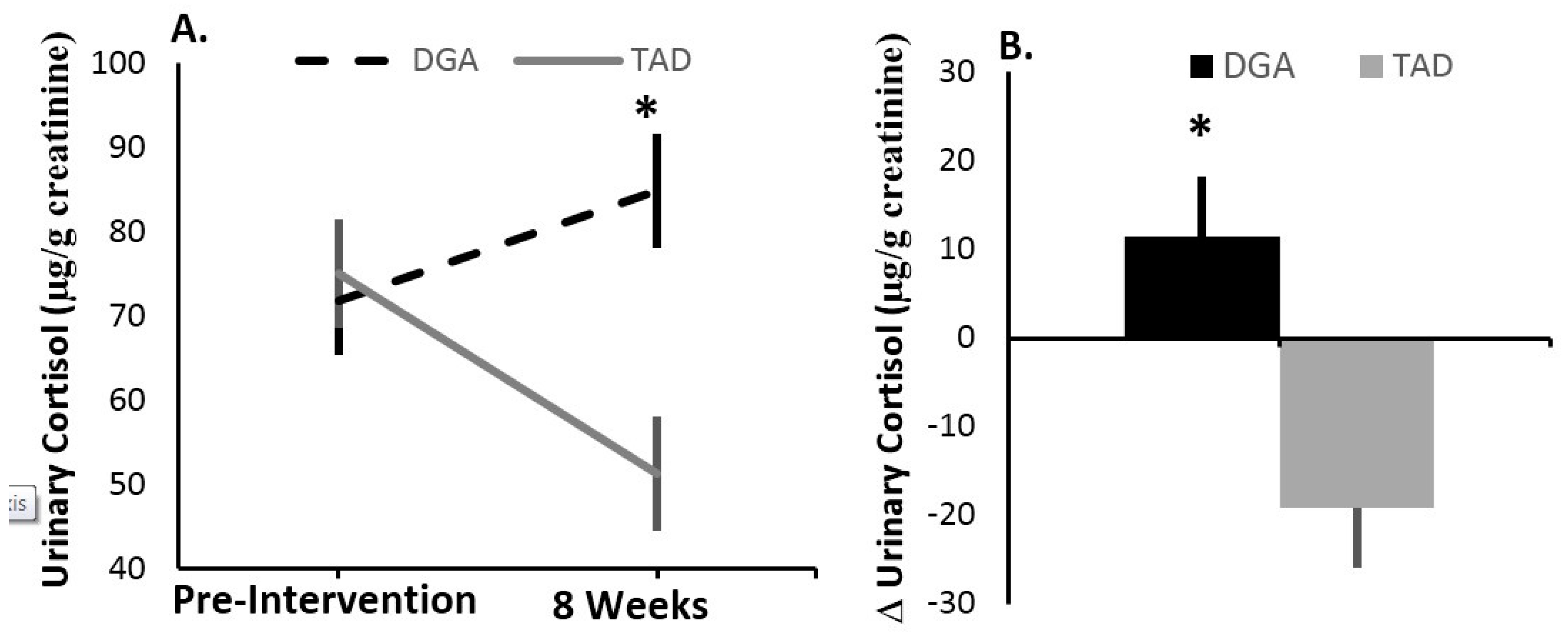
4.1. Stress Load—Effects of the Diet Intervention
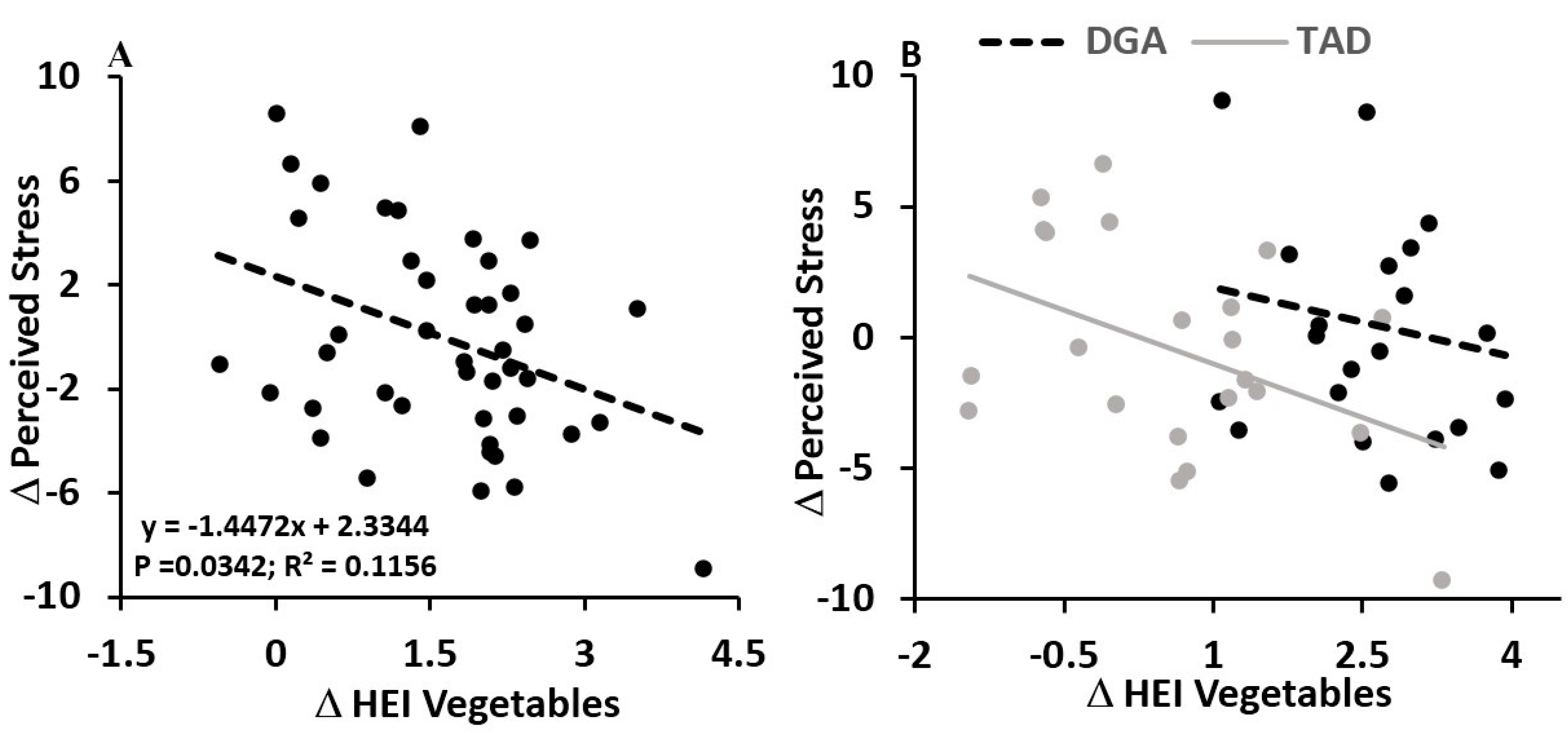
4.2. Diet Quality Effects—Vegetables and Perceived Stress
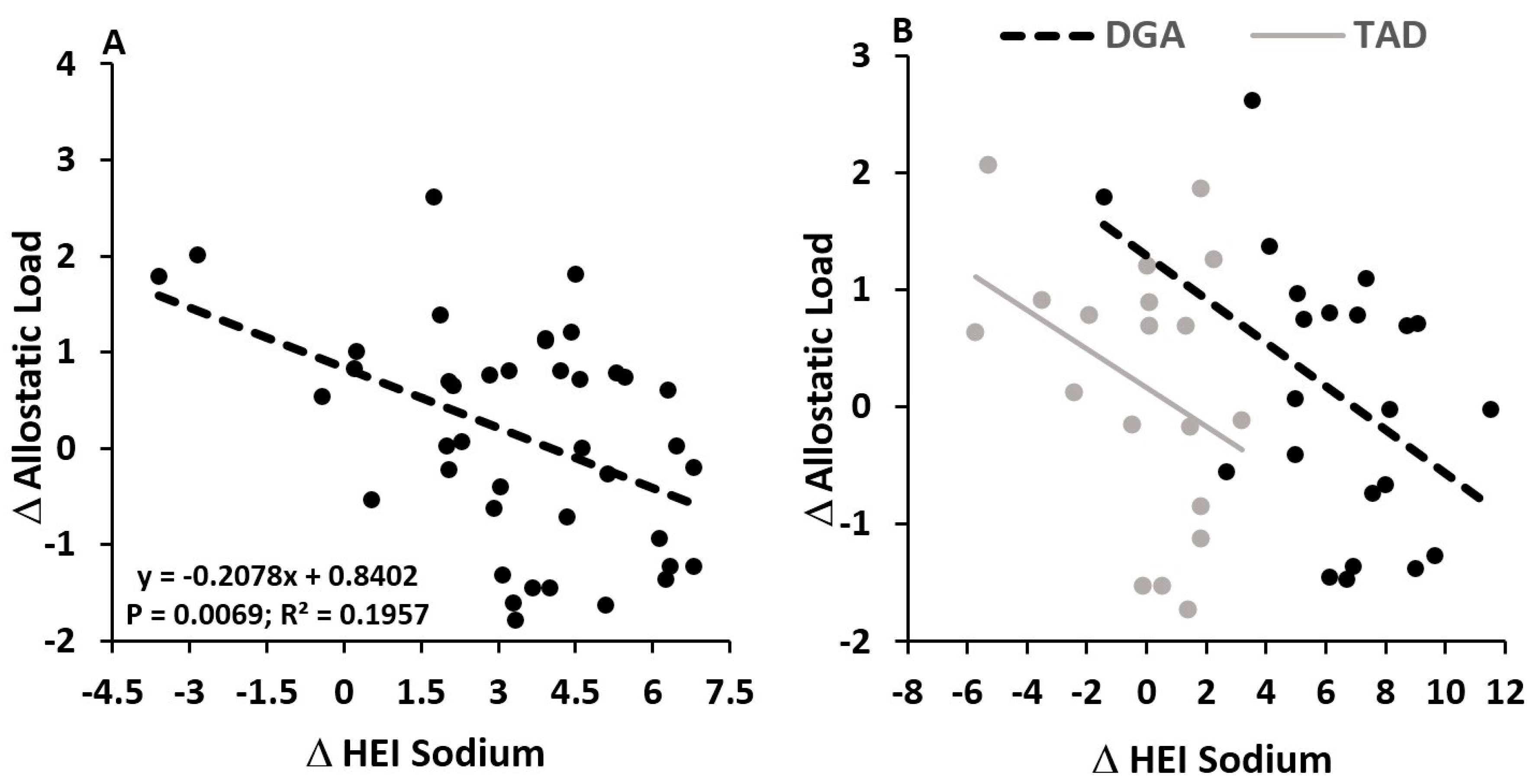
4.3. Diet Quality Effects—Sodium and Allostatic Load
4.4. Overall Implications
4.5. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanza-Martinez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Brody, S.; Preut, R.; Schommer, K.; Schurmeyer, T.H. A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology 2002, 159, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.; Schwartz, C.E.; Yang, H.; Joyner, M.J. Fish oil and neurovascular reactivity to mental stress in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R523–R530. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Kirk, J.; Rowsell, R.; Vitetta, L.; Sali, A.; Scholey, A.B.; Pipingas, A. The effect of multivitamin supplementation on mood and stress in healthy older men. Hum. Psychopharmacol. 2011, 26, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, J.; Fries, E.; Buss, C.; Engert, V.; Tuch, A.; Rutenberg, D.; Hellhammer, D. Effects of soy lecithin phosphatidic acid and phosphatidylserine complex (PAS) on the endocrine and psychological responses to mental stress. Stress 2004, 7, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, J.; Hero, T.; Franz, N.; Contreras, C.; Schubert, M. Omega-3 fatty acids administered in phosphatidylserine improved certain aspects of high chronic stress in men. Nutr. Res. 2012, 32, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, J.; Vogt, D.; Franz, N.; Freitas, U.; Rutenberg, D. A soy-based phosphatidylserine/phosphatidic acid complex (PAS) normalizes the stress reactivity of hypothalamus-pituitary-adrenal-axis in chronically stressed male subjects: A randomized, placebo-controlled study. Lipids Health Dis. 2014, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Jakulj, F.; Zernicke, K.; Bacon, S.L.; van Wielingen, L.E.; Key, B.L.; West, S.G.; Campbell, T.S. A high-fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. J. Nutr. 2007, 137, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Veasey, R.; Watson, A.; Dodd, F.; Jones, E.; Maggini, S.; Haskell, C.F. Effects of high-dose B vitamin complex with vitamin C and minerals on subjective mood and performance in healthy males. Psychopharmacology 2010, 211, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Long, S.J.; Benton, D. Effects of vitamin and mineral supplementation on stress, mild psychiatric symptoms, and mood in nonclinical samples: A meta-analysis. Psychosom. Med. 2013, 75, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.R.; Olivier, B.; de Haan, E.H. Whey protein rich in alpha-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. Am. J. Clin. Nutr. 2002, 75, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.R.; Olivier, B.; Panhuysen, G.E.; Van Der Gugten, J.; Alles, M.S.; Tuiten, A.; Westenberg, H.G.; Fekkes, D.; Koppeschaar, H.F.; de Haan, E.E. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am. J. Clin. Nutr. 2000, 71, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, R.; Schiff, I.; Wurtman, J.; Spiers, P.; McDermott, J.; Wurtman, R. The effect of a carbohydrate-rich beverage on mood, appetite, and cognitive function in women with premenstrual syndrome. Obstet. Gynecol. 1995, 86, 520–528. [Google Scholar] [CrossRef]
- Schubert, M.; Contreras, C.; Franz, N.; Hellhammer, J. Milk-based phospholipids increase morning cortisol availability and improve memory in chronically stressed men. Nutr. Res. 2011, 31, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Schult, J.; Hero, T.; Hellhammer, J. Effects of powdered fertilized eggs on the stress response. Clin. Nutr. 2010, 29, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, J.J.; Brzezinski, A.; Wurtman, R.J.; Laferrere, B. Effect of nutrient intake on premenstrual depression. Am. J. Obstet. Gynecol. 1989, 161, 1228–1234. [Google Scholar] [CrossRef]
- Hilimire, M.R.; DeVylder, J.E.; Forestell, C.A. Fermented foods, neuroticism, and social anxiety: An interaction model. Psychiatry Res. 2015, 228, 203–208. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans; USDA: Washington, DC, USA, 2015.
- Van Horn, L.; Carson, J.A.S.; Appel, L.J.; Burke, L.E.; Economos, C.; Karmally, W.; Lancaster, K.; Lichtenstein, A.H.; Johnson, R.K.; Thomas, R.J.; et al. Recommended Dietary Pattern to Achieve Adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e505–e529. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Look AHEAD Research Group. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef]
- Berk, K.A.; Buijks, H.I.M.; Verhoeven, A.J.M.; Mulder, M.T.; Özcan, B.; van’t Spijker, A.; Timman, R.; Busschbach, J.J.; Sijbrands, E.J. Group cognitive behavioural therapy and weight regain after diet in type 2 diabetes: Results from the randomised controlled POWER trial. Diabetologia 2018, 61, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity 2014, 22, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.M.; French, S.A.; Pagoto, S.L.; Sherwood, N.E. Managing temptation in obesity treatment: A neurobehavioral model of intervention strategies. Appetite 2016, 96, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Elfhag, K.; Rössner, S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes. Rev. 2005, 6, 67–85. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.S.; Wing, R.R.; Davidson, T.; Epstein, L.; Goodpaster, B.; Hall, K.D.; Levin, B.E.; Perri, M.G.; Rolls, B.J.; Rosenbaum, M.; et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity 2015, 23, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Papandonatos, G.; Fava, J.L.; Gorin, A.A.; Phelan, S.; McCaffery, J.; Tate, D.F. Maintaining large weight losses: The role of behavioral and psychological factors. J. Consult. Clin. Psychol. 2008, 76, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.B.; Cronin, F.J.; Krebs-Smith, S.M. Let the pyramid guide your food choices: Capturing the total diet concept. J. Nutr. 2001, 131, 461s–472s. [Google Scholar] [CrossRef] [PubMed]
- George, G.C.; Milani, T.J.; Hanss-Nuss, H.; Freeland-Graves, J.H. Compliance with dietary guidelines and relationship to psychosocial factors in low-income women in late postpartum. J. Am. Diet. Assoc. 2005, 105, 916–926. [Google Scholar] [CrossRef] [PubMed]
- American Psychological Association. Stress in America: 2013; American Psychological Association: Washington, DC, USA, 2013. [Google Scholar]
- Dallman, M.F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 2010, 21, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.; Tulloch, A.; Gold, M.S.; Avena, N.M. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat. Rev. Endocrinol. 2014, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Wansink, B. From mindless eating to mindlessly eating better. Physiol. Behav. 2010, 100, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.W.; Ryan, R.M.; Creswell, J.D. Mindfulness: Theoretical Foundations and Evidence for Its Salutary Effects. Psychol. Inq. 2007, 18, 211–237. [Google Scholar] [CrossRef]
- American Psychological Association. Stress in America: The State of Our Nation. Stress in AmericaTM Survey; American Psychological Association: Washington, DC, USA, 2017. [Google Scholar]
- Saad, L. Eight in 10 Americans Afflicted by Stress; Gallup: Washington, DC, USA, 2017. [Google Scholar]
- Mason, A.E.; Epel, E.S.; Aschbacher, K.; Lustig, R.H.; Acree, M.; Kristeller, J.; Cohn, M.; Dallman, M.; Moran, P.J.; Bacchetti, P.; et al. Reduced reward-driven eating accounts for the impact of a mindfulness-based diet and exercise intervention on weight loss: Data from the SHINE randomized controlled trial. Appetite 2016, 100, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.E.; Epel, E.S.; Kristeller, J.; Moran, P.J.; Dallman, M.; Lustig, R.H.; Acree, M.; Bacchetti, P.; Laraia, B.A.; Hecht, F.M.; et al. Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: Data from the SHINE randomized controlled trial. J. Behav. Med. 2016, 39, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Adams, S.H.; Allen, L.H.; Laugero, K.D.; Newman, J.W.; Stephensen, C.B.; Burnett, D.J.; Witbracht, M.; Welch, L.C.; Que, E.S. A randomized controlled-feeding trial based on the Dietary Guidelines for Americans on cardiometabolic health indexes. Am. J. Clin. Nutr. 2018, 108, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Lutz, L.J.; Gaffney-Stomberg, E.; Williams, K.W.; McGraw, S.M.; Niro, P.J.; Karl, J.P.; Cable, S.J.; Cropper, T.L.; McClung, J.P. Adherence to the Dietary Guidelines for Americans Is Associated with Psychological Resilience in Young Adults: A Cross-Sectional Study. J. Acad. Nutr. Dietet. 2017, 117, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S. The Automated Self-Administered 24-h dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Dietet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the healthy eating index: HEI-2010. J. Acad. Nutr. Dietet. 2013, 113, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Guenther, P.M.; Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Buckman, D.W.; Dodd, K.W.; Casavale, K.O.; Carroll, R.J. The Healthy Eating Index-2010 Is a Valid and Reliable Measure of Diet Quality According to the 2010 Dietary Guidelines for Americans. J. Nutr. 2013, 144, 399–407. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004, 1032, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators: Central role of the brain. Dialog. Clin. Neurosci. 2006, 8, 367–381. [Google Scholar]
- Juster, R.-P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.E.; McEwen, B.S.; Rowe, J.W.; Singer, B.H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl. Acad. Sci. USA 2001, 98, 4770–4775. [Google Scholar] [CrossRef] [PubMed]
- Karlamangla, A.S.; Singer, B.H.; McEwen, B.S.; Rowe, J.W.; Seeman, T.E. Allostatic load as a predictor of functional decline: MacArthur studies of successful aging. J. Clin. Epidemiol. 2002, 55, 696–710. [Google Scholar] [CrossRef]
- McEwen, B.S.; Seeman, T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.; Epel, E.; Gruenewald, T.; Karlamangla, A.; McEwen, B.S. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann. N. Y. Acad. Sci. 2010, 1186, 223–239. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
- Juster, R.-P.; Sindi, S.; Marin, M.-F.; Perna, A.; Hashemi, A.; Pruessner, J.C.; Lupien, S.J. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology 2011, 36, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.C.; Jimenez, J.A.; Shivpuri, S.; Espinosa de los Monteros, K.; Mills, P.J. Domains of chronic stress, lifestyle factors, and allostatic load in middle-aged Mexican-American women. Ann. Behav. Med. 2011, 41, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. Perceived stress scale. In Measuring Stress: A Guide for Health and Social Scientists; Oxford University Press: Oxford, UK, 1994; pp. 235–283. [Google Scholar]
- Roberti, J.W.; Harrington, L.N.; Storch, E.A. Further psychometric support for the 10-item version of the perceived stress scale. J. Coll. Counsel. 2006, 9, 135–147. [Google Scholar] [CrossRef]
- Lee, E.-H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs. Res. 2012, 6, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.J.-D. Denise, Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. J. Appl. Soc. Psychol. 2012, 42, 1320–1334. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Mann, T.; Vinas, D.; Hunger, J.M.; DeJager, J.; Taylor, S.E. Low Calorie Dieting Increases Cortisol. Psychosom. Med. 2010, 72, 357–364. [Google Scholar] [CrossRef] [PubMed]
- French, S.A.; Jeffery, R.W. Consequences of dieting to lose weight: Effects on physical and mental health. Health Psychol. 1994, 13, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Polivy, J. Psychological Consequences of Food Restriction. J. Am. Diet. Assoc. 1996, 96, 589–592. [Google Scholar] [CrossRef]
- Mann, T.; Tomiyama, A.J.; Westling, E.; Lew, A.M.; Samuels, B.; Chatman, J. Medicare’s search for effective obesity treatments: Diets are not the answer. Am. Psychol. 2007, 62, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A.; Shapiro, J.R.; Lundgren, J.D.; Spataro, L.E.; Frye, C.A. Self-reported dietary restraint is associated with elevated levels of salivary cortisol. Appetite 2002, 38, 13–17. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.A.; Barr, S.I.; Prior, J.C. Cognitive dietary restraint is associated with higher urinary cortisol excretion in healthy premenopausal women. Am. J. Clin. Nutr. 2001, 73, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rideout, C.A.; Linden, W.; Barr, S.I. High cognitive dietary restraint is associated with increased cortisol excretion in postmenopausal women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 628–633. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef] [PubMed]
- Tryon, M.S.; Stanhope, K.L.; Epel, E.S.; Mason, A.E.; Brown, R.; Medici, V.; Havel, P.J.; Laugero, K.D. Excessive Sugar Consumption May Be a Difficult Habit to Break: A View From the Brain and Body. J. Clin. Endocrinol. Metab. 2015, 100, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Exebio, J.C.; Zarini, G.G.; Exebio, C.; Huffman, F.G. Healthy Eating Index scores associated with symptoms of depression in Cuban-Americans with and without type 2 diabetes: A cross sectional study. Nutr. J. 2011, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, M.F.; Cremer, A.; Hotchkiss, L.; Cotugna, N.; Evans, M.K.; Zonderman, A.B. Higher HEI-2005 scores associated with reduced symptoms of depression in an urban population: Findings from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. J. Am. Diet. Assoc. 2010, 110, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Milajerdi, A.; Dorosty-Motlagh, A. Association of the Alternative Healthy Eating Index (AHEI-2010) with depression, stress and anxiety among Iranian military personnel. J. R. Army Med. Corps 2018, 164, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Valipour, G.; Esmaillzadeh, A.; Azadbakht, L.; Afshar, H.; Hassanzadeh, A.; Adibi, P. Adherence to the DASH diet in relation to psychological profile of Iranian adults. Eur. J. Nutr. 2017, 56, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Algren, M.H.; Ekholm, O.; Nielsen, L.; Ersboll, A.K.; Bak, C.K.; Andersen, P.T. Associations between perceived stress, socioeconomic status, and health-risk behaviour in deprived neighbourhoods in Denmark: A cross-sectional study. BMC Public Health 2018, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Barrington, W.E.; Ceballos, R.M.; Bishop, S.K.; McGregor, B.A.; Beresford, S.A. Perceived stress, behavior, and body mass index among adults participating in a worksite obesity prevention program, Seattle, 2005–2007. Prev. Chronic Dis. 2012, 9, E152. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Almeida, O.P.; English, D.R.; Giles, G.G.; Flicker, L. Patterns of dietary intake and psychological distress in older Australians: Benefits not just from a Mediterranean diet. Int. Psychogeriatr. 2013, 25, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Ding, D.; Mihrshahi, S. Fruit and vegetable consumption and psychological distress: Cross-sectional and longitudinal analyses based on a large Australian sample. BMJ Open 2017, 7, e014201. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.; Rohrmann, S.; Vandeleur, C.L.; Mohler-Kuo, M.; Eichholzer, M. Associations between fruit and vegetable consumption and psychological distress: Results from a population-based study. BMC Psychiatry 2015, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, Y.; Li, F.; Zhang, D. Fruit and vegetable consumption and the risk of depression: A meta-analysis. Nutrition 2016, 32, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Blanchflower, D.G.; Oswald, A.J.; Stewart-Brown, S. Is Psychological Well-Being Linked to the Consumption of Fruit and Vegetables? Soc. Indic. Res. 2013, 114, 785–801. [Google Scholar] [CrossRef]
- McMartin, S.E.; Jacka, F.N.; Colman, I. The association between fruit and vegetable consumption and mental health disorders: Evidence from five waves of a national survey of Canadians. Prev. Med. 2013, 56, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Tell, G.S.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: The Hordaland Homocysteine Study. Arch. Gen. Psychiatry 2003, 60, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Tucker, K.L. Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. J. Med. Investig. 2005, 52, 252–258. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M. Zinc deficiency and child development. Am. J. Clin. Nutr. 1998, 68 (Suppl. 2), 464s–469s. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.C.; Lobato, K.R.; Binfare, R.W.; Ferreira, P.K.; Rosa, A.O.; Santos, A.R.; Rodrigues, A.L. Evidence for the involvement of the monoaminergic system in the antidepressant-like effect of magnesium. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gareri, P.; Mattace, R.; Nava, F.; De Sarro, G. Role of calcium in brain aging. Gen. Pharmacol. 1995, 26, 1651–1657. [Google Scholar] [CrossRef]
- Bottiglieri, T. Homocysteine and folate metabolism in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.; Reynolds, E.H. Homocysteine, folate, methylation, and monoamine metabolism in depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Dubowitz, T.; Heron, M.; Bird, C.E.; Lurie, N.; Finch, B.K.; Basurto-Dávila, R.; Hale, L.; Escarce, J.J. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. Am. J. Clin. Nutr. 2008, 87, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, C.B.; Giskes, K.; de Bruijn, G.-J.; Wendel-Vos, W.; Brug, J.; Van Lenthe, F.J. Environmental determinants of fruit and vegetable consumption among adults: A systematic review. Br. J. Nutr. 2006, 96, 620–635. [Google Scholar] [PubMed]
- Yeh, M.-C.; Ickes, S.B.; Lowenstein, L.M.; Shuval, K.; Ammerman, A.S.; Farris, R.; Katz, D.L. Understanding barriers and facilitators of fruit and vegetable consumption among a diverse multi-ethnic population in the USA. Health Promot. Int. 2008, 23, 42–51. [Google Scholar] [CrossRef] [PubMed]
- McEWEN, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; Noel, S.E.; Tucker, K.L. A meat, processed meat, and French fries dietary pattern is associated with high allostatic load in Puerto Rican older adults. J. Am. Diet. Assoc. 2011, 111, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; Bhupathiraju, S.; Tucker, K.L. Higher adherence to a diet score based on American Heart Association recommendations is associated with lower odds of allostatic load and metabolic syndrome in Puerto Rican adults. J. Nutr. 2013, 143, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Campino, C.; Carvajal, C.A.; Olivieri, O.; Guidi, G.; Faccini, G.; Vohringer, P.A.; Cerda, J.; Owen, G.; Kalergis, A.M.; et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin. Endocrinol. 2014, 80, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, E.N.; Lugibihl, K.; Laves, M.; Janulis, M. Reciprocal Variations in Urinary Cortisol and Aldosterone in Response to Increased Salt Intake in Humans. J. Clin. Endocrinol. Metab. 1966, 26, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Wambach, G.; Bleienheuft, C.; Bonner, G. Sodium loading raises urinary cortisol in man. J. Endocrinol. Investig. 1986, 9, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Lewicka, S.; Nowicki, M.; Vecsei, P. Effect of sodium restriction on urinary excretion of cortisol and its metabolites in humans. Steroids 1998, 63, 401–405. [Google Scholar] [CrossRef]
- Incollingo Rodriguez, A.C.; Epel, E.S.; White, M.L.; Standen, E.C.; Seckl, J.R.; Tomiyama, A.J. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology 2015, 62, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Marmar, C.R.; Pipkin, S.S.; Moos, R.; Browner, W.S.; Whooley, M.A. Depression and 24-h Urinary Cortisol in Medical Outpatients with Coronary Heart Disease: The Heart and Soul Study. Biol. Psychiatry 2004, 56, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.; Mason, P.A.; Buckingham, J.C.; Gordon, R.D.; Morton, J.J.; Nicholls, M.G.; Semple, P.F.; Tree, M. The interaction of sodium and potassium status, of acth and of angiotensin II in the control of corticosteroid secretion. J. Steroid Biochem. 1979, 11, 1039–1042. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.T.; Nieman, L.K.; Feelders, R.A. Comorbidities in Cushing’s disease. Pituitary 2015, 18, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Tryon, M.S.; DeCant, R.; Laugero, K.D. Having your cake and eating it too: A habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiol. Behav. 2013, 114–115, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.C.; Epel, E.S. Stress, eating and the reward system. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef] [PubMed]
| Stress Marker | Diet | Pre-Intervention | 8 Weeks | 8-Week Change |
|---|---|---|---|---|
| Perceived Stress | DGA | 10.92 ± 1.47 | 13.23 ± 1.00 | 0.53 ± 1.00 |
| TAD | 14.48 ± 1.40 | 12.13 ± 1.00 | −0.57 ± 1.00 | |
| Allostatic Load | DGA | 2.42 ± 0.37 | 2.42 ± 0.26 | 0.001 ± 0.26 |
| TAD | 2.41 ± 0.40 | 2.52 ± 0.28 | 0.105 ± 0.28 |
| Stress Marker | Diet | Pre-Intervention | 8 Weeks | 8-Week Change |
|---|---|---|---|---|
| ** Perceived Stress | DGA | 10.92 ± 1.47 | 14.87 ± 1.20 a | 2.16 ± 1.20 b |
| TAD | 14.48 ± 1.40 | 10.50 ± 1.20 | −2.21 ± 1.20 | |
| *** Allostatic Load | DGA | 2.42 ± 0.37 | 3.11 ± 0.34 a | 0.70 ± 0.34 b |
| TAD | 2.41 ± 0.40 | 1.71 ± 0.38 | −0.70 ± 0.38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltani, H.; Keim, N.L.; Laugero, K.D. Diet Quality for Sodium and Vegetables Mediate Effects of Whole Food Diets on 8-Week Changes in Stress Load. Nutrients 2018, 10, 1606. https://doi.org/10.3390/nu10111606
Soltani H, Keim NL, Laugero KD. Diet Quality for Sodium and Vegetables Mediate Effects of Whole Food Diets on 8-Week Changes in Stress Load. Nutrients. 2018; 10(11):1606. https://doi.org/10.3390/nu10111606
Chicago/Turabian StyleSoltani, Hoda, Nancy L. Keim, and Kevin D. Laugero. 2018. "Diet Quality for Sodium and Vegetables Mediate Effects of Whole Food Diets on 8-Week Changes in Stress Load" Nutrients 10, no. 11: 1606. https://doi.org/10.3390/nu10111606
APA StyleSoltani, H., Keim, N. L., & Laugero, K. D. (2018). Diet Quality for Sodium and Vegetables Mediate Effects of Whole Food Diets on 8-Week Changes in Stress Load. Nutrients, 10(11), 1606. https://doi.org/10.3390/nu10111606




