Fish Intake in Pregnancy and Offspring Metabolic Parameters at Age 9–16—Does Gestational Diabetes Modify the Risk?
Abstract
1. Introduction
2. Materials and Methods
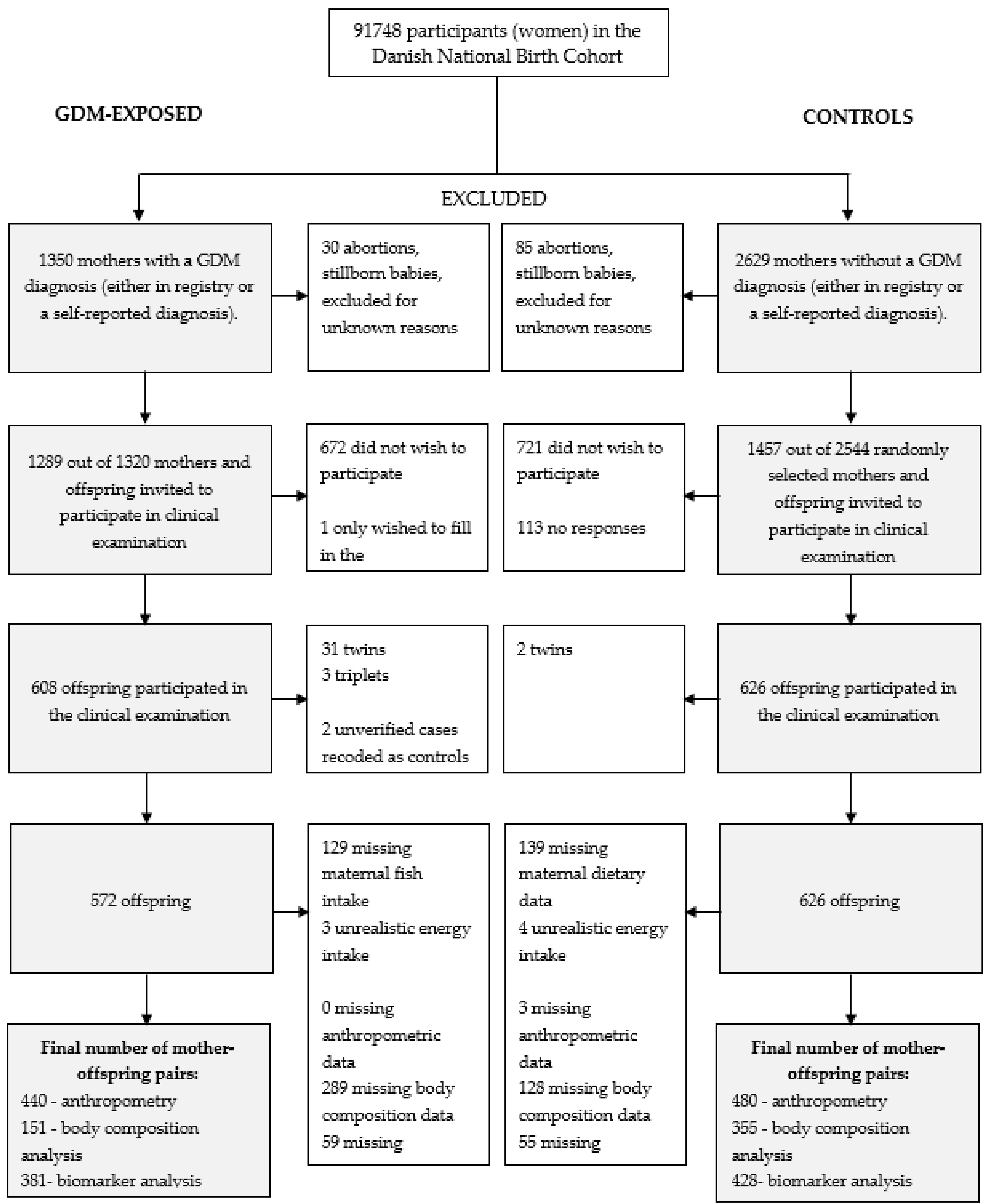
2.1. Study Population
2.2. Maternal Dietary Intake
2.2.1. Food Frequency Questionnaire Data
2.2.2. Interview Data
2.3. Offspring Follow-Up
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Maternal Fish Intake and Offspring Metabolic Outcomes in the GDM-Exposed Group
3.3. Maternal Fish Intake and Offspring Metabolic Outcomes in the Control Group
3.4. Maternal Dietary n-3 LCPUFA Intake in GW 25 and Offspring Metabolic Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahluwalia, N.; Dalmasso, P.; Rasmussen, M.; Lipsky, L.; Currie, C.; Haug, E.; Kelly, C.; Damsgaard, M.T.; Due, P.; Tabak, I.; et al. Trends in overweight prevalence among 11-, 13- and 15-year-olds in 25 countries in Europe, Canada and USA from 2002 to 2010. Eur. J. Public Health 2015, 25 (Suppl. 2), 28–32. [Google Scholar] [CrossRef]
- De Ferranti, S.D.; Osganian, S.K. Epidemiology of paediatric metabolic syndrome and type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2007, 4, 285–296. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care 2000, 23, 381–389. [Google Scholar] [CrossRef]
- Sinha, R.; Fisch, G.; Teague, B.; Tamborlane, W.V.; Banyas, B.; Allen, K.; Savoye, M.; Rieger, V.; Taksali, S.; Barbetta, G.; et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002, 346, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V. Omega-3 fatty acids and the cardiometabolic syndrome. J. Cardiometabolic Syndr. 2008, 3, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Brunner, S.; Amann-Gassner, U. The role of dietary fatty acids for early human adipose tissue growth. Am. J. Clin. Nutr. 2013, 98, 549s–555s. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern. Child Nutr. 2011, 7 (Suppl. 2), 112–123. [Google Scholar] [CrossRef] [PubMed]
- D’Asti, E.; Long, H.; Tremblay-Mercier, J.; Grajzer, M.; Cunnane, S.C.; Di Marzo, V.; Walker, C.D. Maternal dietary fat determines metabolic profile and the magnitude of endocannabinoid inhibition of the stress response in neonatal rat offspring. Endocrinology 2010, 151, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Hollander, K.S.; Tempel Brami, C.; Konikoff, F.M.; Fainaru, M.; Leikin-Frenkel, A. Dietary enrichment with alpha-linolenic acid during pregnancy attenuates insulin resistance in adult offspring in mice. Arch. Physiol. Biochem. 2014, 120, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Nookaew, I.; Khoomrung, S.; Andersson, L.; Larsson, I.; Hulthen, L.; Jansson, N.; Jakubowicz, R.; Nilsson, S.; Sandberg, A.S.; et al. A maternal diet of fatty fish reduces body fat of offspring compared with a maternal diet of beef and a post-weaning diet of fish improves insulin sensitivity and lipid profile in adult C57BL/6 male mice. Acta Physiol. 2013, 209, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, F.L.; Fernandes, F.S.; Tavares do Carmo, M.G.; Herrera, E. Sex-dependent nutritional programming: Fish oil intake during early pregnancy in rats reduces age-dependent insulin resistance in male, but not female, offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R313–R320. [Google Scholar] [CrossRef] [PubMed]
- Siemelink, M.; Verhoef, A.; Dormans, J.A.; Span, P.N.; Piersma, A.H. Dietary fatty acid composition during pregnancy and lactation in the rat programs growth and glucose metabolism in the offspring. Diabetologia 2002, 45, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Soulimane-Mokhtari, N.A.; Guermouche, B.; Yessoufou, A.; Saker, M.; Moutairou, K.; Hichami, A.; Merzouk, H.; Khan, N.A. Modulation of lipid metabolism by n-3 polyunsaturated fatty acids in gestational diabetic rats and their macrosomic offspring. Clin. Sci. 2005, 109, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, A.; Soulaimann, N.; Merzouk, S.A.; Moutairou, K.; Ahissou, H.; Prost, J.; Simonin, A.M.; Merzouk, H.; Hichami, A.; Khan, N.A. N-3 fatty acids modulate antioxidant status in diabetic rats and their macrosomic offspring. Int. J. Obes. 2006, 30, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, S.W.; Wijga, A.H.; van Rossem, L.; Gehring, U.; Koppelman, G.H.; Smit, H.A.; Boer, J.M. Maternal fish consumption during pregnancy and BMI in children from birth up to age 14 years: The PIAMA cohort study. Eur. J. Nutr. 2016, 55, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Osmond, C.; Phillips, D.I.; Godfrey, K.M. Maternal BMI, parity, and pregnancy weight gain: Influences on offspring adiposity in young adulthood. J. Clin. Endocrinol. Metab. 2010, 95, 5365–5369. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Quinn, S.; Dwyer, T.; Ponsonby, A.L.; Jones, G. Maternal diet, breastfeeding and adolescent body composition: A 16-year prospective study. Eur. J. Clin. Nutr. 2012, 66, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, N.; Roumeliotaki, T.; Oken, E.; Barros, H.; Basterrechea, M.; Charles, M.A.; Eggesbo, M.; Forastiere, F.; Gaillard, R.; Gehring, U.; et al. Fish Intake in Pregnancy and Child Growth: A Pooled Analysis of 15 European and US Birth Cohorts. JAMA Pediatr. 2016, 170, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Campoy, C.; Escolano-Margarit, M.V.; Ramos, R.; Parrilla-Roure, M.; Csabi, G.; Beyer, J.; Ramirez-Tortosa, M.C.; Molloy, A.M.; Decsi, T.; Koletzko, B.V. Effects of prenatal fish-oil and 5-methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. Am. J. Clin. Nutr. 2011, 94, 1880s–1888s. [Google Scholar] [CrossRef] [PubMed]
- De Vries, P.S.; Gielen, M.; Rizopoulos, D.; Rump, P.; Godschalk, R.; Hornstra, G.; Zeegers, M.P. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Helland, I.B.; Smith, L.; Blomen, B.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Effect of supplementing pregnant and lactating mothers with n-3 very-long-chain fatty acids on children’s IQ and body mass index at 7 years of age. Pediatrics 2008, 122, e472–e479. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Harvey, N.C.; Robinson, S.M.; Ntani, G.; Davies, J.H.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Calder, P.C.; Cooper, C. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J. Clin. Endocrinol. Metab. 2013, 98, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Rytter, D.; Bech, B.H.; Christensen, J.H.; Schmidt, E.B.; Henriksen, T.B.; Olsen, S.F. Intake of fish oil during pregnancy and adiposity in 19-y-old offspring: Follow-up on a randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Rytter, D.; Bech, B.H.; Halldorsson, T.; Christensen, J.H.; Schmidt, E.B.; Danielsen, I.; Henriksen, T.B.; Olsen, S.F. No association between the intake of marine n-3 PUFA during the second trimester of pregnancy and factors associated with cardiometabolic risk in the 20-year-old offspring. Br. J. Nutr. 2013, 110, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.D.; Wang, M.; Martorell, R.; Neufeld, L.M.; Flores-Ayala, R.; Rivera, J.A.; Ramakrishnan, U. Growth to age 18 months following prenatal supplementation with docosahexaenoic acid differs by maternal gravidity in Mexico. J. Nutr. 2011, 141, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Rytter, D.; Schmidt, E.B.; Bech, B.H.; Christensen, J.H.; Henriksen, T.B.; Olsen, S.F. Fish oil supplementation during late pregnancy does not influence plasma lipids or lipoprotein levels in young adult offspring. Lipids 2011, 46, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Courville, A.B.; Harel, O.; Lammi-Keefe, C.J. Consumption of a DHA-containing functional food during pregnancy is associated with lower infant ponderal index and cord plasma insulin concentration. Br. J. Nutr. 2011, 106, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Much, D.; Brunner, S.; Vollhardt, C.; Schmid, D.; Sedlmeier, E.M.; Bruderl, M.; Heimberg, E.; Bartke, N.; Boehm, G.; Bader, B.L.; et al. Effect of dietary intervention to reduce the n-6/n-3 fatty acid ratio on maternal and fetal fatty acid profile and its relation to offspring growth and body composition at 1 year of age. Eur. J. Clin. Nutr. 2013, 67, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lucia Bergmann, R.; Bergmann, K.E.; Haschke-Becher, E.; Richter, R.; Dudenhausen, J.W.; Barclay, D.; Haschke, F. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J. Perinat. Med. 2007, 35, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Muhlhausler, B.S.; Gibson, R.A.; Makrides, M. Effect of long-chain polyunsaturated fatty acid supplementation during pregnancy or lactation on infant and child body composition: A systematic review. Am. J. Clin. Nutr. 2010, 92, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.; Iglesia, I.; Bel-Serrat, S.; Moreno, L.A. Effect of n-3 long chain polyunsaturated fatty acids during the perinatal period on later body composition. Br. J. Nutr. 2012, 107 (Suppl. 2), S117–S128. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Pagan, A.; Prieto-Sanchez, M.T.; Blanco-Carnero, J.E.; Gil-Sanchez, A.; Parrilla, J.J.; Demmelmair, H.; Koletzko, B.; Larque, E. Materno-fetal transfer of docosahexaenoic acid is impaired by gestational diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E826–E833. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Bendel, R.B.; Couch, S.C.; Philipson, E.H.; Cheruku, S.; Lammi-Keefe, C.J. Fetal erythrocyte phospholipid polyunsaturated fatty acids are altered in pregnancy complicated with gestational diabetes mellitus. Lipids 2000, 35, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Bendel, R.B.; Couch, S.C.; Philipson, E.H.; Thomsen, K.; Zhang, X.; Lammi-Keefe, C.J. Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: Relations with maternal factors. Am. J. Clin. Nutr. 1999, 70, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Schmidt, L.; Damm, P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Melbye, M.; Olsen, S.F.; Sorensen, T.I.; Aaby, P.; Andersen, A.M.; Taxbol, D.; Hansen, K.D.; Juhl, M.; Schow, T.B.; et al. The Danish National Birth Cohort--its background, structure and aim. Scand. J. Public Health 2001, 29, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, F.B.; Olsen, S.F.; Vaag, A.; Gore-Langton, R.; Chavarro, J.E.; Bao, W.; Yeung, E.; Bowers, K.; Grunnet, L.G.; et al. Rationale, design, and method of the Diabetes & Women’s Health study—A study of long-term health implications of glucose intolerance in pregnancy and their determinants. Acta Obstet. Gynecol. Scand. 2014, 93, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Houshmand-Oeregaard, A.; Granstrom, C.; Langhoff-Roos, J.; Damm, P.; Bech, B.H.; Vaag, A.A.; Zhang, C. Diagnosing gestational diabetes mellitus in the Danish National Birth Cohort. Acta Obstet. Gynecol. Scand. 2017, 96, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Damm, P.; Handberg, A.; Kuhl, C.; Beck-Nielsen, H.; Molsted-Pedersen, L. Insulin receptor binding and tyrosine kinase activity in skeletal muscle from normal pregnant women and women with gestational diabetes. Obstet. Gynecol. 1993, 82, 251–259. [Google Scholar] [PubMed]
- Olsen, S.F.; Mikkelsen, T.B.; Knudsen, V.K.; Orozova-Bekkevold, I.; Halldorsson, T.I.; Strom, M.; Osterdal, M.L. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr. Perinat. Epidemiol. 2007, 21, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Mikkelsen, T.B.; Osler, M.; Olsen, S.F. Validity of protein, retinol, folic acid and n-3 fatty acid intakes estimated from the food-frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutr. 2006, 9, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.W.; Hanson, R.L.; Knowler, W.C.; Moffett, C.; Enos, G.; Infante, A.M.; Krakoff, J.; Looker, H.C. Childhood predictors of young-onset type 2 diabetes. Diabetes 2007, 56, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Hanson, R.L.; Lindsay, R.S.; Pettitt, D.J.; Imperatore, G.; Gabir, M.M.; Roumain, J.; Bennett, P.H.; Knowler, W.C. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: A study of discordant sibships. Diabetes 2000, 49, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, D.J.; Baird, H.R.; Aleck, K.A.; Bennett, P.H.; Knowler, W.C. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N. Engl. J. Med. 1983, 308, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Bennett, P.H.; Hamman, R.F.; Miller, M. Diabetes incidence and prevalence in Pima Indians: A 19-fold greater incidence than in Rochester, Minnesota. Am. J. Epidemiol. 1978, 108, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, K.F.; Brussaard, J.H.; Kruizinga, A.G.; Telman, J.; Lowik, M.R. Socio-economic status, dietary intake and 10 y trends: The Dutch National Food Consumption Survey. Eur. J. Clin. Nutr. 2003, 57, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Levitan, E.B.; Gillman, M.W. Maternal smoking during pregnancy and child overweight: Systematic review and meta-analysis. Int. J. Obes. 2008, 32, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Mihalopoulos, N.L.; Holubkov, R.; Young, P.; Dai, S.; Labarthe, D.R. Expected changes in clinical measures of adiposity during puberty. J. Adolesc. Health 2010, 47, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.A.; Cutfield, W.S. Exercise in pregnancy: Weighing up the long-term impact on the next generation. Exerc. Sport Sci. Rev. 2011, 39, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.Y.; Tint, M.T.; Aris, I.M.; Chen, L.W.; Quah, P.L.; Tan, K.H.; Yeo, G.S.; Fortier, M.V.; Yap, F.; Shek, L.; et al. Maternal plasma phosphatidylcholine polyunsaturated fatty acids during pregnancy and offspring growth and adiposity. Prostaglandins Leukot. Essent. Fatty Acids 2017, 121, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Silventoinen, K.; Baker, J.L.; Sorensen, T.I. Growth in height in childhood and risk of coronary heart disease in adult men and women. PLoS ONE 2012, 7, e30476. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health & Human Services, U.S. Food and Drug Administration. Fish: What Pregnant Women and Parents Should Know. Available online: www.fda.gov/food/foodborneillnesscontaminants/metals/ucm393070.htm (accessed on 6 May 2016).
- Grunnet, L.G.; Hansen, S.; Hjort, L.; Madsen, C.M.; Kampmann, F.B.; Thuesen, A.C.B.; Granstromi, C.; Strom, M.; Maslova, E.; Frikke-Schmidt, R.; et al. Adiposity, Dysmetabolic Traits, and Earlier Onset of Female Puberty in Adolescent Offspring of Women with Gestational Diabetes Mellitus: A Clinical Study Within the Danish National Birth Cohort. Diabetes Care 2017, 40, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Maslova, E.; Hansen, S.; Grunnet, L.G.; Strom, M.; Bjerregaard, A.A.; Hjort, L.; Kampmann, F.B.; Madsen, C.M.; Baun Thuesen, A.C.; Bech, B.H.; et al. Maternal protein intake in pregnancy and offspring metabolic health at age 9-16 y: Results from a Danish cohort of gestational diabetes mellitus pregnancies and controls. Am. J. Clin. Nutr. 2017, 106, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.S.; Harreiter, J.; Damm, P.; Corcoy, R.; Chico, A.; Simmons, D.; Vellinga, A.; Dunne, F. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabet. Med. 2012, 29, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Contreras, R.; Chen, W.; Sacks, D.A. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care 2008, 31, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, A.J.; Jaddoe, V.W.V.; Voortman, T.; Demmelmair, H.; Koletzko, B.; Gaillard, R. Maternal plasma polyunsaturated fatty acid levels during pregnancy and childhood lipids and insulin levels. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Levy, E.; Fraser, W.D.; Julien, P.; Delvin, E.; Montoudis, A.; Spahis, S.; Garofalo, C.; Nuyt, A.M.; Luo, Z.C. Circulating docosahexaenoic acid levels are associated with fetal insulin sensitivity. PLoS ONE 2014, 9, e85054. [Google Scholar] [CrossRef] [PubMed]
- Rump, P.; Popp-Snijders, C.; Heine, R.J.; Hornstra, G. Components of the insulin resistance syndrome in seven-year-old children: Relations with birth weight and the polyunsaturated fatty acid content of umbilical cord plasma phospholipids. Diabetologia 2002, 45, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Drouillet, P.; Forhan, A.; De Lauzon-Guillain, B.; Thiebaugeorges, O.; Goua, V.; Magnin, G.; Schweitzer, M.; Kaminski, M.; Ducimetiere, P.; Charles, M.A. Maternal fatty acid intake and fetal growth: Evidence for an association in overweight women. The ‘EDEN mother-child’ cohort (study of pre- and early postnatal determinants of the child’s development and health). Br. J. Nutr. 2009, 101, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Hakola, L.; Takkinen, H.M.; Niinisto, S.; Ahonen, S.; Erlund, I.; Rautanen, J.; Veijola, R.; Ilonen, J.; Toppari, J.; Knip, M.; et al. Maternal fatty acid intake during pregnancy and the development of childhood overweight: A birth cohort study. Pediatr. Obes. 2017, 12 (Suppl. 1), 26–37. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; van den Hooven, E.H.; Braun, K.V.; van den Broek, M.; Bramer, W.M.; Chowdhurry, R.; Franco, O.H. Effects of polyunsaturated fatty acid intake and status during pregnancy, lactation, and early childhood on cardiometabolic health: A systematic review. Prog. Lipid Res. 2015, 59, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.R.; Harvey, N.C.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Robinson, S.M. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: Findings from the Southampton Women’s Survey. Am. J. Clin. Nutr. 2012, 96, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Veena, S.R.; Winder, N.R.; Hill, J.C.; Noonan, K.; Boucher, B.J.; Karat, S.C.; Fall, C.H. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: The Mysore Parthenon Study. Am. J. Clin. Nutr. 2011, 93, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Rodriguez, A.; Valvi, D.; Iniguez, C.; Esplugues, A.; Vioque, J.; Marina, L.S.; Jimenez, A.; Espada, M.; Dehli, C.R.; et al. Deficit of vitamin D in pregnancy and growth and overweight in the offspring. Int. J. Obes. 2015, 39, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.N.; Laughon, S.K.; Catov, J.M.; Olsen, J.; Bech, B.H. First trimester coffee and tea intake and risk of gestational diabetes mellitus: A study within a national birth cohort. BJOG 2015, 122, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, C.; Maindal, H.T.; Kristensen, J.K.; Ovesen, P.G.; Witte, D.R. National study of the prevalence of gestational diabetes mellitus among Danish women from 2004 to 2012. Scand. J. Public Health 2017, 45, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Sundhedsstyrelsen. Information Til Gravide. Available online: https://www.sst.dk/da/sundhed-og-livsstil/graviditet-og-foedsel/information-til-gravide (accessed on 9 October 2018).
| Categories of Total Seafood Intake | |||||
| 0–0.5 oz/day | >0.5–1 oz/day | 1–1.5 oz/day | >1.5 oz/day | p1 | |
| GDM-exposed (n = 443) | % or means (SD) | % or means (SD) | % or means (SD) | % or means (SD) | |
| Lean fish intake (oz/day) | 0.1 (0.1) | 0.2 (0.2) | 0.4 (0.2) | 0.6 (0.4) | <0.0001 |
| Oily fish intake (oz/day) | 0.0 (0.1) | 0.1 (0.1) | 0.2 (0.2) | 0.3 (0.3) | <0.0001 |
| Marine n-3 LCPUFA (g/day) | 0.2 (0.1) | 0.4 (0.2) | 0.5 (0.2) | 0.8 (0.5) | <0.0001 |
| Total fat intake (g/day) | 83.4 (18.2) | 81.9 (15.8) | 79.5 (16.1) | 79.2 (16.0) | 0.20 |
| Carbohydrate intake (g/day) | 317.4 (36.9) | 313.6 (38.3) | 317.8 (35.3) | 309.8 (35.4) | 0.33 |
| Protein intake (g/day) | 88.3 (15.8) | 91.7 (12.4) | 95.5 (13.6) | 102.7 (15.7) | <0.0001 |
| Maternal age (years) | 31.2 (4.6) | 32.1 (4.2) | 32.4 (4.1) | 32.2 (4.5) | 0.12 |
| Nulliparous (%) | 44 | 36 | 38 | 29 | 0.32 |
| High/medium sociodemographic position (%) | 49 | 45 | 56 | 57 | 0.63 |
| No physical activity (min/week) (%) | 65 | 60 | 67 | 68 | 0.51 |
| Pre-pregnancy BMI 18.6–24.9 kg/m2 (%) | 35 | 41 | 42 | 46 | 0.05 |
| Gestational weight gain (kg/week) | 0.4 (0.4) | 0.4 (0.3) | 0.4 (0.3) | 0.4 (0.4) | 0.72 |
| Non-smokers in pregnancy (%) | 71 | 70 | 71 | 78 | 0.78 |
| Breastfeeding ≥10 months (%) | 17 | 21 | 33 | 28 | 0.34 |
| Birth weight (g) | 3786 (623) | 3709 (719) | 3766 (471) | 3801 (590) | 0.75 |
| Gestational age (days) | 277 (11) | 276 (11) | 278 (11) | 278 (12) | 0.57 |
| Categories of Total Seafood Intake | |||||
| 0–0.5 oz/day | >0.5–1 oz/day | 1–1.5 oz/day | >1.5 oz/day | p1 | |
| Controls (n = 487) | % or means (SD) | % or means (SD) | % or means (SD) | % or means (SD) | |
| Lean fish intake (oz/day) | 0.1 (0.1) | 0.3 (0.2) | 0.4 (0.2) | 0.6 (0.4) | <0.0001 |
| Oily fish intake (oz/day) | 0.0 (0.1) | 0.1 (0.1) | 0.2 (0.2) | 0.4 (0.3) | <0.0001 |
| Marine n-3 LCPUFA (g/day) | 0.2 (0.1) | 0.4 (0.2) | 0.5 (0.3) | 0.8 (0.4) | <0.0001 |
| Total fat intake (g/day) | 78.1 (16.0) | 80.0 (16.0) | 79.3 (14.9) | 78.8 (12.8) | 0.75 |
| Carbohydrate intake (g/day) | 330.2 (34.1) | 319.8 (36.5) | 318.2 (32.9) | 316.6 (30.0) | 0.01 |
| Protein intake (g/day) | 84.7 (13.8) | 90.0 (12.4) | 93.3 (12.9) | 94.9 (13.7) | <0.0001 |
| Maternal age (years) | 30.2 (3.8) | 31.2 (4.1) | 31.7 (4.4) | 31.7 (4.2) | 0.07 |
| Nulliparous (%) | 54 | 52 | 49 | 47 | 0.82 |
| High/medium sociodemographic position (%) | 63 | 60 | 70 | 69 | 0.10 |
| No physical activity (min/week) (%) | 59 | 60 | 60 | 56 | 0.94 |
| Pre-pregnancy BMI 18.6–24.9 kg/m2 (%) | 67 | 74 | 74 | 81 | 0.35 |
| Gestational weight gain (kg/week) | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.2) | 0.92 |
| Non-smokers in pregnancy (%) | 77 | 78 | 79 | 81 | 0.70 |
| Breastfeeding ≥10 months (%) | 29 | 31 | 21 | 34 | 0.17 |
| Birth weight (g) | 3619 (473) | 3587 (482) | 3594 (551) | 3537 (519) | 0.65 |
| Gestational age (days) | 281 (10) | 281 (10) | 281 (13) | 280 (11) | 0.82 |
| Categories of Total Seafood Intake | |||||
|---|---|---|---|---|---|
| 0–0.5 oz/day | >0.5–1 oz/day | 1–1.5 oz/day | >1.5 oz/day | p1 | |
| Metabolic Measures in the Offspring | n = 142 (32%) | n = 136 (31%) | n = 96 (22%) | n = 69 (16%) | |
| BMI, kg/m2 (n = 440) | |||||
| Unadjusted RGM (95% CI) | 1 (ref) | 0.98 (0.94, 1.02) | 0.97 (0.93, 1.02) | 0.99 (0.94, 1.04) | 0.69 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 0.98 (0.94, 1.02) | 0.99 (0.94, 1.03) | 1.01 (0.96, 1.05) | 0.65 |
| Waist circumference, cm (n = 438) | |||||
| Unadjusted RGM (95% CI) | 1 (ref) | 0.99 (0.96, 1.03) | 0.97 (0.93, 1.01) | 1.00 (0.95, 1.04) | 0.39 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 0.99 (0.96, 1.02) | 0.98 (0.94, 1.01) | 1.00 (0.96, 1.04) | 0.49 |
| Total fat mass, % (n = 151) | |||||
| Unadjusted RGM (95% CI) | 1 (ref) | 1.04 (0.93, 1.17) | 0.99 (0.87, 1.13) | 1.07 (0.92, 1.25) | 0.68 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.07 (0.96, 1.20) | 0.95 (0.84, 1.07) | 1.12 (0.96, 1.28) | 0.12 |
| Abdominal fat mass, % (n = 151) | |||||
| Unadjusted RGM (95% CI) | 1 (ref) | 1.09 (0.88, 1.36) | 0.97 (0.76, 1.22) | 1.16 (0.88, 1.54) | 0.52 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.12 (0.91, 1.38) | 0.93 (0.75, 1.17) | 1.26 (0.96, 1.63) | 0.13 |
| Total cholesterol, mmol/L (n = 381) | |||||
| Unadjusted mean Δ (95% CI) | 0 (ref) | −0.05 (−0.23, 0.14) | 0.09 (−0.11, 0.29) | −0.13 (−0.36, 0.10) | 0.31 |
| Adjusted mean Δ2 (95% CI) | 0 (ref) | −0.01 (−0.19, 0.17) | 0.00 (−0.20, 0.20) | −0.09 (−0.31, 0.13) | 0.86 |
| LDL, mmol/L (n = 381) | |||||
| Unadjusted mean Δ (95% CI) | 0 (ref) | −0.01 (−0.18, 0.16) | 0.09 (−0.09, 0.28) | −0.15 (−0.36, 0.06) | 0.20 |
| Adjusted mean Δ2 (95% CI) | 0 (ref) | 0.02 (−0.15, 0.19) | 0.02 (−0.16, 0.21) | −0.10 (−0.30, 0.10) | 0.65 |
| HDL, mmol/L (n = 381) | |||||
| Unadjusted mean Δ (95% CI) | 0 (ref) | −0.03 (−0.12, 0.06) | −0.02 (−0.12, 0.08) | 0.05 (−0.06, 0.17) | 0.52 |
| Adjusted mean Δ2 (95% CI) | 0 (ref) | −0.03 (−0.13, 0.06) | −0.05 (−0.16, 0.05) | 0.04 (−0.07, 0.16) | 0.43 |
| TG, mmol/L (n = 381) | |||||
| Unadjusted RGM (95% CI) | 1 (ref) | 0.93 (0.84, 1.03) | 0.95 (0.84, 1.06) | 0.85 (0.75, 0.97) | 0.11 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 0.92 (0.83, 1.03) | 0.96 (0.86, 1.08) | 0.89 (0.78, 1.01) | 0.26 |
| HOMA-IR (n = 361) | |||||
| Unadjusted RGM (95% CI) | 1 (ref) | 0.99 (0.86, 1.15) | 0.91 (0.77, 1.07) | 1.00 (0.83, 1.20) | 0.70 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.00 (0.87, 1.16) | 0.97 (0.83, 1.14) | 1.02 (0.85, 1.22) | 0.97 |
| MetS z score, SD (n = 359) | |||||
| Unadjusted mean Δ (95% CI) | 0 (ref) | −1.33 (−3.12, 0.45) | −1.62 (−3.73, 0.50) | −1.46 (−3.83, 0.91) | 0.37 |
| Adjusted mean Δ2 (95% CI) | 0 (ref) | −1.17 (−2.93, 0.59) | −0.91 (−2.98, 1.16) | −1.23 (−3.54, 1.07) | 0.56 |
| Categories of Consistent Fish Intake | ||||||
|---|---|---|---|---|---|---|
| Metabolic measures in the offspring | >2 times/week | 1–2 times/week | Weekly | Monthly | Never | p1 |
| n = 29 (16%) | n = 41 (22%) | n = 68 (37%) | n = 39 (21%) | n = 8 (4%) | ||
| BMI, kg/m2 (n = 143) | ||||||
| Unadjusted RGM (95% CI) | 1 (ref) | 1.08 (0.98, 1.20) | 1.09 (0.99, 1.20) | 1.03 (0.92, 1.14) | 1.22 (1.02, 1.46) | 0.11 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.07 (0.96, 1.20) | 1.06 (0.96, 1.16) | 0.98 (0.88, 1.08) | 1.28 (1.06, 1.55) | 0.02 |
| Waist circumference, cm (n = 143) | ||||||
| Unadjusted RGM (95% CI) | 1 (ref) | 1.06 (0.98, 1.15) | 1.05 (0.98, 1.13) | 1.01 (0.93, 1.09) | 1.21 (1.06, 1.39) | 0.05 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.04 (0.96, 1.14) | 1.02 (0.95, 1.11) | 0.97 (0.90, 1.05) | 1.22 (1.05, 1.40) | 0.02 |
| Total fat mass, % (n = 44) | ||||||
| Unadjusted RGM (95% CI) | 1 (ref) | 1.09 (0.86, 1.39) | 1.08 (0.86, 1.36) | 0.99 (0.74, 1.31) | 1.26 (0.82, 1.93) | 0.75 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.16 (0.79, 1.72) | 1.06 (0.77, 1.48) | 0.98 (0.66, 1.45) | 1.57 (0.51, 4.85) | 0.76 |
| Abdominal fat mass, % (n = 44) | ||||||
| Unadjusted RGM (95% CI) | 1 (ref) | 1.30 (0.84, 2.03) | 1.16 (0.76, 1.79) | 1.01 (0.59, 1.72) | 1.62 (0.73, 3.60) | 0.61 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.39 (0.65, 2.97) | 1.14 (0.60, 2.16) | 0.96 (0.44, 2.12) | 2.44 (0.27, 22.20) | 0.74 |
| Total cholesterol, mmol/L (n = 130) | ||||||
| Unadjusted mean Δ (95% CI) | 0 (ref) | 0.14 (−0.29, 0.57) | 0.20 (−0.20, 0.60) | −0.06 (−0.49, 0.38) | 0.14 (−0.59, 0.87) | 0.61 |
| Adjusted mean Δ2 (95% CI) | 0 (ref) | 0.24 (−0.24, 0.72) | 0.25 (−0.20, 0.70) | −0.01 (−0.48, 0.46) | 0.21 (−0.64, 1.05) | 0.56 |
| LDL, mmol/L (n = 130) | ||||||
| Unadjusted mean Δ (95% CI) | 0 (ref) | 0.15 (−0.26, 0.57) | 0.17 (−0.22, 0.56) | 0.02 (−0.40, 0.44) | 0.08 (−0.63, 0.79) | 0.85 |
| Adjusted mean Δ2 (95% CI) | 0 (ref) | 0.16 (−0.31, 0.63) | 0.19 (−0.26, 0.63) | 0.01 (−0.45, 0.48) | 0.16 (−0.68, 0.99) | 0.85 |
| HDL, mmol/L (n = 130) | ||||||
| Unadjusted mean Δ (95% CI) | 0 (ref) | −0.15 (−0.37, 0.07) | −0.01 (−0.21, 0.20) | −0.15 (−0.37, 0.08) | −0.02 (−0.40, 0.35) | 0.34 |
| Adjusted mean Δ2 (95% CI) | 0 (ref) | −0.09 (−0.32, 0.15) | 0.01 (−0.21, 0.24) | −0.11 (−0.34, 0.12) | −0.06 (−0.48, 0.36) | 0.62 |
| TG, mmol/L (n = 130) | ||||||
| Unadjusted RGM (95% CI) | 1 (ref) | 1.17 (0.90, 1.54) | 1.21 (0.94, 1.57) | 1.21 (0.92, 1.60) | 1.72 (1.08, 2.72) | 0.22 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.34 (0.98, 1.80) | 1.31 (0.99, 1.75) | 1.26 (0.94, 1.70) | 1.77 (1.03, 3.03) | 0.20 |
| HOMA-IR (n = 124) | ||||||
| Unadjusted RGM (95% CI) | 1 (ref) | 1.22 (0.88, 1.68) | 1.52 (1.13, 2.08) | 1.15 (0.82, 1.60) | 2.23 (1.30, 3.82) | 0.01 |
| Adjusted RGM 2 (95% CI) | 1 (ref) | 1.20 (0.84, 1.72) | 1.54 (1.09, 2.14) | 1.07 (0.76, 1.51) | 2.16 (1.17, 3.97) | 0.01 |
| MetS z score, SD (n = 123) | ||||||
| Unadjusted mean Δ (95% CI) | 0 (ref) | 4.29 (−0.60, 9.17) | 6.24 (1.66, 10.8) | 2.23 (−2.74, 7.20) | 12.47 (4.34, 20.60) | 0.01 |
| Adjusted mean Δ2 (95% CI) | 0 (ref) | 4.23 (−0.85, 9.30) | 5.94 (1.13, 10.75) | 1.04 (−3.91, 5.99) | 12.91 (4.07, 21.75) | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslova, E.; Hansen, S.; Strøm, M.; Halldorsson, T.I.; Grunnet, L.G.; Vaag, A.A.; Olsen, S.F. Fish Intake in Pregnancy and Offspring Metabolic Parameters at Age 9–16—Does Gestational Diabetes Modify the Risk? Nutrients 2018, 10, 1534. https://doi.org/10.3390/nu10101534
Maslova E, Hansen S, Strøm M, Halldorsson TI, Grunnet LG, Vaag AA, Olsen SF. Fish Intake in Pregnancy and Offspring Metabolic Parameters at Age 9–16—Does Gestational Diabetes Modify the Risk? Nutrients. 2018; 10(10):1534. https://doi.org/10.3390/nu10101534
Chicago/Turabian StyleMaslova, Ekaterina, Susanne Hansen, Marin Strøm, Thorhallur I. Halldorsson, Louise G. Grunnet, Allan A. Vaag, and Sjurdur F. Olsen. 2018. "Fish Intake in Pregnancy and Offspring Metabolic Parameters at Age 9–16—Does Gestational Diabetes Modify the Risk?" Nutrients 10, no. 10: 1534. https://doi.org/10.3390/nu10101534
APA StyleMaslova, E., Hansen, S., Strøm, M., Halldorsson, T. I., Grunnet, L. G., Vaag, A. A., & Olsen, S. F. (2018). Fish Intake in Pregnancy and Offspring Metabolic Parameters at Age 9–16—Does Gestational Diabetes Modify the Risk? Nutrients, 10(10), 1534. https://doi.org/10.3390/nu10101534





