Dietary Interventions in Pollen-Related Food Allergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria, Information Sources, and Search
2.3. Study Selection
2.4. Data Collection
2.5. Risk of Bias Assessment
2.6. Synthesis of Results
3. Results
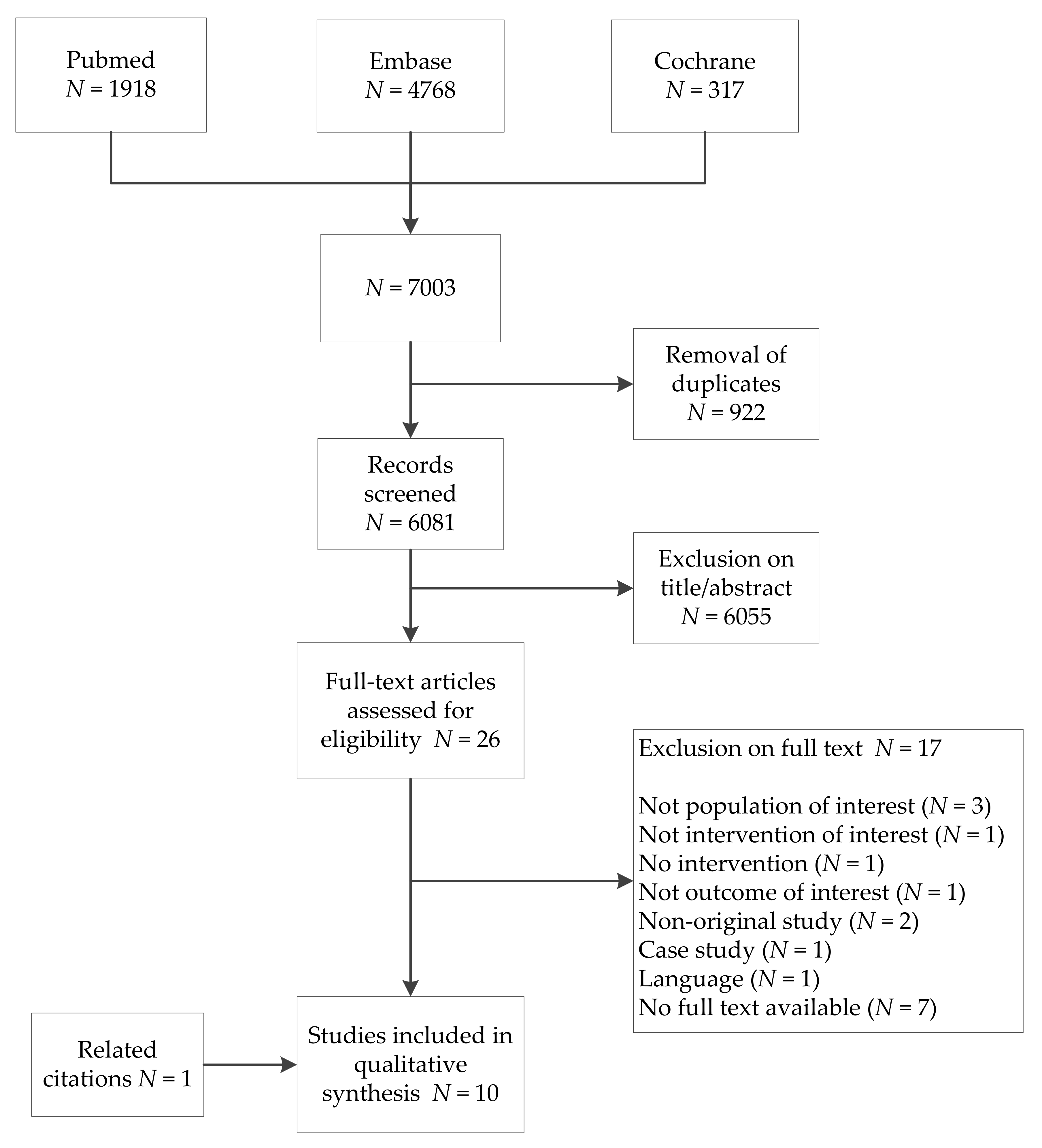
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
3.4. Synthesis of Results and Level of Evidence
3.4.1. Oral Immunotherapy
3.4.2. (Heat) Processing
3.4.3. Hypoallergenic Cultivars
4. Discussion
4.1. Oral Immunotherapy
4.2. (Heat) Processing
4.3. Consumption of Hypoallergenic Cultivars
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
- 1.
- food*[Title/Abstract] OR fruit[MeSH Terms] OR fruit*[Title/Abstract] OR rosaceae[MeSH Terms] OR rosaceae*[Title/Abstract] OR apple*[Title/Abstract] OR malus*[Title/Abstract] OR apricot*[Title/Abstract] OR cherr*[Title/Abstract] OR peach*[Title/Abstract] OR plum*[Title/Abstract] OR nectarin*[Title/Abstract] OR prunus*[Title/Abstract] OR pear*[Title/Abstract] OR pyrus*[Title/Abstract] OR actinidia[MeSH Terms] OR actinidia*[Title/Abstract] OR kiwi*[Title/Abstract] OR mangifera[MeSH Terms] OR mangifera*[Title/Abstract] OR mango*[Title/Abstract] OR diospyros[MeSH Terms] OR diospyros*[Title/Abstract] OR artocarpus[MeSH Terms] OR artocarpus*[Title/Abstract] OR jackfruit*[Title/Abstract] OR litchi[MeSH Terms] OR lychee*[Title/Abstract] OR litch*[Title/Abstract] OR leechee*[Title/Abstract] OR vitis[MeSH Terms] OR vitis*[Title/Abstract] OR grape*[Title/Abstract] OR ficus[MeSH Terms] OR ficus*[Title/Abstract] OR fig*[Title/Abstract] OR fabaceae[MeSH Terms] OR fabaceae*[Title/Abstract] OR legume*[Title/Abstract] OR soy food[MeSH Terms] OR soybeans[MeSH Terms] OR soy*[Title/Abstract] OR soj*[Title/Abstract] OR bean*[Title/Abstract] OR vegetable[MeSH Terms] OR vegetable*[Title/Abstract] OR daucus carota[MeSH Terms] OR daucus carota*[Title/Abstract] OR carrot*[Title/Abstract] OR apium graveolens[MeSH Terms] OR apium graveolen*[Title/Abstract] OR celer*[Title/Abstract] OR nuts[MeSH Terms] OR nut[Title/Abstract] OR nuts[Title/Abstract] OR corylus[MeSH Terms] OR corylus*[Title/Abstract] OR hazelnut*[Title/Abstract] OR arachis[MeSH Terms] OR arachis*[Title/Abstract] OR peanut*[Title/Abstract] OR solanum tuberosum[MeSH Terms] OR solanum tuberosum*[Title/Abstract] OR spices[MeSH Terms] OR spice*[Title/Abstract] OR herb*[Title/Abstract] OR sunflower seed*[Title/Abstract]
- 2.
- hypersensitivities[MeSH Terms] OR hypersensitiv*[Title/Abstract] OR allergens[MeSH Terms] OR allerg*[Title/Abstract] OR cross reactions[MeSH Terms] OR cross react*[Title/Abstract] OR crossreact*[Title/Abstract] OR ige mediat*[Title/Abstract] OR sensitis*[Title/Abstract] OR sensitis*[Title/Abstract]
- 3.
- 1 AND 2
- 4.
- food hypersensitivities[MeSH Terms]
- 5.
- 3 OR 4
- 6.
- pollen[MeSH Terms] OR pollen*[Title/Abstract] OR trees[MeSH Terms] OR tree*[Title/Abstract] OR orchard*[Title/Abstract] OR plane*[Title/Abstract] OR betulaceae[MeSH Terms] OR alnus*[Title/Abstract] OR alder*[Title/Abstract] OR betula*[Title/Abstract] OR birch*[Title/Abstract] OR corylus*[Title/Abstract] OR hazel*[Title/Abstract] OR filbert*[Title/Abstract] OR hornbeam*[Title/Abstract] OR quercus[MeSH Terms] OR quercus*[Title/Abstract] OR oak*[Title/Abstract] OR poaceae[MeSH Terms] OR poaceae*[Title/Abstract] OR grass*[Title/Abstract] OR timothy*[Title/Abstract] OR artemisia[MeSH Terms] OR artemisia*[Title/Abstract] OR ambrosia[MeSH Terms] OR ambrosia*[Title/Abstract] OR mugwort*[Title/Abstract] OR ragweed*[Title/Abstract] OR plant weeds[MeSH Terms] OR weed*[Title/Abstract]
- 7.
- 5 AND 6
- 8.
- oral allergy syndrom*[Title/Abstract]) OR pollen food syndrom*[Title/Abstract]
- 9.
- 7 OR 8
- 10.
- immunotherapy[MeSH Terms] OR immunotherap*[Title/Abstract]
- 11.
- oral[Title/Abstract] AND tolerance[Title/Abstract] AND induc*[Title/Abstract]
- 12.
- 10 OR 11
- 13.
- heating[MeSH Terms] OR heat*[Title/Abstract] OR cooking[MeSH Terms] OR cook*[Title/Abstract] OR roast*[Title/Abstract] OR baked[Title/Abstract] OR baking[Title/Abstract] OR microwav*[Title/Abstract] OR pasteuriz*[Title/Abstract] OR pasteuris*[Title/Abstract] OR process*[Title/Abstract] OR dehydrat*[Title/Abstract] OR dried[Title/Abstract] OR spice*[Title/Abstract] OR herb*[Title/Abstract]
- 14.
- hypoallergen*[Title/Abstract] OR hypo allergen*[Title/Abstract] OR low allergen*[Title/Abstract] OR reduced allergen*[Title/Abstract] OR cultiv*[Title/Abstract] OR variet*[Title/Abstract]
- 15.
- diet[Title/Abstract]) OR diets[Title/Abstract] OR dietary[Title/Abstract] OR avoid*[Title/Abstract] or trace[Title/Abstract] OR traces[Title/Abstract]
- 16.
- 12 OR 13 OR 14 OR 15
- 17.
- 9 AND 16
References
- Bircher, A.J.; Van Melle, G.; Haller, E.; Curty, B.; Frei, P.C. Ige to food allergens are highly prevalent in patients allergic to pollens, with and without symptoms of food allergy. Clin. Exp. Allergy 1994, 24, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Cervera, L.; Sanz, M.L.; Diaz-Perales, A.; Fernandez, J. Plant food allergy in patients with pollinosis from the Mediterranean area. Int. Arch. Allergy Immunol. 2012, 159, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Osterballe, M.; Hansen, T.K.; Mortz, C.G.; Bindslev-Jensen, C. The clinical relevance of sensitization to pollen-related fruits and vegetables in unselected pollen-sensitized adults. Allergy 2005, 60, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Edenharter, G.; Worm, M.; Ehlers, I.; Reimann, S.; Hantke, T.; Roehr, C.C.; Bergmann, K.E.; Niggemann, B. Prevalence of adverse reactions to food in Germany—A population study. Allergy 2004, 59, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Enrique, E.; Knulst, A.C.; Mari, A.; Muraro, A.; Ollert, M.; Poulsen, L.K.; et al. Position paper of the eaaci: Food allergy due to immunological cross-reactions with common inhalant allergens. Allergy 2015, 70, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Jappe, U.; Kleine-Tebbe, J.; Schafer, C.; Reese, I.; Saloga, J.; Treudler, R.; Zuberbier, T.; Wassmann, A.; Fuchs, T.; et al. Food allergies resulting from immunological cross-reactivity with inhalant allergens: Guidelines from the German society for allergology and clinical immunology (dgaki), the german dermatology society (ddg), the association of German allergologists (aeda) and the society for pediatric allergology and environmental medicine (gpa). Allergo. J. Int. 2014, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Epidemiology and risk factors for the development of food allergy. Pediatr. Ann. 2008, 37, 552–558. [Google Scholar] [PubMed]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C. Prevalence of food allergy: An overview. Proc. Nutr. Soc. 2005, 64, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. Eaaci food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Ramachandran, S.; Smith, G.P.; Stevenson, M.L.; Pomeranz, M.K.; Cohen, D.E. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis 2015, 26, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sicherer, S.H.; Nowak-Wegrzyn, A. A survey on the management of pollen-food allergy syndrome in allergy practices. J. Allergy Clin. Immunol. 2003, 112, 784–788. [Google Scholar] [CrossRef]
- Kinaciyan, T.; Nagl, B.; Faustmann, S.; Frommlet, F.; Kopp, S.; Wolkersdorfer, M.; Wohrl, S.; Bastl, K.; Huber, H.; Berger, U.; et al. Efficacy and safety of 4 months of sublingual immunotherapy with recombinant mal d 1 and bet v 1 in patients with birch pollen-related apple allergy. J. Allergy Clin. Immunol. 2018, 141, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Gernez, Y.; Nowak-Wegrzyn, A. Immunotherapy for food allergy: Are we there yet? J. Allergy Clin. Immunol. Pract. 2017, 5, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.; Rachid, R. The heterogeneity of oral immunotherapy clinical trials: Implications and future directions. Curr. Allergy Asthma Rep. 2016, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Asarnoj, A.; Moverare, R.; Ostblom, E.; Poorafshar, M.; Lilja, G.; Hedlin, G.; van Hage, M.; Ahlstedt, S.; Wickman, M. Ige to peanut allergen components: Relation to peanut symptoms and pollen sensitization in 8-year-olds. Allergy 2010, 65, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Jankiewicz, A.; Baltes, W.; Bögl, W.; Dehne, L.; Jamin, A.; Hoffmann, A.; Haustein, D.; Vieths, S. Influence of food processing on the immunochemical stability of celery allergens. J. Sci. Food Agric. 1997, 359–370. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. Robins-i: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- How to Grade the Quality of the Evidence. Available online: https://www.google.com.tw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwi8pNuf5P3dAhXFAIgKHZN9B0EQFjAAegQIBRAC&url=https%3A%2F%2Fcc.cochrane.org%2Fsites%2Fcc.cochrane.org%2Ffiles%2Fpublic%2Fuploads%2Fhow_to_grade.pdf&usg=AOvVaw2juZxoBHf5FSRuWAiHVkLa (accessed on 8 August 2018).
- Bouvier, M.; Van Der Brempt, X.; Nosbaum, A.; Codier, J.M.; Cherih, C.; Frappaz, A.; Berion, C.; Grande, S.; Pralong, P.; Nicolas, J.F.; et al. L’induction de tolérance orale dans l’allergie aux rosacées. Revue Française d’Allergologie 2014, 54, 127–133. [Google Scholar] [CrossRef]
- Kopac, P.; Rudin, M.; Gentinetta, T.; Gerber, R.; Pichler, C.; Hausmann, O.; Schnyder, B.; Pichler, W.J. Continuous apple consumption induces oral tolerance in birch-pollen-associated apple allergy. Allergy 2012, 67, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Ballmer-Weber, B.K.; Hoffmann, A.; Wuthrich, B.; Luttkopf, D.; Pompei, C.; Wangorsch, A.; Kastner, M.; Vieths, S. Influence of food processing on the allergenicity of celery: Dbpcfc with celery spice and cooked celery in patients with celery allergy. Allergy 2002, 57, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Bohle, B.; Zwolfer, B.; Heratizadeh, A.; Jahn-Schmid, B.; Antonia, Y.D.; Alter, M.; Keller, W.; Zuidmeer, L.; van Ree, R.; Werfel, T.; et al. Cooking birch pollen-related food: Divergent consequences for ige- and t cell-mediated reactivity in vitro and in vivo. J. Allergy Clin. Immunol. 2006, 118, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.S.; Ballmer-Weber, B.K.; Luttkopf, D.; Skov, P.S.; Wuthrich, B.; Bindslev-Jensen, C.; Vieths, S.; Poulsen, L.K. Roasted hazelnuts--allergenic activity evaluated by double-blind, placebo-controlled food challenge. Allergy 2003, 58, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Hompes, S.; Fiedler, E.M.; Illner, A.K.; Zuberbier, T.; Vieths, S. Impact of native, heat-processed and encapsulated hazelnuts on the allergic response in hazelnut-allergic patients. Clin. Exp. Allergy 2009, 39, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Marzban, G.; Martinelli, A.; Zaccarini, M.; Machado, M.L. Search for low-allergenic apple cultivars for birch-pollen-allergic patients: Is there a correlation between in vitro assays and patient response? Eur. Ann. Allergy Clin. Immunol. 2006, 38, 94–98. [Google Scholar] [PubMed]
- Bolhaar, S.T.; van de Weg, W.E.; van Ree, R.; Gonzalez-Mancebo, E.; Zuidmeer, L.; Bruijnzeel-Koomen, C.A.; Fernandez-Rivas, M.; Jansen, J.; Hoffmann-Sommergruber, K.; Knulst, A.C.; et al. In vivo assessment with prick-to-prick testing and double-blind, placebo-controlled food challenge of allergenicity of apple cultivars. J. Allergy Clin. Immunol. 2005, 116, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Kootstra, H.S.; Vlieg-Boerstra, B.J.; Dubois, A.E. Assessment of the reduced allergenic properties of the Santana apple. Ann. Allergy Asthma Immunol. 2007, 99, 522–525. [Google Scholar] [CrossRef]
- Vlieg-Boerstra, B.J.; van de Weg, W.E.; van der Heide, S.; Kerkhof, M.; Arens, P.; Heijerman-Peppelman, G.; Dubois, A.E. Identification of low allergenic apple cultivars using skin prick tests and oral food challenges. Allergy 2011, 66, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Neudecker, P.; Schweimer, K.; Nerkamp, J.; Scheurer, S.; Vieths, S.; Sticht, H.; Rosch, P. Allergic cross-reactivity made visible: Solution structure of the major cherry allergen pru av 1. J. Biol. Chem. 2001, 276, 22756–22763. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.N.; Sancho, A.I.; Rigby, N.M.; Jenkins, J.A.; Mackie, A.R. Impact of food processing on the structural and allergenic properties of food allergens. Mol. Nutr. Food Res. 2009, 53, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Datema, M.R.; Zuidmeer-Jongejan, L.; Asero, R.; Barreales, L.; Belohlavkova, S.; de Blay, F.; Bures, P.; Clausen, M.; Dubakiene, R.; Gislason, D.; et al. Hazelnut allergy across Europe dissected molecularly: A europrevall outpatient clinic survey. J. Allergy Clin. Immunol. 2015, 136, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Mutschlechner, S.; Wopfner, N.; Gadermaier, G.; Briza, P.; Ferreira, F. Pollen-food syndromes associated with weed pollinosis: An update from the molecular point of view. Allergy 2006, 61, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Van der Maas, M.P.; Schen, M.F. Development of a protocol that allows safe consumption of the hypoallergenic apple cultivar Santana. Acta. Hortic. 2009, 549–552. [Google Scholar] [CrossRef]
- Geroldinger-Simic, M.; Zelniker, T.; Aberer, W.; Ebner, C.; Egger, C.; Greiderer, A.; Prem, N.; Lidholm, J.; Ballmer-Weber, B.K.; Vieths, S.; et al. Birch pollen-related food allergy: Clinical aspects and the role of allergen-specific ige and igg4 antibodies. J. Allergy Clin. Immunol. 2011, 127, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Kiewning, D.; Schmitz-Eiberger, M. Effects of long-term storage on mal d 1 content of four apple cultivars with initial low mal d 1 content. J. Sci. Food Agric. 2014, 94, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rivas, M.; Cuevas, M. Peels of rosaceae fruits have a higher allergenicity than pulps. Clin. Exp. Allergy 1999, 29, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Nybom, H.; Cervin-Hoberg, C.; Andersson, M. Oral challenges with four apple cultivars result in significant differences in oral allergy symptoms. Int. Arch. Allergy Immunol. 2013, 161, 258–264. [Google Scholar] [CrossRef] [PubMed]
| Study Information | Study Design | Relevant Study Population | Method of Intervention | Method of Outcome Measurement | Outcomes Reported |
|---|---|---|---|---|---|
| Oral Immunotherapy | |||||
| Bouvier et al.; Lyon hospital allergy clinic (F); 1 May 2012–1 February 2013 [21] | NS, comparison of participants’ allergic reactions before and after oral immunotherapy | 52 subjects (age 8–63 years; 17% <18 years) with IgE-sensitisation to birch pollen and apple; and OAS to Rosaceae foods according to history | All subjects underwent oral immunotherapy with increasing doses of fresh Golden Delicious (GD) apple. 1. Initial dose escalation with nine doses from 0.1 mg to 16 g. Portion increased every 20–30 min. 2. Build-up phase starting at 16 g and increasing to half an apple (64 g) 3 times per week up until 24 weeks after start. 3. Maintenance phase consisting of half an apple 3 times a week up until 48 weeks after start. | Patient history at 48 weeks follow-up | 1. Number of subjects that achieved tolerance of 64 g of apple after 48 weeks. 2. Number of subjects that was tolerant to other Rosaceae fruits after oral immunotherapy with apple. |
| Kopac et al.; University hospital Bern allergy clinic (CH); December 2009–August 2010 [22] | RCT | 40 subjects (age 18–61 years) with IgE-sensitisation to birch pollen and Mal d 1; and challenge-confirmed OAS to Golden Delicious apple | 27 of 40 subjects underwent oral immunotherapy with increasing doses of fresh Golden Delicious (GD) apple. 1. Initial dose escalation with doses from 1 g to 128 g. Portion doubled every 5 min. 2. Build-up phase starting at the largest dose tolerated in the preceding phase to whole apple (150–200 g). Portion doubled every 2–3 weeks. 3. Maintenance phase commencing when a whole apple was tolerated (average 20 weeks) and consisting of at least three apples per week up until 8 months after the start. 13 of 40 subjects remained untreated and formed the control group. | Patient history at 8 months follow-up | 1. Proportion of subjects that achieved tolerance to 128 g of apple after 8 months. 2. Number of subjects that achieved cross-tolerance to other birch pollen cross-reacting fruits/nuts after oral immunotherapy with apple. |
| (Heat) Processing | |||||
| Ballmer-Weber et al.; University hospital Zurich allergy clinic (CH); January 2000–February 2001 [23] | NS, comparison of participants’ allergic reactions to processed and unprocessed variants of the food | 12 subjects (age 21–42 years) with IgE- sensitisation to birch pollen (and mugwort pollen in 9/12 subjects) and celery; and allergic reactions to celery according to history | 1. Cook celery (110 °C; 15 min) 2. Dehydrate celery (celery spice) | Comparison of DBPCFC with processed celery (12 of 12 subjects) to DBPCFC with raw celery (10 of 12 subjects) or convincing history to raw celery (2 of 12 subjects) | 1. Number of subjects with symptoms in response to oral challenge with cooked celery and celery spice 2. Type of symptoms 3. Dose eliciting symptoms |
| Bohle et al.; Hannover Medical School Department of Dermatology and Allergology (D); time NS [24] | NS, comparison of participants’ allergic reactions to processed and unprocessed variants of the food | 5 subjects (age 5–37 years; 20% <18 years) IgE-sensitised to birch pollen with OAS and worsening of atopic dermatitis to carrot, celery or apple according to history | Celery (1 of 5 subjects): boil until soft Carrot (3 of 5 subjects): boil until soft Apple (1 of 5 subjects): pasteurisation (juice) | Comparison of DBPCFC with processed food (4 of 5 subjects) or convincing history to processed food (1of 5 subjects) to DBPCFC with raw food (5 of 5 subjects) | 1. Number of subjects with OAS in response to oral challenge with cooked carrot or celery or apple 2. Type of symptoms |
| Hansen et al.; University hospital Copenhagen (DK), and University hospital Zurich allergy clinics (CH); 1998–2000 [25] | NS, comparison of participants’ allergic reactions to processed and unprocessed variants of the food | 17 subjects (age 14–65 years) with IgE-sensitisation to birch pollen and hazelnut; and OAS to hazelnut according to history or challenge | Roast hazelnut (140 °C; 40 min) | Comparison of DBPCFC with roasted hazelnut (17 of 17 subjects) to DBPCFC with raw hazelnut (16 of 17 subjects) or convincing history to raw hazelnut (1 of 17 subjects) | 1. Number of subjects with symptoms in response to oral challenge with roasted hazelnut 2. Type of symptoms 3. Dose eliciting symptoms |
| Worm et al.; University hospital Charité Berlin dermatology outpatient clinic (D); time NS [26] | NS, comparison of participants’ allergic reactions to processed and unprocessed variants of the food | 82 of 132 included subjects (age 21–65 years) with IgE-sensitisation to birch pollen and hazelnut; and challenge-confirmed hazelnut allergy | Roast hazelnut (144 °C; time unknown) | Comparison of DBPCFC with roasted hazelnut (20 of 82 subjects) to DBPCFC with raw hazelnut (82 of 82 subjects) | 1. Number of subjects with symptoms in response to oral challenge with roasted hazelnut 2. Type of symptoms 3. Dose eliciting symptoms |
| Consumption of Hypoallergenic Cultivars | |||||
| Asero et al.; setting NS; 2004 [27] | NS, comparison of participants’ allergic reactions to low and high allergenic cultivars | 7 of 17 included subjects (age 26–49) with sensitisation to birch pollen and apple; and OAS to apple according to history | Consumption of low allergenic G-198 or Orim apple | Comparison of SBFC with G-198 apple (6 of 7 subjects) or Orim apple (1 of 7 subjects) to SBFC with Golden Delicious apple | 1. Mean symptoms severity score for OAS (Score 0–100) 2. Number of subjects reporting NO symptoms in response to oral challenge |
| Bolhaar et al.; University Medical Centre Utrecht department of dermatology and allergology (NL); time NS [28] | NS, comparison of participants’ allergic reactions to low and high allergenic cultivars | 5 of 23 included subjects (age > 18 years) with a history of rhinoconjunctivitis during birch pollen season, sensitisation to apple, and OAS to apple according to history | Consumption of low allergenic Santana apple | Comparison of DBPCFC with Santana apple to DBPCFC with Golden Delicious apple | 1. Mean symptom severity score for OAS (VAS 0–100) 2. Quantities needed to provoke similar VAS score for Santana apples as for Golden Delicious apples |
| Kootstra et al.; University Medical Centre Groningen allergy outpatient clinic (NL); February–May 2005 [29] | NS, comparison of participants’ allergic reactions to low and high allergenic cultivars | 15 subjects (age > 18 years) with sensitisation to birch pollen and apple; and challenge-confirmed OAS to apple | Consumption of low allergenic Santana apple | Comparison of SBFC with Santana apple to SBFC with Golden Delicious apple as a positive control and SBFC with Topaz apple as a negative control | 1. Maximum symptom severity score (VAS, range not described) at dose 1. 2. Number of subjects reporting NO symptoms in response to oral challenge. |
| Vlieg-Boerstra et al.; University Medical Centre Groningen allergy outpatient clinic (NL); 2006–2008 [30] | NS, comparison of participants’ allergic reactions to low and high allergenic cultivars | 33 subjects (age 18–52 years) with sensitisation to birch pollen in 32/33 subjects; and challenge-confirmed OAS to apple | Consumption of low allergenic Elise, Santana and Pink Lady apples | Comparison of SBFC with Elise, Santana, Pink Lady and Golden Delicious apple | 1. Cumulative symptom severity score (VAS, range not described) at dose 1. 2. Number of subjects reporting NO symptoms in response to oral challenge. |
| Confounding | Selection | Classification of Interventions | Deviations from Interventions | Missing Data | Outcome Measurement | Selection of Reported Results | Overall Risk | |
|---|---|---|---|---|---|---|---|---|
| Bouvier et al. [21] | ● | ● | ● | ● | ● | ● | ● | ● |
| Kopac et al. [22] | ● | ● | ● | ● | ● | ● | ● | ● |
| Ballmer-Weber et al. [23] | ● | ● | ● | ● | ● | ● | ? | ● |
| Bohle et al. [24] | ● | ● | ● | ● | ● | ● | ● | ● |
| Hansen et al. [25] | ● | ? | ● | ● | ● | ● | ● | ● |
| Worm et al. [26] | ● | ● | ● | ● | ● | ● | ● | ● |
| Asero et al. [27] | ● | ● | ● | ● | ● | ● | ● | ● |
| Bolhaar et al. [28] | ● | ● | ● | ● | ● | ? | ● | ● |
| Kootstra et al. [29] | ● | ● | ● | ● | ● | ● | ● | ● |
| Vlieg-Boerstra et al. [30] | ● | ● | ● | ● | ● | ● | ● | ● |
| Source | Number of Subjects | Build-Up Phase Completed N (%) | Maintenance Phase Completed N (%) | Frequency of Achieved Tolerance at Final Follow-Up N (%) * | Tolerated Dose | Frequency of Achieved Tolerance to Other Birch Pollen Cross-Reacting Foods |
|---|---|---|---|---|---|---|
| Bouvier et al. [21] | 52 Active: 52 Control: NA | 46/52 (88.5) Week 24; 1 non-responder; 5 drop-outs | 42/52 (80.8) Week 48; 4 missings | 42/52 (80.8) | No information | 41/42 subjects reported tolerance to other Rosaceae fruits (cherries, peaches), kiwi fruit, nuts, and peanuts. One apple-tolerant subject reported being unable to consume carrots. |
| Kopac et al. [22] | 40 Active: 27 Control: 13 | 17/27 (63.0) Week 20 (range 7–30); 5 non-responders; 5 drop-outs | 17/27 (63.0) Month 8 (T8); 0 missings | Active: 17/27 (63.0) Control: 0/13 (0.0) p (active vs. control) = 0.0001 | Active: Responders (N = 17): Median tolerated dose ΔT8-T0: 126 (69–127) g p (Δmedian tolerated dose) = 0.0009 Non-responders (N = 5): Median tolerated dose ΔT8-T0: 8 (0–60) g p (Δmedian tolerated dose) = NA Control: Median tolerated dose ΔT8-T0: 0 (−24–6) gp (Δmedian tolerated dose) = NA | Of subjects who reported symptoms to cross-reactive fruits in the active group and who completed protocol, 29% could tolerate pear where they could previously not. 27% could tolerate cherries, 23% hazelnuts, 14% walnuts, 18% peaches. In the control group 1 patient could tolerate pear where he could previously not; no other changes were observed. |
| Source | Food and Number of Subjects | Frequency of NO Symptoms in DBPCFC with Processed Food N (%) | Frequency of Symptoms in DBPCFC with Processed Food N (%) | Symptom Severity in DBPCFC with Raw vs. Processed Food | Eliciting Dose in DBPCFC with Raw vs. Processed Food |
|---|---|---|---|---|---|
| Ballmer-Weber et al. [23] | Cooked celery: 11 Celery spice: 5 | Cooked celery: 5/11 (45.5) Celery spice: 0/5 (0) | Cooked celery: 6/11 (54.5) Celery spice: 5/5 (100.0) | Raw celery: 6× mild, 3× moderate, 3× severe Cooked celery: 5× no symptoms, 5× mild; 1× severe Celery spice: 2× mild; 3× moderate | Raw celery: 7× 0.7 g; 3× 28.5 g Cooked celery: 3× 0.9 g, 2× 1.8 g, 1× 34.5 g Celery spice: 3× 0.16 g, 1× 0.32 g, 1× 5.85 g * |
| Bohle et al. [24] | Carrot: 3 Celery: 1 Apple: 1 | Cooked food: 5/5 (100.0) | Cooked food: 0/5 (0.0) | Raw food: 5× mild Cooked food: 5× no symptoms | No information |
| Hansen et al. [25] | Hazelnut: 17 | Roasted hazelnut: 12/17 (70.6) | Roasted hazelnut: 5/17 (29.4) | Raw hazelnut: 17× mild Roasted hazelnut: 12× no symptoms, 5× mild | Raw hazelnut: Median dose Copenhagen (N = 10) 2 g; Zurich (N = 7) 2.6 g Roasted hazelnut: Median dose Copenhagen (N = 4) 7 g; Zurich (N = 1) 5.2 g p (roasted vs. raw) = NA |
| Worm et al. [26] | Hazelnut: 82 | Roasted hazelnut: 3/20 (15.0) | Roasted hazelnut: 17/20 (85.0) | Raw hazelnut: 78× mild, 4× unclear Roasted hazelnut: 17× mild | Raw hazelnut: Median dose 0.1 g, range 0.01–2.0 g Roasted hazelnut: Median dose 0.23 g, range 0.01–10.0 g p (roasted vs. raw) = 0.009 |
| Source | Number of Subjects | Food | Frequency of NO Symptoms to Highest Dose in FC N (%) | Symptom Severity in FC with Various Cultivars | Dose Eliciting Symptoms in FC with Various Cultivars |
|---|---|---|---|---|---|
| Asero et al. [27] | 7 | High allergenic Golden Delicious vs. low allergenic G-198/ Orim | GD: 0/7 (0.0) G-198/Orim: 0/7 (0.0) (p = NA) | GD: median VAS score 2/10; range 2–8. G-198/Orim: median VAS score 4/10; range 2–8. 2/7 patients reported more severe OAS to GD than to G-198. 2/7 patients reported more severe symptoms to G-198 than to GD. 3/7 subjects reported identical severity of symptoms to both apple cultivars. | NA, only 1 dose (15 g) |
| Bolhaar et al. [28] | 5 | High allergenic Golden Delicious vs. low allergenic Santana | No information | Mean VAS score after dose 1 (5 g): Santana < GD (p > 0.05); Mean VAS score after dose 2 (40 g): Santana < GD (p < 0.05); Mean VAS score after dose 3 (120 g): Santana < GD (p < 0.05) | “The quantities needed to provoke a similar VAS score were on average 30 times higher for Santana than for GD apples (p < 0.001)” |
| Kootstra et al. [29] | 15 | High allergenic Golden Delicious vs. low allergenic Santana and Topaz | GD: 1/15 (6.7) Topaz: 1/15 (6.7) Santana: 8/15 (53.5) p (Santana vs. GD/Topaz) = 0.002 | Maximum VAS score after dose 1 (20 g): Santana < GD (p = 0.017), Santana < Topaz (p = 0.004) | No information |
| Vlieg-Boerstra et al. [30] | 33 | High allergenic Golden Delicious vs. Low-allergenic Elise, Santana and Pink Lady | 6–16%; no significant differences between GD, Elise, Santana and Pink Lady (p = NA). | Cumulative VAS score after dose 1 (15 g): Elise < Santana (p = 0.02), Elise < PL (p = 0.04), Elise < GD (p < 0.001); Santana < GD (p = 0.05) | No information |
| Outcome | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall GRADE |
|---|---|---|---|---|---|---|---|
| Oral Immunotherapy | |||||||
| Frequency of achieved tolerance to apple at final follow-up [21,22] | 3 | −1 | −1 | 0 | −1 | 0 | VERY LOW |
| (Heat) Processing | |||||||
| Frequency of (NO) symptoms in DBPCFC with processed food [23,24,25,26] | 2 | −1 | −1 | −1 | −1 | 0 | VERY LOW |
| Symptom severity in DBPCFC with raw vs. processed food [23,24,25,26] | 2 | −1 | −1 | −1 | −1 | 0 | VERY LOW |
| Dose eliciting symptoms in DBPCFC with raw vs. processed food [23,25,26] | 2 | −1 | −1 | −1 | −1 | 0 | VERY LOW |
| Consumption of Hypoallergenic Cultivars | |||||||
| Frequency of NO symptoms to highest dose in FC [27,29,30] | 2 | −1 | −2 | 0 | −1 | 0 | VERY LOW |
| Severity of symptoms in FC with various cultivars [27,28,29,30] | 2 | −1 | −2 | 0 | −1 | 0 | VERY LOW |
| Dose eliciting symptoms in FC with various cultivars [28] | 2 | −1 | −2 | 0 | −1 | 0 | VERY LOW |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyons, S.A.; Dijk, A.M.v.; Knulst, A.C.; Alquati, E.; Le, T.-M.; Os-Medendorp, H.V. Dietary Interventions in Pollen-Related Food Allergy. Nutrients 2018, 10, 1520. https://doi.org/10.3390/nu10101520
Lyons SA, Dijk AMv, Knulst AC, Alquati E, Le T-M, Os-Medendorp HV. Dietary Interventions in Pollen-Related Food Allergy. Nutrients. 2018; 10(10):1520. https://doi.org/10.3390/nu10101520
Chicago/Turabian StyleLyons, Sarah A., Anne M. van Dijk, André C. Knulst, Eleonora Alquati, Thuy-My Le, and Harmieke Van Os-Medendorp. 2018. "Dietary Interventions in Pollen-Related Food Allergy" Nutrients 10, no. 10: 1520. https://doi.org/10.3390/nu10101520
APA StyleLyons, S. A., Dijk, A. M. v., Knulst, A. C., Alquati, E., Le, T.-M., & Os-Medendorp, H. V. (2018). Dietary Interventions in Pollen-Related Food Allergy. Nutrients, 10(10), 1520. https://doi.org/10.3390/nu10101520





