Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
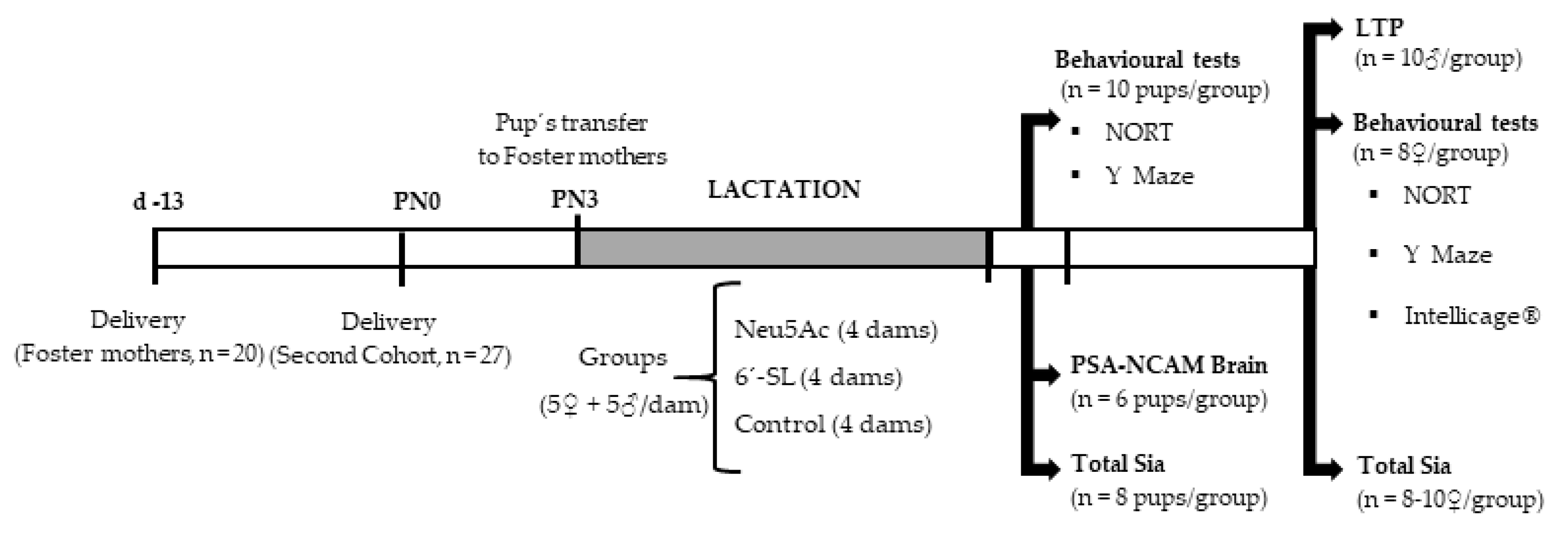
2.2. Experimental Design
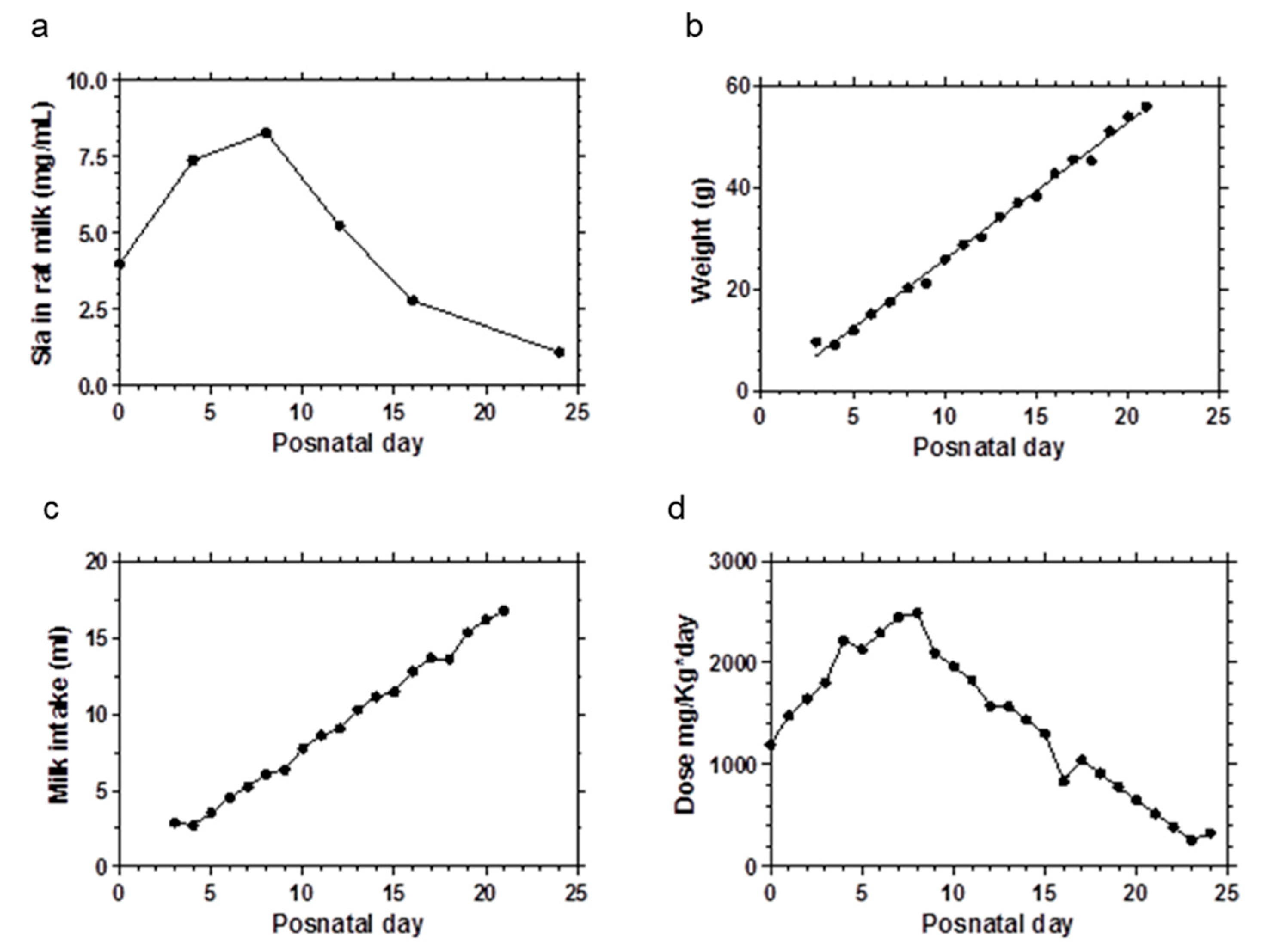
2.3. Sialic Acid Doses
2.4. Analytical Determination of Sialic Acid Content by HPLC
2.5. Western Blotting
2.6. Long-Term Potentiation Measurement
2.7. Classical Behavioral Tests
2.7.1. Novel Object Recognition Test
2.7.2. Y Maze with Blocked Arm Test
2.8. IntelliCage® Protocol
2.9. Statistical Analysis
3. Results
3.1. Sialic Acid Content in Brain
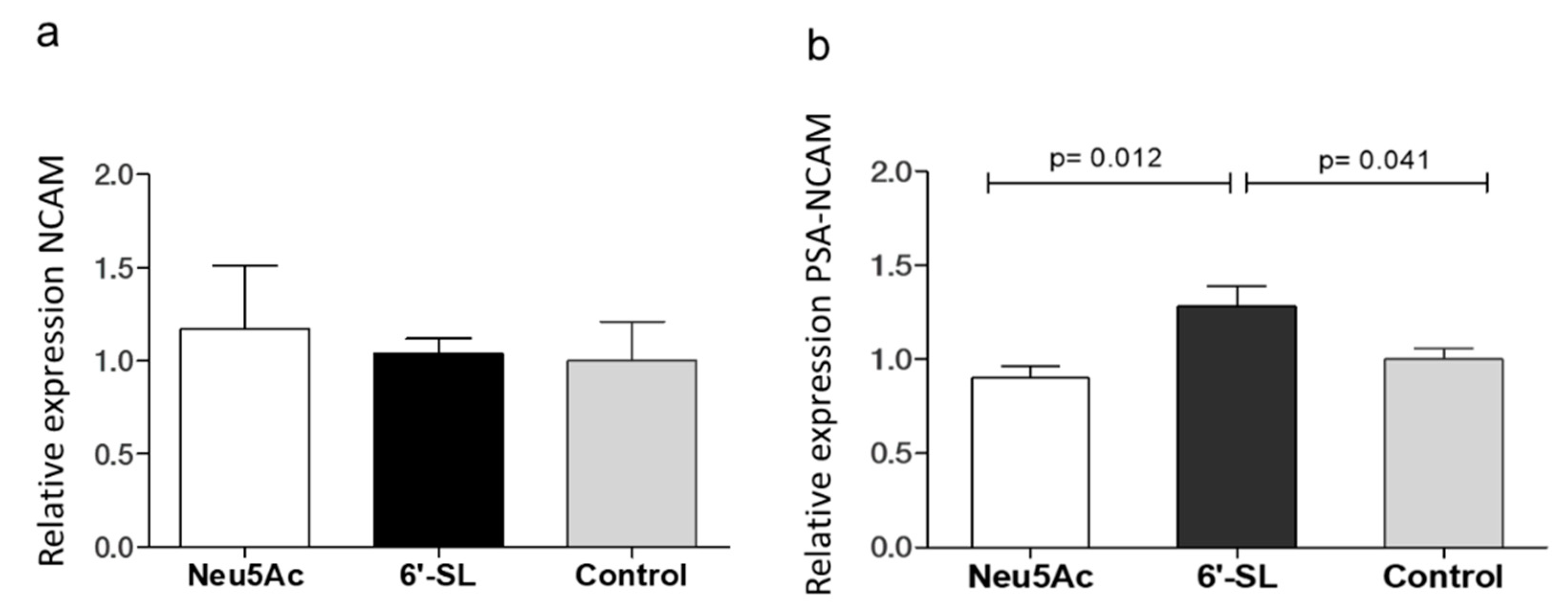
3.2. Western Blot
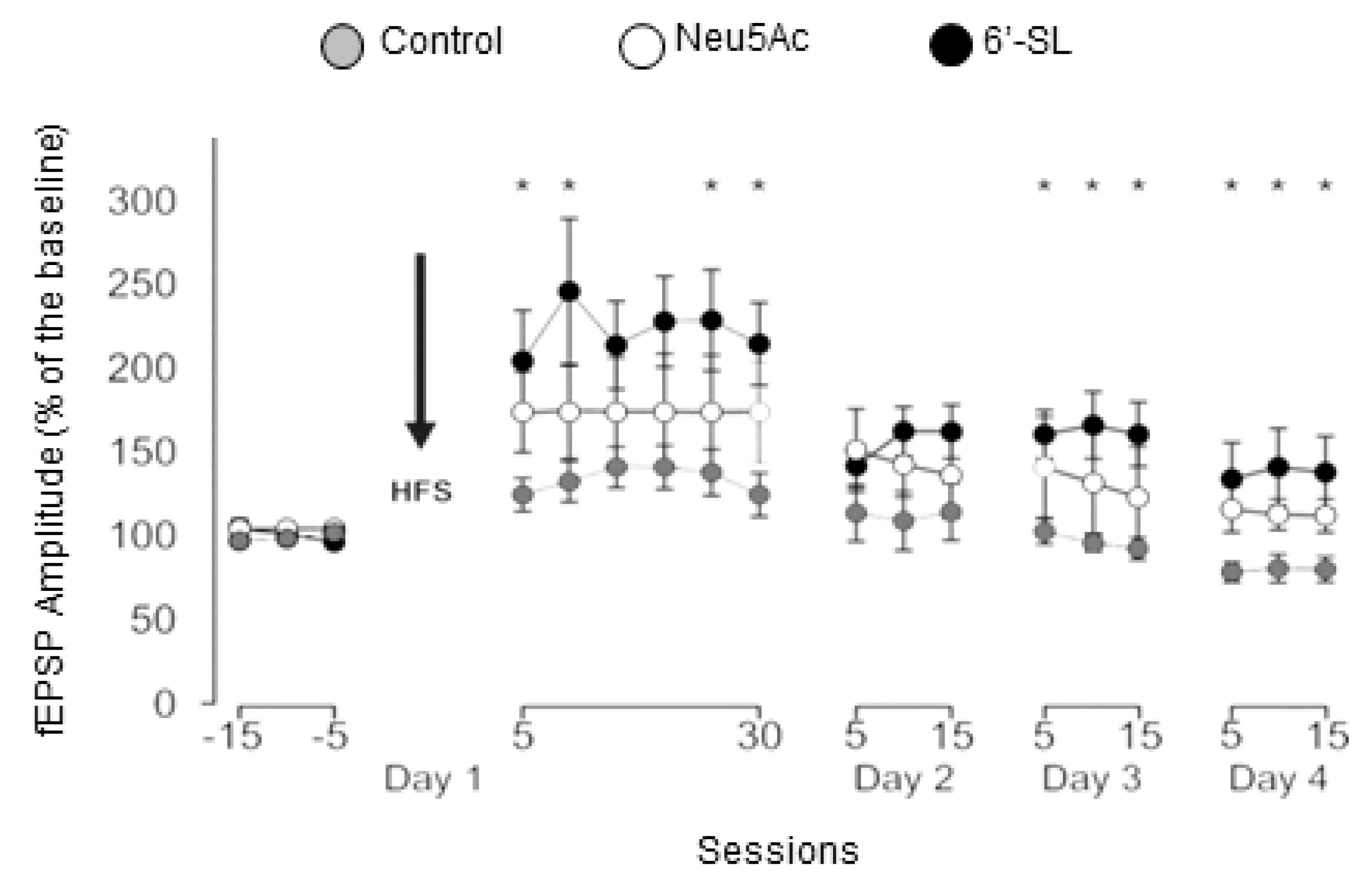
3.3. In Vivo LTP
3.4. Classical Behavioral Tests
3.4.1. NORT
3.4.2. Y Maze with Blocked Arm Test
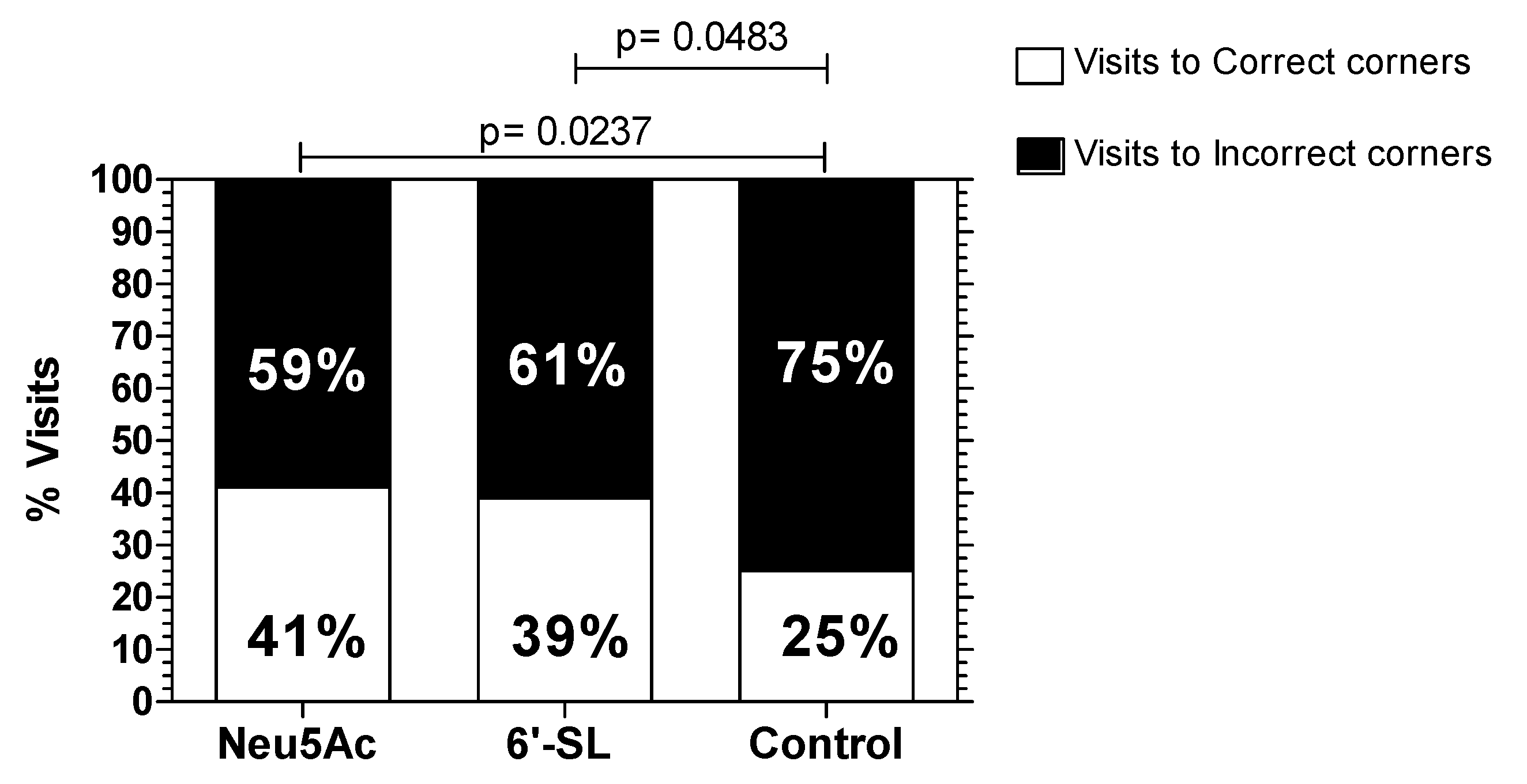
3.5. IntelliCage® Protocol
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, J.W.; Johnstone, B.M.; Remley, D.T. Breast-feeding and cognitive development: A meta-analysis. Am. J. Clin. Nutr. 1999, 70, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Schauer, R. Sialic acids. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef] [PubMed]
- Ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.V.; Pierani, P.; Zampini, L.; Carloni, I.; Carlucci, A.; Gabrielli, O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. Suppl. 1999, 88, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 2012, 64, 83–99. [Google Scholar] [PubMed]
- Kiskini, A.; Difilippo, E. Oligosaccharides in goat milk: Structure, health effects and isolation. Cell. Mol. Biol. 2013, 59, 25–30. [Google Scholar] [PubMed]
- Martin-Sosa, S.; Martin, M.J.; Garcia-Pardo, L.A.; Hueso, P. Sialyloligosaccharides in human and bovine milk and in infant formulas: Variations with the progression of lactation. J. Dairy Sci. 2003, 86, 52–59. [Google Scholar] [CrossRef]
- Peterson, R.; Cheah, W.Y.; Grinyer, J.; Packer, N. Glycoconjugates in human milk: Protecting infants from disease. Glycobiology 2013, 23, 1425–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, S.; De Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Jennewein, S.; Hufner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Koromyslova, A.; Singh, B.K.; Hansman, S.; Jennewein, S.; Schroten, H.; Hansman, G.S. Structural basis for norovirus inhibition by human milk oligosaccharides. J. Virol. 2016, 90, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates cd14 expression in human enterocytes, thereby attenuating lps-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienenstock, J.; Buck, R.H.; Linke, H.; Forsythe, P.; Stanisz, A.M.; Kunze, W.A. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS ONE 2013, 8, e76236. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Sugawara, M.; Kawakami, H. Sialic acid in human milk: Composition and functions. Acta Paediatr. Taiwan 2001, 42, 11–17. [Google Scholar] [PubMed]
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. Int. Rev. J. 2012, 3, 465S–472S. [Google Scholar] [CrossRef] [PubMed]
- Asakuma, S.; Akahori, M.; Kimura, K.; Watanabe, Y.; Nakamura, T.; Tsunemi, M.; Arai, I.; Sanai, Y.; Urashima, T. Sialyl oligosaccharides of human colostrum: Changes in concentration during the first three days of lactation. Biosci. Biotechnol. Biochem. 2007, 71, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; McVeagh, P.; Petocz, P.; Brand-Miller, J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 2003, 78, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Chichlowski, M.; German, J.B.; Lebrilla, C.; Mills, D.A. The influence of milk oligosaccharides on microbiota of infants: Opportunities for formulas. Annu. Rev. Food Sci. Technol. 2011, 2, 331–351. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Freeman, S.L.; Lebrilla, C.B.; Mills, D.A. Human milk oligosaccharides: Evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr. Workshop Ser. Pediatr. Program. 2008, 62, 205–218. [Google Scholar] [PubMed]
- Jantscher-Krenn, E.; Marx, C.; Bode, L. Human milk oligosaccharides are differentially metabolised in neonatal rats. Br. J. Nutr. 2013, 110, 640–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, B.L.; Winick, M. Effects of administration of n-acetylneuraminic acid (nana) on brain nana content and behavior. J. Nutr. 1980, 110, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, E.; Barranco, A.; Ramirez, M.; Gruart, A.; Delgado-Garcia, J.M.; Jimenez, M.L.; Buck, R.; Rueda, R. Dietary 2′-fucosyllactose enhances operant conditioning and long-term potentiation via gut-brain communication through the vagus nerve in rodents. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′sialyllactose and 6′sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant gut microbiome associated with cognitive development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; ILSI Europe a.i.s.b.l. The influence of children’s diet on their cognition and behavior. Eur. J. Nutr. 2008, 47 (Suppl. 3), 25–37. [Google Scholar] [CrossRef]
- Sprenger, N.; Duncan, P.I. Sialic acid utilization. Adv. Nutr. 2012, 3, 392S–397S. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.J.; Messer, M. Intestinal neuraminidase activity of suckling rats and other mammals. Relationship to the sialic acid content of milk. Biochem. J. 1978, 170, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, H.D.; Thomas, J.D. Artificial Rearing. In Alcohol: Methods and Protocols; Nagy, L.E., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 447, pp. 85–100. [Google Scholar]
- Hara, S.; Takemori, Y.; Yamaguchi, M.; Nakamura, M.; Ohkura, Y. Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal. Biochem. 1987, 164, 138–145. [Google Scholar] [CrossRef]
- Martin, M.J.; Vazquez, E.; Rueda, R. Application of a sensitive fluorometric HPLC assay to determine the sialic acid content of infant formulas. Anal. Bioanal. Chem. 2007, 387, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Gruart, A.; Munoz, M.D.; Delgado-Garcia, J.M. Involvement of the ca3-ca1 synapse in the acquisition of associative learning in behaving mice. J. Neurosci. 2006, 26, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, E.; Barranco, A.; Ramirez, M.; Gruart, A.; Delgado-García, J.M.; Martinez-Lara, E.; Blanco, S.; Martin, M.J.; Castanys-Muñoz, E.; Buck, R.H.; et al. Effects of a human milk oligosacharide, 2′-fucosyllactose, on hippocampal long term potentiation and learning capabilities in rodents. JNB 2015, 26, 455–465. [Google Scholar] [PubMed]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Delgado-Garcia, J.M.; Buck, R.; Rueda, R.; Martin, M.J. Oral supplementation of 2′-fucosyllactose during lactation improves memory and learning in rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Wolf, A.; Bauer, B.; Abner, E.L.; Ashkenazy-Frolinger, T.; Hartz, A.M. A comprehensive behavioral test battery to assess learning and memory in 129s6/tg2576 mice. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Dellu, F.; Mayo, W.; Cherkaoui, J.; Le Moal, M.; Simon, H. A two-trial memory task with automated recording: Study in young and aged rats. Brain Res. 1992, 588, 132–139. [Google Scholar] [CrossRef]
- Wang, B.; Downing, J.A.; Petocz, P.; Brand-Miller, J.; Bryden, W.L. Metabolic fate of intravenously administered n-acetylneuraminic acid-6-14c in newborn piglets. Asia Pac. J. Clin. Nutr. 2007, 16, 110–115. [Google Scholar] [PubMed]
- Carlson, S.E.; House, S.G. Oral and intraperitoneal administration of n-acetylneuraminic acid: Effect on rat cerebral and cerebellar n-acetylneuraminic acid. J. Nutr. 1986, 116, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, S.A.; Gottipati, B.S.; Gajewski, B.J.; Carlson, S.E. Dietary sialic acid and cholesterol influence cortical composition in developing rats. J. Nutr. 2013, 143, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A.; Chichlowski, M.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary sialyllactose does not influence measures of recognition memory or diurnal activity in the young pig. Nutrients 2018, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Fleming, S.A.; Labhart, B.; Chichlowski, M.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary sialyllactose influences sialic acid concentrations in the prefrontal cortex and magnetic resonance imaging measures in corpus callosum of young pigs. Nutrients 2017, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary isomers of sialyllactose increase ganglioside sialic acid concentrations in the corpus callosum and cerebellum and modulate the colonic microbiota of formula-fed piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; De Bartolo, P.; Caporali, P.; Laricchiuta, D.; Foti, F.; Ronci, M.; Rossi, C.; Neri, C.; Spalletta, G.; Caltagirone, C.; et al. N-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front. Aging Neurosci. 2014, 6, 220. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Honma, H.; Saitoh, Y.; Yasuda, Y.; Yokogoshi, H. Effects of dietary sialic acid in n-3 fatty acid-deficient dams during pregnancy and lactation on the learning abilities of their pups after weaning. J. Nutr. Sci. Vitaminol. (Tokyo) 2013, 59, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Leussis, M.P.; Bolivar, V.J. Habituation in rodents: A review of behavior, neurobiology, and genetics. Neurosci. Biobehav. Rev. 2006, 30, 1045–1064. [Google Scholar] [CrossRef] [PubMed]
- Aggleton, J.P.; Albasser, M.M.; Aggleton, D.J.; Poirier, G.L.; Pearce, J.M. Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behav. Neurosci. 2010, 124, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Albasser, M.M.; Davies, M.; Futter, J.E.; Aggleton, J.P. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav. Neurosci. 2009, 123, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.L.; Lönnerdal, B.; Clegg, M.; Hurley, L.S. Developmental changes in composition of rat milk: Trace elements, minerals, protein, carbohydrate and fat. J. Nutr. 1981, 111, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Anjos, T.; Altmae, S.; Emmett, P.; Tiemeier, H.; Closa-Monasterolo, R.; Luque, V.; Wiseman, S.; Perez-Garcia, M.; Lattka, E.; Demmelmair, H.; et al. Nutrition and neurodevelopment in children: Focus on nutrimenthe project. Eur. J. Nutr. 2013, 52, 1825–1842. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.G.; Artola, A.; Gerardy-Schahn, R.; Becker, T.; Welzl, H.; Schachner, M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J. Neurosci. Res. 1996, 45, 143–152. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.M.; Wang, J.T.; Chiu, T.H. Effects of exogenous gm1 ganglioside on ltp in rat hippocampal slices perfused with different concentrations of calcium. Neurosci. Lett. 1992, 141, 227–230. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Wu, G.; Lu, Z.H.; Kozireski-Chuback, D.; Fang, Y. The role of gm1 and other gangliosides in neuronal differentiation. Overview and new finding. Ann. N. Y. Acad. Sci. 1998, 845, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Microbiota regulation of the mammalian gut-brain axis. Adv. Appl. Microbiol. 2015, 91, 1–62. [Google Scholar] [PubMed]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human milk oligosaccharides inhibit the adhesion to caco-2 cells of diarrheal pathogens: Escherichia coli, vibrio cholerae, and salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Nöhle, U.; Schauer, R. Metabolism of sialic acids from exogenously administered sialyllactose and mucin in mouse and rat. Hoppe Seylers Z. Physiol. Chem. 1984, 365, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, T.; Malicdan, M.C.; Cho, A.; Hayashi, Y.K.; Nonaka, I.; Mine, T.; Yamamoto, T.; Nishino, I.; Noguchi, S. Sialyllactose ameliorates myopathic phenotypes in symptomatic gne myopathy model mice. Brain 2014, 137, 2670–2679. [Google Scholar] [CrossRef] [PubMed]

| Sia Concentration (µg Neu5Ac/mg Brain) | ||||
|---|---|---|---|---|
| Neu5Ac Group | 6′-SL Group | Control Group | One-Way ANOVA p-Value | |
| Pups rats (n = 8/group) | 1.399 ± 0.067 | 1.405 ± 0.124 | 1.422 ± 0.058 | p = 0.8336 |
| 1-year old rats (n = 8–10/group) | 1.018 ± 0.106 | 1.067 ± 0.025 | 0.9745 ± 0.096 | p = 0.2143 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveros, E.; Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Buck, R.; Rueda, R.; Martín, M.J. Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats. Nutrients 2018, 10, 1519. https://doi.org/10.3390/nu10101519
Oliveros E, Vázquez E, Barranco A, Ramírez M, Gruart A, Delgado-García JM, Buck R, Rueda R, Martín MJ. Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats. Nutrients. 2018; 10(10):1519. https://doi.org/10.3390/nu10101519
Chicago/Turabian StyleOliveros, Elena, Enrique Vázquez, Alejandro Barranco, María Ramírez, Agnes Gruart, Jose María Delgado-García, Rachael Buck, Ricardo Rueda, and María J. Martín. 2018. "Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats" Nutrients 10, no. 10: 1519. https://doi.org/10.3390/nu10101519
APA StyleOliveros, E., Vázquez, E., Barranco, A., Ramírez, M., Gruart, A., Delgado-García, J. M., Buck, R., Rueda, R., & Martín, M. J. (2018). Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats. Nutrients, 10(10), 1519. https://doi.org/10.3390/nu10101519





