Effect of Fermentation with Different Lactic Acid Bacteria Starter Cultures on Biogenic Amine Content and Ripening Patterns in Dry Fermented Sausages
Abstract
1. Introduction
2. Materials and Methods
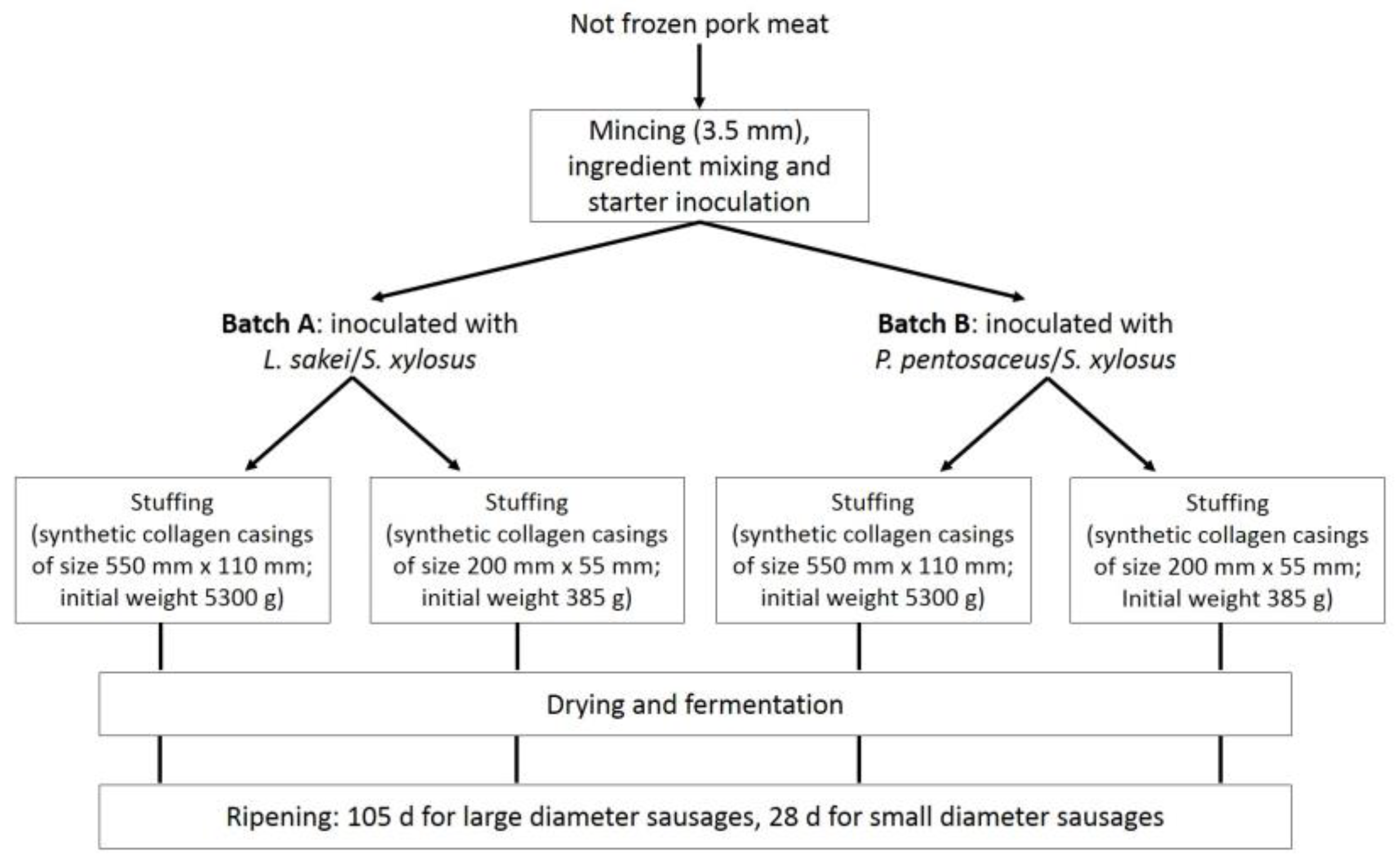
2.1. Sausage Manufacturing
2.2. Weight Losses and pH
2.3. Biogenic Amine
2.4. Proteolysis of Myofibrillar Proteins
2.5. Lipid Characterization
2.6. Statistical Analysis
3. Results
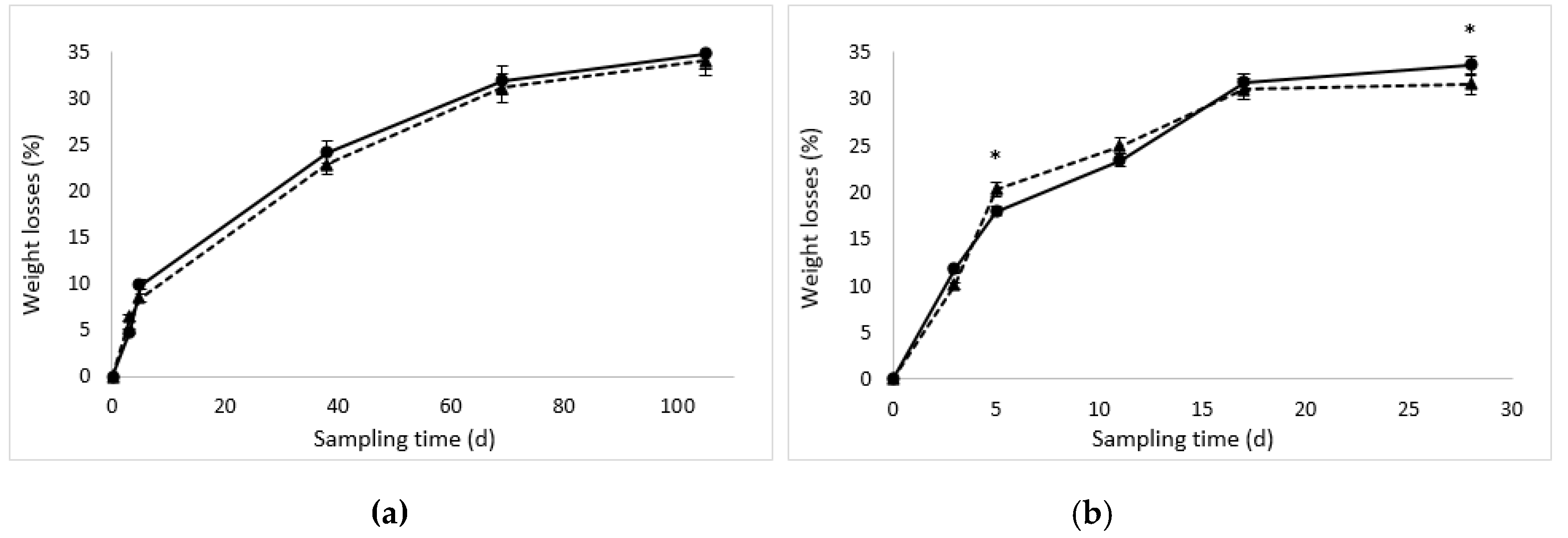
3.1. Weight Losses
3.2. Biogenic Amine Content
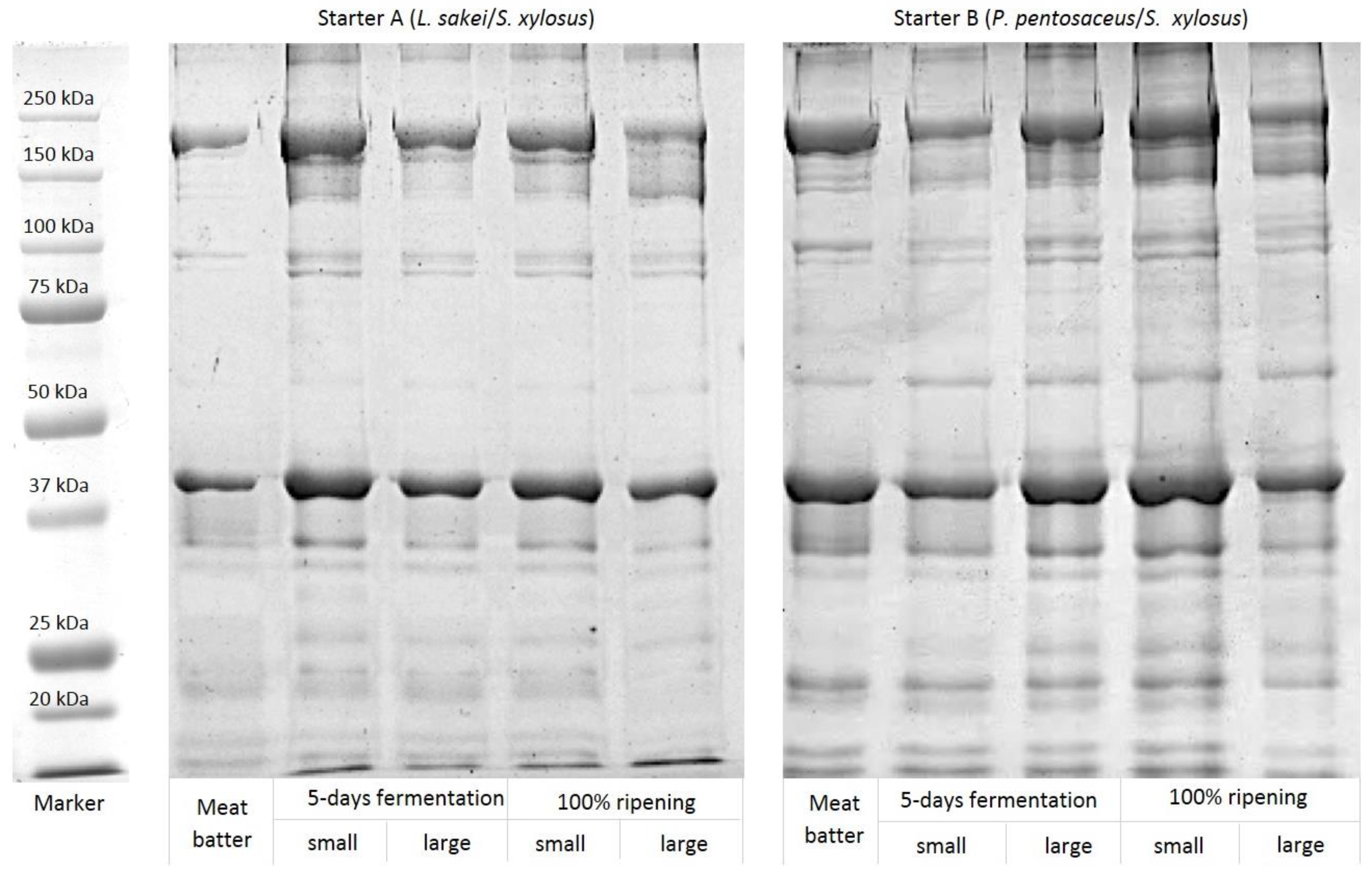
3.3. Proteolysis of Myofibrillar Proteins
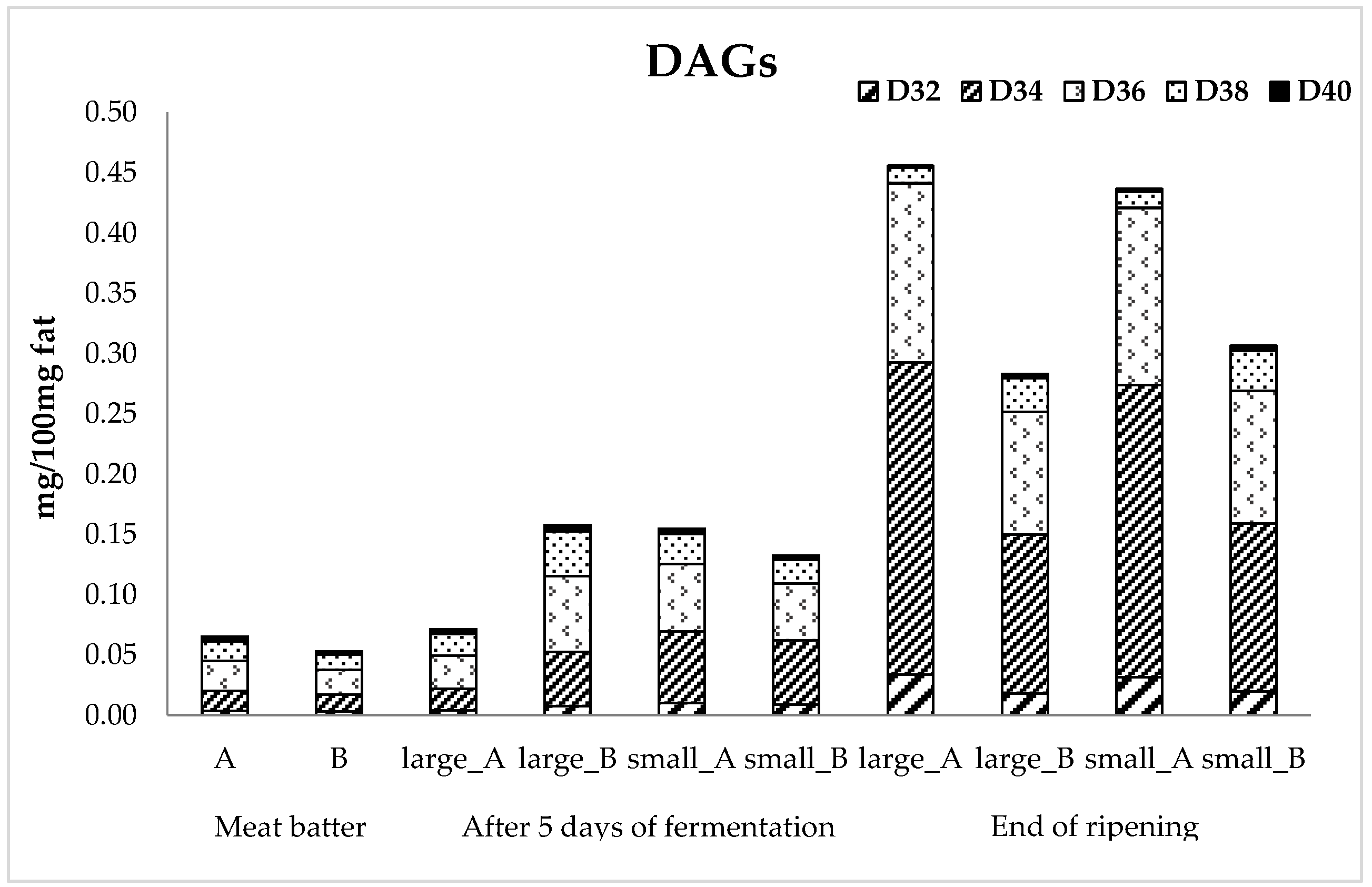
3.4. Lipid Fraction
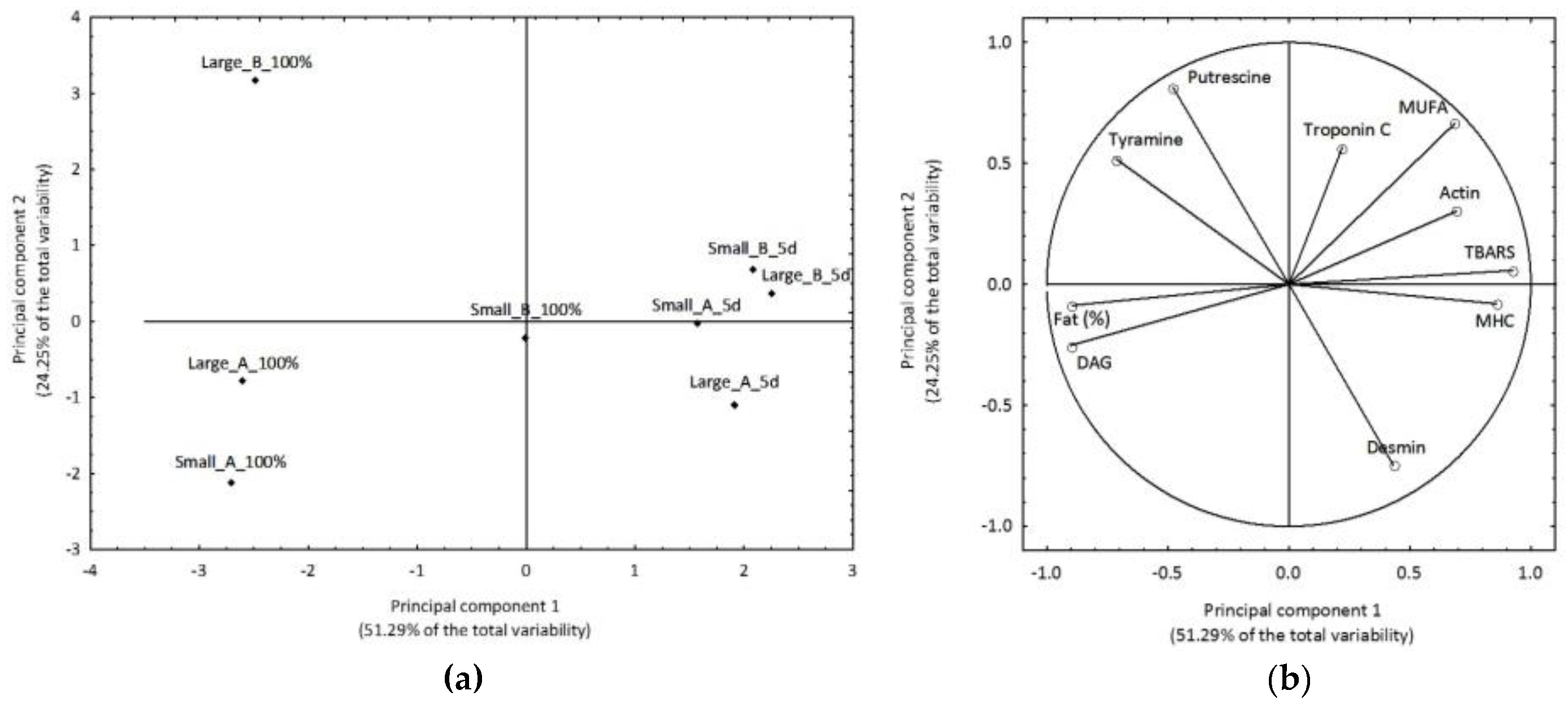
3.5. Three-way ANOVA and Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leroy, F.; Geyzen, A.; Janssens, M.; De Vust, L.; Scholliers, P. Meat fermentation at the crossroads of innovation and tradition: A historical outlook. Trends Food Sci. Technol 2013, 31, 130–137. [Google Scholar] [CrossRef]
- Toldrá, F. Handbook of Food Science, Technology and Engineering; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 4. [Google Scholar]
- Shabbir, M.A.; Raza, A.; Anjum, F.M.; Khan, M.R.; Suleria, H.A. Effect of thermal treatment on meat proteins with special reference to heterocyclic aromatic amines (HAAs). Crit. Rev. Food Sci. Nutr. 2015, 55, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, B.A.; Campagnol, P.C.B.; da Cruz, A.G.; Morgano, M.A.; Wagner, R.; Pollonio, M.A.R. Is there a potential consumer market for low-sodium fermented sausages? J. Food Sci. 2015, 80, S1093–S1099. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L.; Rantsiou, K. Meat fermentation. In Handbook of Meat and Meat Processing, 2nd ed.; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Diaz, O.; Fernandez, M.; De Fernando, G.D.G.; de la Hoz, L.; Ordoñez, J.A. Proteolysis in Dry Fermented Sausages: The Effect of Selected Exogenous Proteases. Meat Sci. 1997, 46, 115–128. [Google Scholar] [CrossRef]
- Berardo, A.; Devreese, B.; De Maere, H.; Stavropoulou, D.A.; Van Royen, G.; Leroy, F.; De Smet, S. Actin proteolysis during ripening of dry fermented sausages at different pH values. Food Chem. 2017, 221, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Saccani, G.; Fornelli, G.; Zanardi, E. Characterization of textural properties and changes of myofibrillar and sarcoplasmic proteins in salame felino during ripening. Int. J. Food Prop. 2013, 16, 1460–1471. [Google Scholar] [CrossRef][Green Version]
- Stavropoulou, D.A.; Borremans, W.; De Vuyst, L.; De Smet, S.; Leroy, F. Amino acid conversions by coagulase-negative staphylococci in a rich medium: Assessment of inter- and intraspecies heterogeneity. Int. J. Food Microbiol. 2015, 212, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.M.; Sentandreu, M.A.; Vignolo, G.M.; Fadda, S.G. Proteomic and peptidomic insights on myofibrillar protein hydrolysis in a sausage model during fermentation with autochthonous starter cultures. Food Res. Int. 2015, 78, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Di Monaco, R.; Cavella, S.; Toldrà, F.; Ercolini, D.; Villani, F. Proteolytic and lipolytic starter cultures and their effect on traditional fermented sausages ripening and sensory traits. Food Microbiol. 2008, 25, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Mainar, M.; Stavropoulou, D.A.; Leroy, F. Exploring the metabolic heterogeneity of coagulase-negative staphylococci to improve the quality and safety of fermented meats: A review. Int. J. Food Microbiol. 2017, 247, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Franco, D. Fat effect on physico-chemical, microbial and textural changes through the manufactured of dry-cured foal sausage Lipolysis, proteolysis and sensory properties. Meat Sci. 2012, 92, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Gallego, M.; Escudero, E.; Reig, M.; Aristoy, M.C.; Toldrá, F. Small peptides hydrolysis in dry-cured meats. Int. J. Food Microbiol. 2015, 212, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological factors affecting biogenic amine content in foods: A review. Front Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]
- Cid, S.B.; Buncic, S.; McLauchlin, J.; Maradona, M.P.; Rauscher-Gabernig, E.; Spano, G. Scientific opinion on risk-based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393–2486. [Google Scholar]
- Ordoñez, J.A.; Hierro, E.M.; Bruna, J.M.; de la Hoz, L. Changes in the components of dry-fermented sausages during ripening. Crit. Rev. Food Sci. Nutr. 1999, 39, 329–367. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Shabbir, M.A.; Khan, M.I.; Suleria, H.A.R.; Sultan, S. Effect of Thermal Treatments on the Formation of Heterocyclic Aromatic Amines in Various Meats. J. Food Process Preserv. 2015, 39, 376–383. [Google Scholar] [CrossRef]
- Anastasio, A.; Draisci, R.; Pepe, T.; Mercogliano, R.; Delli Quadri, F.; Luppi, G.; Cortesi, M.L. Development of biogenic amines during the ripening of Italian dry sausages. J. Food Prot. 2010, 73, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Montanari, C.; Bargossi, E.; Gardini, A.; Lanciotti, R.; Magnani, R.; Gardini, F.; Tabanelli, G. Correlation between volatile profiles of Italian fermented sausages and their size and starter culture. Food Chem. 2016, 192, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, G.; Bargossi, E.; Gardini, A.; Lanciotti, R.; Magnani, R.; Gardini, F.; Montanari, C. Physico-chemical and microbiological characterisation of Italian fermented sausages in relation to their size. J. Sci. Food Agric. 2016, 96, 2773–2781. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.; Puglisi, E.; Cocconcelli, P.S. Comparing natural and selected starter cultures in meat and cheese fermentations. Curr. Opin. Food Sci. 2015, 2, 118–122. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Sanmartin, C.; Quartacci, M.F.; Andrich, G. Kinetics of d-glucose and d-fructose conversion during the alcoholic fermentation promoted by Saccharomyces cerevisiae. Biosci. Bioeng. 2013, 115, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zinnai, A.; Venturi, F.; Quartacci, M.F.; Andrich, G.A. Mathematical model to describe malolactic fermentation. Ital. J. Food Sci. 2011, 23, 80–89. [Google Scholar]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanellia, G.; Gardini, F. Effects of the diameter on physico-chemical, microbiological and volatile profile in dry fermented sausages produced with two different starter cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]
- Coloretti, F.; Chiavari, C.; Armaforte, E.; Carri, S.; Castagnetti, G.B. Combined use of starter cultures and preservatives to control production of biogenic amines and improve sensorial profile in low-acid salami. J. Agric. Food Chem. 2008, 56, 11238–11244. [Google Scholar] [CrossRef] [PubMed]
- Martuscelli, M.; Crudele, M.A.; Gardini, F.; Suzzi, G. Biogenic amine formation and oxidation by Staphylococcus xylosus strains from artisanal fermented sausages. Lett. Appl. Microbiol. 2000, 31, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Tabanelli, G.; Lanciotti, R.; Montanari, C.; Luppi, M.; Coloretti, F.; Chiavari, C.; Grazia, L. Biogenic amine content and aromatic profile of Salama da sugo, a typical cooked fermented sausage produced in Emilia-Romagna Region (Italy). Food Control. 2013, 32, 638–643. [Google Scholar] [CrossRef]
- Hughes, M.C.; Kerry, J.P.; Arendt, E.K.; Kenneally, P.M.; McSweeney, P.L.; O’Neill, E.E. Characterization of proteolysis during the ripening of semi-dry fermented sausages. Meat Sci. 2002, 62, 205–216. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Christie, W.W. A simple procedure of rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [PubMed]
- Ben Lajnef, H.; Pasini, F.; Politowicz, J.; Tlili, N.; Khaldi, A.; Caboni, M.F.; Nasri, N. Lipid characterization of Eryngium maritimum seeds grown in Tunisia. Ind. Crop. Prod. 2017, 105, 47–52. [Google Scholar] [CrossRef]
- Bortolomeazzi, R.; Frega, N.; Lercker, G. Rapid determination of free and esterified sterols using prepacked silica columns. Ital. J. Food Sci. 1990, 2, 265–268. [Google Scholar]
- Sweeley, C.C.; Bentley, R.; Makita, M.; Wells, W.W. Gas–liquid chromatography of trimethylsilyl derivatives of sugars and related substances. J. Am. Chem. Soc. 1963, 85, 2497–2507. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [PubMed]
- Bao, Y.; Ertbjerg, P. Relationship between oxygen concentration, shear force and protein oxidation in modified atmosphere packaged pork. Meat Sci. 2015, 110, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Suzzi, G.; Gardini, F. Biogenic amines in dry fermented sausages: A review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef]
- Wunderlichová, L.; Buňková, L.; Koutnỳ, M.; Jaňcová, P.; Buňka, F. Formation, degradation, and detoxification of putrescine by foodborne bacteria: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1012–1033. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Pietroniro, R.; Doria, F.; Pessione, E.; Garcia-Moruno, E. Putrescine production from different amino acid precursors by lactic acid bacteria from wine and cider. Int. J. Food Microbiol. 2013, 165, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Rimaux, T.; Rivière, A.; Illeghems, K.; Weckx, S.; De Vuyst, L.; Leroy, F. Expression of the arginine deiminase pathway genes in Lactobacillus sakei is strain dependent and is affected by the environmental pH. Appl. Environ. Microbiol. 2012, 78, 4874–4883. [Google Scholar] [CrossRef] [PubMed]
- Van Ba, H.; Seo, H.W.; Kim, J.H.; Cho, S.H.; Kim, Y.S.; Ham, J.S.; Park, B.Y.; Kim, H.W.; Kim, T.B.; Seong, P.N. The effects of starter culture types on the technological quality, lipid oxidation and biogenic amines in fermented sausages. LWT Food Sci. Technol. 2016, 74, 191–198. [Google Scholar] [CrossRef]
- Miguélez-Arrizado, M.J.; Bover-Cid, S.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Biogenic amines in Spanish fermented sausages as a function of diameter and artisanal or industrial origin. J. Sci. Food Agric. 2006, 86, 549–557. [Google Scholar] [CrossRef]
- Komprda, T.; Sládková, P.; Dohnal, V. Biogenic amine content in dry fermented sausage as influenced by a producer, spice mix, starter culture, sausages diameter and time of ripening. Meat Sci. 2009, 83, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Bosch-Fusté, J.; Vidal-Carou, M.C. Influence of technological conditions of sausage fermentation on the aminogenic activity of L. curvatus CTC273. Food Microbiol. 2012, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Martuscelli, M.; Caruso, M.C.; Galgano, F.; Crudele, M.A.; Favati, F.; Guerzoni, M.E.; Suzzi, G. Effects of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis. Int. J. Food Microbiol. 2001, 64, 105–117. [Google Scholar] [CrossRef]
- Emborg, J.; Dalgaard, P. Modelling the effect of temperature, carbon dioxide, water activity and pH on growth and histamine formation by Morganella psychrotolerans. Int. J. Food Microbiol. 2008, 128, 226–233. [Google Scholar] [CrossRef] [PubMed]
- La Gioia, F.; Rizzotti, L.; Rossi, F.; Gardini, F.; Tabanelli, G.; Torriani, S. Identification of a Tyrosine Decarboxylase (tdcA) Gene in Streptococcus thermophilus 1TT45: Analysis of its Expression and Tyramine Production in Milk. Appli. Environ. Microbiol. 2011, 77, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Buňková, L.; Buňka, F.; Pollaková, E.; Podešvová, T.; Dráb, V. The effect of lactose, NaCl and an aero/anaerobic environment on the tyrosine decarboxylase activity of Lactococcus lactis subsp. cremoris and Lactococcus lactis subsp. lactis. Int. J. Food Microbiol. 2011, 147, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Escudero, E.; Toldrá, F. Bioactive peptides and free amino acids profiles in different types of European dry-fermented sausages. Int. J. Food Microbiol. 2018, 276, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Candogan, K.; Wardlaw, F.B.; James, C.; Acton, A. Effect of starter culture on proteolytic changes during processing of fermented beef sausages. Food Chem. 2009, 116, 731–737. [Google Scholar] [CrossRef]
- Soriano, A.; Cruz, B.; Gomez, L.; Mariscal, C.; Garca Ruiz, A. Proteolysis, physicochemical characteristics and free fatty acid composition of dry sausages made with deer (Cervus elaphus) or wild boar (Sus scrofa) meat: A preliminary study. Food Chem. 2006, 96, 173–184. [Google Scholar] [CrossRef]
- Aro Aro, J.M.; Nyam-Osor, P.; Tsuji, K.; Shimada, K.; Fukushima, M.; Sekikawa, M. The effect of starter cultures on proteolytic changes and amino acid content in fermented sausages. Food Chem. 2010, 119, 279–285. [Google Scholar] [CrossRef]
- Lomiwes, D.; Hurst, S.M.; Dobbie, P.; Frost, D.A.; Hurst, R.D.; Young, O.A.; Farouk, M.M. The protection of bovine skeletal myofibrils from proteolytic damage post mortem by small heat shock proteins. Meat Sci. 2014, 97, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Ikonic, P.; Tasica, T.; Petrovic, L.; Skaljac, S.; Jokanovic, M.; Mandic, A.; Ikonic, B. Proteolysis and biogenic amines formation during the ripening of Petrovská klobása, traditional dry-fermented sausage from Northern Serbia. Food Control. 2013, 30, 69–75. [Google Scholar] [CrossRef]
- Bonoli, M.; Caboni, M.F.; Rodriguez-Estrada, M.T.; Lercker, G. Effect of processing technology on the quality and composition of lipids of precooked chicken patties. Int. J. Food Sci. Technol. 2008, 43, 296–308. [Google Scholar] [CrossRef]
- Gambacorta, G.; Sinigaglia, M.; Schena, A.; Baiano, A.; Lamacchia, C.; Pati, S.; La notte, E. Changes in free fatty acid and diacylglycerol compounds in short-ripening dry-cured sausage. J. Food Lipids 2009, 16, 1–18. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT Food Sci. Technol. 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Rubio, B.; Martínez, B.; García-Cachán, M.D.; Rovira, J.; Jaime, I. Effect of the packaging method and the storage time on lipid oxidation and colour stability on dry fermented sausage salchichón manufactured with raw material with a high level of mono and polyunsaturated fatty acids. Meat Sci. 2008, 80, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Visessanguan, W.; Benjakul, S.; Riebroy, S.; Yarchai, M.; Tapingkae, W. Changes in lipid composition and fatty acid profile of Nham, a Thai fermented pork sausage, during fermentation. Food Chem. 2006, 94, 580–588. [Google Scholar] [CrossRef]
- Chizzolini, R.; Novelli, E.; Zanardi, E. Oxidation in Traditional Mediterranean Meat Products. Meat Sci. 1998, 49, S87–S99. [Google Scholar] [CrossRef]
- Severini, C.; De Pilli, T.; Baiano, A. Partial substitution of pork backfat with extra-virgin olive oil in ‘salami’ products: Effects on chemical, physical and sensorial quality. Meat Sci. 2003, 64, 323–331. [Google Scholar] [CrossRef]
| Sampling Points 1 | Starter Culture 2 | Histamine | Tyramine | Putrescine | Cadaverine |
|---|---|---|---|---|---|
| Meat batter | A | - 3 | - a | - a | - a |
| B | - | - a | - a | - a | |
| Large size sausages | |||||
| After 5 days of fermentation | A | - | - a | 1.74 a | - a |
| B | - | 0.71 a | 2.00 a | 0.58 a | |
| 30% of ripening (38 days) | A | - | 120.97 b | 2.87 a | 3.76 a |
| B | 2.20 | 145.22 c | 40.14 b | 2.05 a | |
| 60% of ripening (69 days) | A | - | 142.63 c | 3.98 a | 9.02 b |
| B | - | 155.67 d | 56.78 c | 7.26 b | |
| 100% of ripening (105 days) | A | 0.22 | 177.32 e | 8.68 a | 1.14 a |
| B | 1.94 | 204.15 f | 128.17 d | 1.95 a | |
| Small size sausages | |||||
| After 5 days of fermentation | A | - | - a | - a | - a |
| B | - | - a | - a | - a | |
| 30% of ripening (11 days) | A | - | 1.67 a | 1.43 a | 1.15 a |
| B | - | 11.73 g | 1.68 a | 0.89 a | |
| 60% of ripening (17 days) | A | - | 3.45 ag | 0.98 a | 1.59 a |
| B | - | 9.89 g | 1.06 a | 0.83 a | |
| 100% of ripening (28 days) | A | - | 6.50 ag | 1.38 a | 2.48 a |
| B | 1.75 | 9.09 g | 2.73 a | 0.95 a | |
| Sampling Points 1 | Starter Culture 2 | MHC (200 kDa) | Desmin (53 kDa) | Actin (42 kDa) | Troponin T (35.2 kDa) | Tropomyosin (34 kDa) | Troponin C (21.6 kDa) |
|---|---|---|---|---|---|---|---|
| Meat batter | A | 7.5 a | 6.0 a | 8.8 a | 6.3 a | 5.6 a | 4.8 a |
| B | 7.7 a | 5.7 a | 7.6 a | 5.9 a | 5.9 a | 5.2 a | |
| Large size sausages | |||||||
| After 5 days of fermentation | A | 7.6 a | 3.6 b | 4.5 b | 4.4 a | - b | - c |
| B | 5.9 ab | 3.6 b | 5.8 b | - b | 4.5 c | 4.1 ab | |
| 100% of ripening (105 days) | A | 3.7 b | 3.5 b | 3.6 b | 4.9 a | 5.0 ac | 3.1 b |
| B | 3.6 b | 2.9 b | 4.2 b | 3.8 a | 3.2 c | 2.9 b | |
| Small size sausages | |||||||
| After 5 days of fermentation | A | 5.0 ab | 3.4 b | 3.9 b | 4.7 a | 4.7 c | 2.9 b |
| B | 5.6 ab | 3.3 b | 5.5 b | - b | 3.9 c | 3.4 b | |
| 100% of ripening (28 days) | A | 3.1 b | 3.4 b | 3.4 b | 4.2 a | 4.4 c | - c |
| B | 4.2 b | 3.6 b | 5.4 b | 4.4 a | 3.7 c | 3.5 b | |
| Sampling Points 1 | Starter Culture 2 | Lipid | MUFAs | PUFAs | SFAs | DAGs | PV | TBARS |
|---|---|---|---|---|---|---|---|---|
| Meat batter | A | 16.95 a | 42.22 a | 10.83 | 32.60 | 0.06 a | 13.23 | 18.58 a |
| B | 16.37 a | 35.49 ab | 10.74 | 26.83 | 0.05 a | 11.59 | 15.99 a | |
| Large size sausages | ||||||||
| After 5 days of fermentation | A | 19.67 b | 29.42 b | 7.41 | 22.73 | 0.07 ab | 14.04 | 19.08 ab |
| B | 15.36 a | 37.37 ab | 10.86 | 28.75 | 0.16 bc | 13.62 | 20.32 ab | |
| 100% of ripening (105 days) | A | 25.23 c | 6.63 c | 10.42 | 30.65 | 0.46 d | 13.56 | 12.52 c |
| B | 26.14 cd | 35.31 ab | 10.35 | 27.87 | 0.28 e | 13.47 | 12.64 c | |
| Small size sausages | ||||||||
| After 5 days of fermentation | A | 19.20 b | 31.13 b | 8.08 | 24.06 | 0.15 bc | 16.48 | 22.35 ab |
| B | 17.48 ab | 33.21 ab | 9.12 | 25.76 | 0.13 bc | 13.84 | 21.81 ab | |
| 100% of ripening (28 days) | A | 28.12 d | 5.24 c | 8.81 | 26.12 | 0.44 d | 14.45 | 12.14 c |
| B | 24.86 cd | 31.56 b | 9.04 | 24.51 | 0.31 e | 13.72 | 13.76 c | |
| Variables | A | B | C | A × B | A × C | B × C | A × B × C |
|---|---|---|---|---|---|---|---|
| Tyramine | ** | ** | ** | ** | ** | ** | - |
| Cadaverine | ** | ** | ** | - | ** | * | ** |
| Putrescine | ** | ** | ** | ** | ** | ** | ** |
| MHC | - | - | * | - | - | - | - |
| Desmin | - | - | - | - | - | - | - |
| Actin | - | - | * | - | - | - | - |
| Troponin T | - | - | * | - | - | - | - |
| Tropomyosin | - | - | ** | - | - | * | * |
| Troponin C | - | ** | ** | - | - | - | ** |
| Lipid | - | ** | ** | - | - | - | * |
| MUFAs | - | ** | ** | - | - | ** | - |
| PUFAs | - | - | - | - | - | - | - |
| SFAs | - | - | - | - | - | - | - |
| DAGs | - | * | ** | - | - | ** | - |
| PV | - | - | - | - | - | - | - |
| TBARS | - | - | * | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasini, F.; Soglia, F.; Petracci, M.; Caboni, M.F.; Marziali, S.; Montanari, C.; Gardini, F.; Grazia, L.; Tabanelli, G. Effect of Fermentation with Different Lactic Acid Bacteria Starter Cultures on Biogenic Amine Content and Ripening Patterns in Dry Fermented Sausages. Nutrients 2018, 10, 1497. https://doi.org/10.3390/nu10101497
Pasini F, Soglia F, Petracci M, Caboni MF, Marziali S, Montanari C, Gardini F, Grazia L, Tabanelli G. Effect of Fermentation with Different Lactic Acid Bacteria Starter Cultures on Biogenic Amine Content and Ripening Patterns in Dry Fermented Sausages. Nutrients. 2018; 10(10):1497. https://doi.org/10.3390/nu10101497
Chicago/Turabian StylePasini, Federica, Francesca Soglia, Massimiliano Petracci, Maria Fiorenza Caboni, Sara Marziali, Chiara Montanari, Fausto Gardini, Luigi Grazia, and Giulia Tabanelli. 2018. "Effect of Fermentation with Different Lactic Acid Bacteria Starter Cultures on Biogenic Amine Content and Ripening Patterns in Dry Fermented Sausages" Nutrients 10, no. 10: 1497. https://doi.org/10.3390/nu10101497
APA StylePasini, F., Soglia, F., Petracci, M., Caboni, M. F., Marziali, S., Montanari, C., Gardini, F., Grazia, L., & Tabanelli, G. (2018). Effect of Fermentation with Different Lactic Acid Bacteria Starter Cultures on Biogenic Amine Content and Ripening Patterns in Dry Fermented Sausages. Nutrients, 10(10), 1497. https://doi.org/10.3390/nu10101497










