Digestive Responses to Fortified Cow or Goat Dairy Drinks: A Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Study Procedures
2.4. Study Treatments
2.5. Biochemical Analysis
2.6. Breath Hydrogen Analysis
2.7. Digestive Symptoms and Appetite Visual Analogue Scales
2.8. Statistical Analysis
3. Results
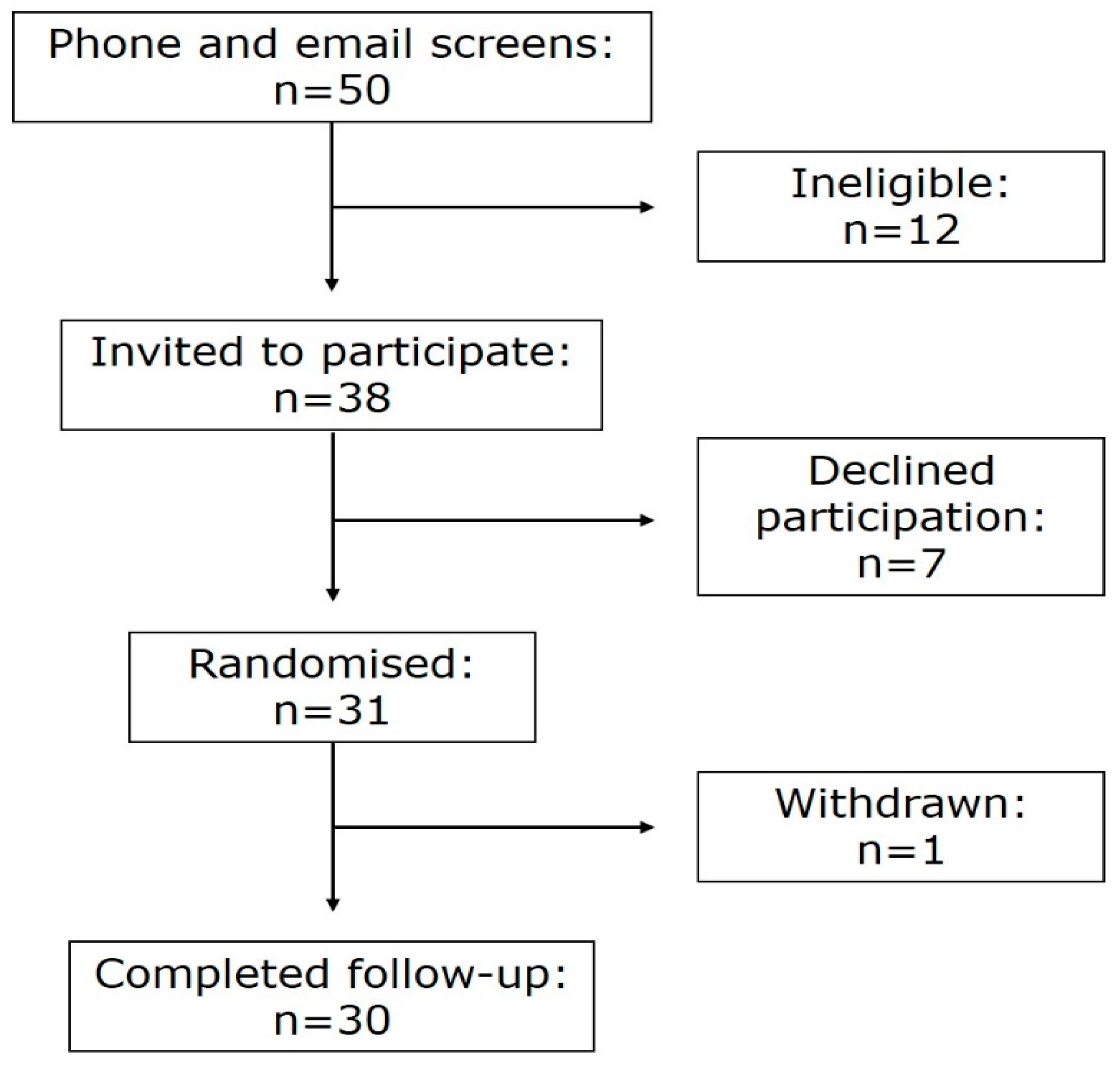
3.1. Demographics
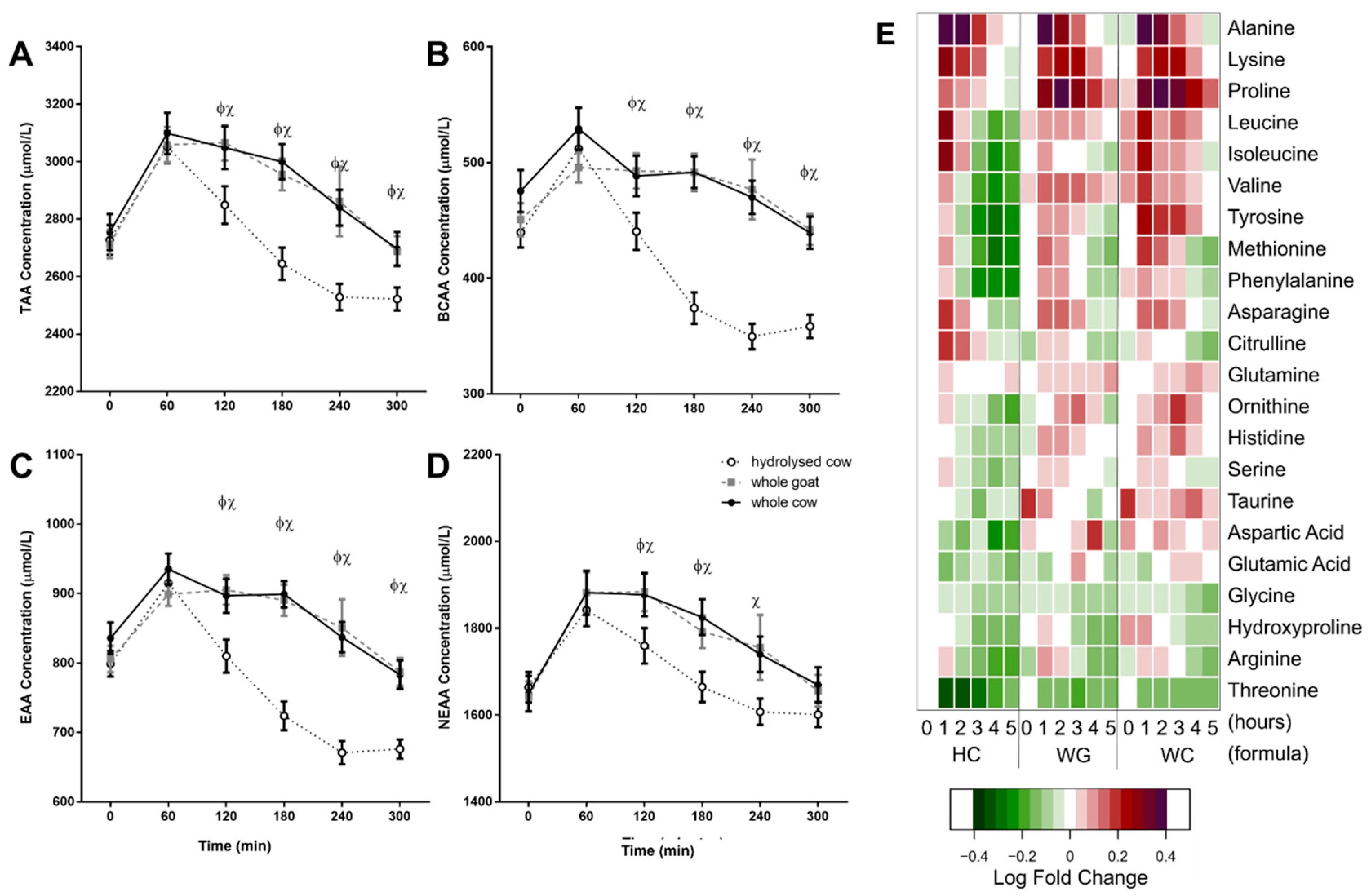
3.2. Postprandial Amino Acid Response
3.3. Postprandial Glycaemic Response
3.4. Plasma Hormone Response
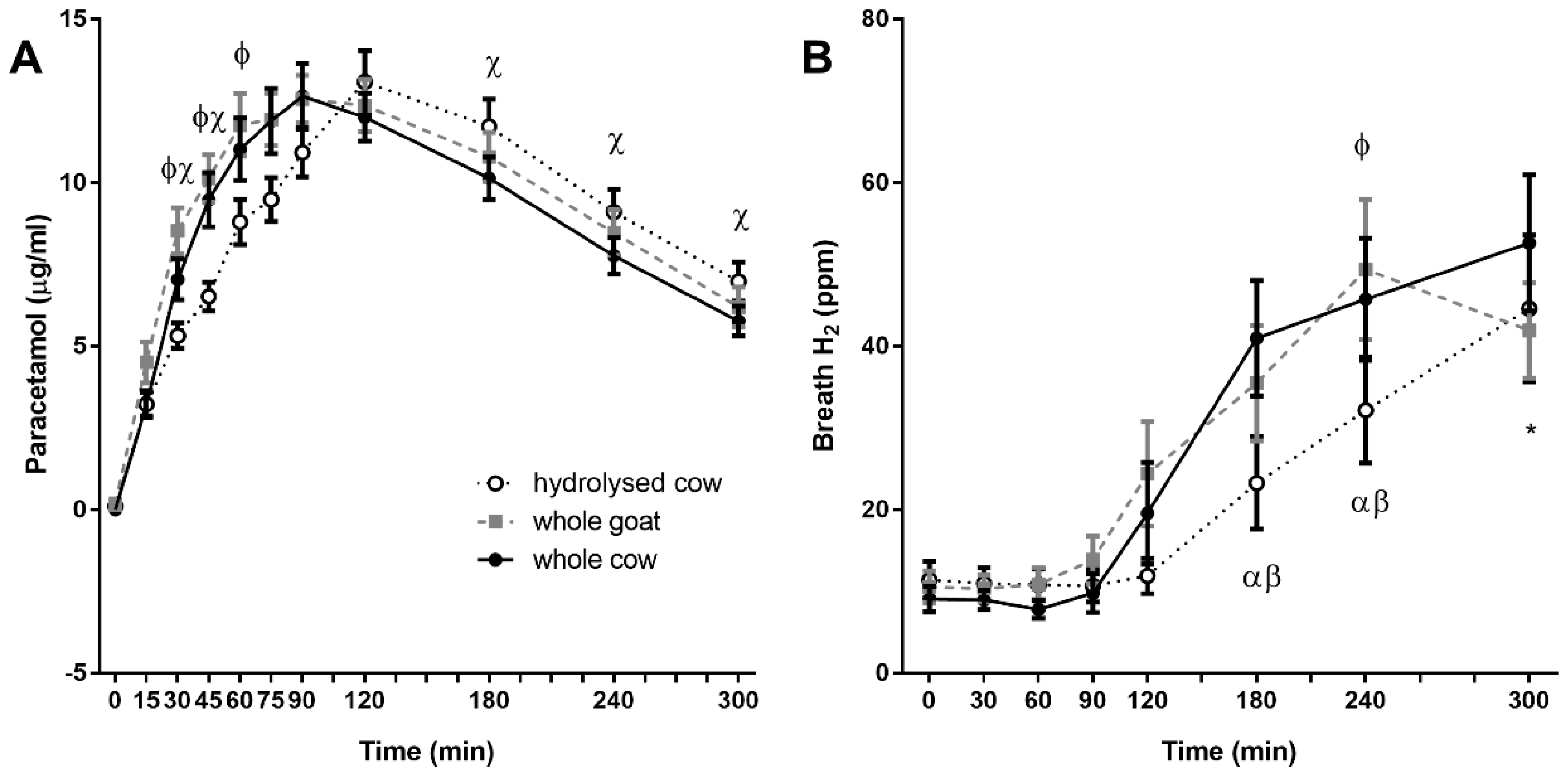
3.5. Plasma Paracetamol Recovery
3.6. Breath Hydrogen
3.7. Digestive Comfort and Appetite Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keast, D.; Fulgoni, V.; Nicklas, T.; O’Neil, C. Food Sources of Energy and Nutrients among Children in the United States: National Health and Nutrition Examination Survey 2003–2006. Nutrients 2013, 5, 283–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.L.; Harris, T.B.; Tylavsky, F.A.; Perry, S.E.; Houston, D.K.; Hue, T.F.; Strotmeyer, E.S.; Sahyoun, N.R.; Health ABC Study. Dietary patterns and survival of older adults. J. Am. Diet. Assoc. 2011, 111, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Haenlein, G.F.W. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Ghisolfi, J.; Fantino, M.; Turck, D.; de Courcy, G.P.; Vidailhet, M. Nutrient intakes of children aged 1–2 years as a function of milk consumption, cows’ milk or growing-up milk. Public Health Nutr. 2013, 16, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Fantino, M.; Gourmet, E. Apports nutritionnels en France en 2005 chez les enfants non allaités âgés de moins de 36 mois. Arch. Pédiatrie 2008, 15, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; De Ronne, N.; Van De Sompel, A.; Huysentruyt, K.; Robert, M.; Rigo, J.; Scheers, I.; Brasseur, D.; Goyens, P. A Belgian consensus-statement on growing-up milks for children 12–36 months old. Eur. J. Pediatr. 2014, 173, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Eichler, K.; Wieser, S.; Rüthemann, I.; Brügger, U. Effects of micronutrient fortified milk and cereal food for infants and children: A systematic review. BMC Public Health 2012, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Harb, T.; David, M.; Davies, P.S.; Hill, R.J. Effect of fortified milk on growth and nutritional status in young children: A systematic review and meta-analysis. Public Health Nutr. 2016, 20, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.; Bigornia, S.; Cullum-Dugan, D.; Schoonmaker, C.; Radziejowska, J.; Phipps, J.; Gokce, N.; Istfan, N.; Meyers, A.; Lenders, C. Milk-Based Nutritional Supplements in Conjunction With Lifestyle Intervention in Overweight Adolescents. Infant. Child Adolesc. Nutr. 2009, 1, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, N.P.; Kim, H.; Wells, A.M.; Kajkenova, O.; Evans, W.J. Effects of Whey and Fortified Collagen Hydrolysate Protein Supplements on Nitrogen Balance and Body Composition in Older Women. J. Am. Diet. Assoc. 2009, 109, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Gade, J.; Beck, A.M.; Bitz, C.; Christensen, B.; Klausen, T.W.; Vinther, A.; Astrup, A. Protein-enriched, milk-based supplement to counteract sarcopenia in acutely ill geriatric patients offered resistance exercise training during and after hospitalisation: study protocol for a randomised, double-blind, multicentre trial. BMJ Open 2018, 8, e019210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceballos, L.S.; Morales, E.R.; de la Torre Adarve, G.; Castro, J.D.; Martínez, L.P.; Sampelayo, M.R.S. Composition of goat and cow milk produced under similar conditions and analyzed by identical methodology. J. Food Compos. Anal. 2009, 22, 322–329. [Google Scholar] [CrossRef]
- Martin, P.; Ollivier-Bousquet, M.; Grosclaude, F. Genetic polymorphism of caseins: A tool to investigate casein micelle organization. Int. Dairy J. 1999, 9, 163–171. [Google Scholar] [CrossRef]
- Martin, P.; Szymanowska, M.; Zwierzchowski, L.; Leroux, C.; Martin, P. The impact of genetic polymorphisms on the protein composition of ruminant milks. Reprod. Nutr. Dev 2002, 42, 433–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caroli, A.M.; Chessa, S.; Erhardt, G.J. Milk Protein Polymorphisms in Cattle: Effect On Animal Breeding And Human Nutrition. J. Dairy Sci. 2009, 92, 5332–5352. [Google Scholar] [CrossRef] [PubMed]
- Pierre, A.; Michel, F.; Le Graët, Y.; Zahoute, L. Casein micelle size in relation with casein composition and αs1, αs2, β and casein contents in goat milk. Le Lait 1998, 78, 591–605. [Google Scholar] [CrossRef]
- Ambrosoli, R.; di Stasio, L.; Mazzocco, P. Content of as1—Casein and Coagulation Properties in Goat Milk. J. Dairy Sci. 1988, 71, 24–28. [Google Scholar] [CrossRef]
- Remeuf, F.; Lenoir, J.; Duby, C.; Normand, A. Etude des relations entre les caractéristiques physico-chimiques des laits de chèvre et leur aptitude à la coagulation par la présure. Le Lait 1989, 69, 499–518. [Google Scholar] [CrossRef] [Green Version]
- Mestawet, T.A.; Girma, A.; Ådnøy, T.; Devold, T.G.; Narvhus, J.A.; Vegarud, G.E. New insights in goat breeds of Ethiopia: High content of αs1-CN and its association with coagulation properties, whey syneresis and micelle size. Small Rumin. Res. 2014, 119, 146–155. [Google Scholar] [CrossRef]
- Thévenot, J.; Cauty, C.; Legland, D.; Dupont, D.; Floury, J. Pepsin diffusion in dairy gels depends on casein concentration and microstructure. Food Chem. 2017, 223, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Greppi, G.F.; Roncada, P.; Fortin, A.R. Protein Components of Goat’s Milk. Dairy Goats Feed. Nutr. 2008, 2, 71–94. [Google Scholar]
- Hodgkinson, A.J.; Wallace, O.A.M.; Boggs, I.; Broadhurst, M.; Prosser, C.G. Gastric digestion of cow and goat milk: Impact of infant and young child in vitro digestion conditions. Food Chem. 2018, 245, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calbet, J.A.L.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Cruchet, S.; Faure, C.; Lee, H.; Di Lorenzo, C.; Staiano, A.; Chundi, X.; Aw, M.; Gutiérrez-Castrellón, P.; Asery, A.; et al. When should we use partially hydrolysed formulae for frequent gastrointestinal symptoms and allergy prevention? Acta Paediatr. 2014, 103, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.M.; Boirie, Y.; van Loon, L.J.C. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, R.; Foong, R.-X.M.; Thapar, N.; Kritas, S.; Shah, N. Systematic review of the impact of feed protein type and degree of hydrolysis on gastric emptying in children. BMC Gastroenterol. 2015, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. 2013. Available online: http://www.randomizer.org (accessed on 21 September 2015).
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.; Quartero, A.O.; Numans, M.E. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig. Dis. Sci. 2001, 46, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.M.; D’Souza, R.F.; Pundir, S.; Pileggi, C.A.; Thorstensen, E.B.; Barnett, M.P.G.; Markworth, J.F.; Cameron-Smith, D.; Mitchell, C. Older adults have delayed amino acid absorption after a high protein mixed breakfast meal. J. Nutr. Heal. Aging 2015, 19, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.M.; Darragh, A.J.J.; Hendriks, W.H.H.; Prosser, C.G.G.; Lowry, D. True Ileal Amino Acid Digestibility of Goat and Cow Milk Infant Formulas. J. Dairy Sci. 2006, 89, 2408–2413. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; MacLean, D.A. Role of caloric content on gastric emptying in humans. J. Physiol. 1997, 498, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Billeaud, C.; Guillet, J.; Sandler, B. Gastric emptying in infants with or without gastro-oesophageal reflux according to the type of milk. Eur. J. Clin. Nutr. 1990, 44, 577–583. [Google Scholar] [PubMed]
- Dickinson, J.M.; Rasmussen, B.B. Essential amino acid sensing, signaling, and transport in the regulation of human muscle protein metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 83–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.D.; Cruthirds, C.L.; Lockwood, C.M.; Pappan, K.; Childs, T.E.; Company, J.M.; Brown, J.D.; Toedebusch, R.G.; Booth, F.W.; Cruthirds, C.; et al. Comparing serum responses to acute feedings of an extensively hydrolyzed whey protein concentrate versus a native whey protein concentrate in rats: A metabolomics approach. Appl. Physiol. Nutr. Metab. 2014, 39, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martín, E.; García-Escobar, E.; Ruiz de Adana, M.-S.; Lima-Rubio, F.; Peláez, L.; Caracuel, A.-M.; Bermúdez-Silva, F.-J.; Soriguer, F.; Rojo-Martínez, G.; Olveira, G.; et al. Comparison of the Effects of Goat Dairy and Cow Dairy Based Breakfasts on Satiety, Appetite Hormones, and Metabolic Profile. Nutrients 2017, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C. How to interpret hydrogen breath tests. J. Neurogastroenterol. Motil. 2011, 17, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Sherbon, J.W. Alphas1-casein, milk composition and coagulation properties of goat milk. Small Rumin. Res. 2000, 38, 123–134. [Google Scholar] [CrossRef]
- Hadary, G.; Sommer, H.H.; Gonce, J.E. The Relationship Between the Curd Tension of Milk and Gastric Emptying Time in Children. J. Dairy Sci. 1943, 26, 259–269. [Google Scholar] [CrossRef]
- Fox, M.; Georgi, G.; Boehm, G.; Menne, D.; Fried, M.; Thumshirn, M. Dietary protein precipitation properties have effects on gastric emptying in healthy volunteers. Clin. Nutr. 2004, 23, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Milsap, R.L.; Jusko, W.J. Pharmacokinetics in the infant. Environ. Health Perspect. 1994, 102, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, E.; Minekus, M.; van Aken, G.A.; van de Heijning, B.; Knol, J.; Bartke, N.; Oozeer, R.; van der Beek, E.M.; Ludwig, T. Development of the Digestive System-Experimental Challenges and Approaches of Infant Lipid Digestion. Food Dig. 2012, 3, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Hauser, B.; Blecker, U.; Suys, B.; Peeters, S.; Keymolen, K.; Loeb, H. The nutritional value of a whey hydrolysate formula compared with a whey-predominant formula in healthy infants. J. Pediatr. Gastroenterol. Nutr. 1993, 17, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Maggio, L.; Zuppa, A.A.; Sawatzki, G.; Valsasina, R.; Schubert, W.; Tortorolo, G. Higher urinary excretion of essential amino acids in preterm infants fed protein hydrolysates. Acta Paediatr. 2007, 94, 75–84. [Google Scholar] [CrossRef]
- Giovannini, M.; Agostoni, C.; Fiocchi, A.; Bellú, R.; Trojan, S.; Riva, E. Antigen-reduced infant formulas versus human milk: Growth and metabolic parameters in the first 6 months of life. J. Am. Coll. Nutr. 1994, 13, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Hernell, O.; Lönnerdal, B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: Plasma amino acids, hematology, and trace elements. Am. J. Clin. Nutr. 2003, 78, 296–301. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.; Burd, N.A.; Coffey, V.G.; Baker, S.K.; Burke, L.M.; Hawley, J.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am. J. Clin. Nutr. 2011, 94, 795–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutherfurd, S.M.; Moughan, P.J.; Lowry, D.; Prosser, C.G. Amino acid composition determined using multiple hydrolysis times for three goat milk formulations. Int. J. Food Sci. Nutr. 2008, 59, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Prosser, C.G.; McLaren, R.D.; Frost, D.; Agnew, M.; Lowry, D.J. Composition of the non-protein nitrogen fraction of goat whole milk powder and goat milk-based infant and follow-on formulae. Int. J. Food Sci. Nutr. 2008, 59, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Aliaga, I.; Alferez, M.J.M.; Barrionuevo, M.; Nestares, T.; Sanz Sampelayo, M.R.; Campos, M.S. Study of Nutritive Utilization of Protein and Magnesium in Rats with Resection of the Distal Small Intestine. Beneficial Effect of Goat Milk. J. Dairy Sci. 2003, 86, 2958–2966. [Google Scholar] [CrossRef]
- Ceballos, L.S.; Morales, E.R.; Martínez, L.P.; Extremera, F.G.; Sanz Sampelayo, M.R. Utilization of nitrogen and energy from diets containing protein and fat derived from either goat milk or cow milk. J. Dairy Res. 2009, 76, 497. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, H.; Kruger, M.; Prosser, C.; Lowry, D. The effect of formulated goats’ milk on calcium bioavailability in male growing rats. J. Sci. Food Agric. 2010, 90, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Darragh, A.J.; Hendriks, W.H.; Prosser, C.G.; Lowry, D. Mineral Retention in Three-Week-Old Piglets Fed Goat and Cow Milk Infant Formulas. J. Dairy Sci. 2006, 89, 4520–4526. [Google Scholar] [CrossRef]
- Nestares, T.; Barrionuevo, M.; Díaz-Castro, J.; López-Aliaga, I.; Alférez, M.J.M.; Campos, M.S. Calcium-enriched goats’ milk aids recovery of iron status better than calcium-enriched cows’ milk, in rats with nutritional ferropenic anaemia. J. Dairy Res. 2008, 75, 153–159. [Google Scholar] [CrossRef] [PubMed]
- López Aliaga, I.; Alférez, M.J.M.; Barrionuevo, M.; Lisbona, F.; Campos, M.S. Influence of goat and cow milk on the digestive and metabolic utilization of calcium and iron. J. Physiol. Biochem. 2000, 56, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Alférez, M.J.M.; Barrionuevo, M.; López Aliaga, I.; Sanz-Sampelayo, M.R.; Lisbona, F.; Robles, J.C.; Campos, M.S. Digestive utilization of goat and cow milk fat in malabsorption syndrome. J. Dairy Res. 2001, 68, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.; Rotherham, B.; Sharpe, S.; Scragg, R.; Thompson, J.; Andrews, J.; Wall, C.; Murphy, J.; Lowry, D. Randomized, double-blind comparison of growth in infants receiving goat milk formula versus cow milk infant formula. J. Paediatr. Child Health 2005, 41, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.J.; Sullivan, T.; Gibson, R.A.; Lönnerdal, B.; Prosser, C.G.; Lowry, D.J.; Makrides, M. Nutritional adequacy of goat milk infant formulas for term infants: A double-blind randomised controlled trial. Br. J. Nutr. 2014, 111, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Y.; Dai, Z.; Zhang, Y.; Li, Y.; Wang, J. Comparison of growth and nutritional status in infants receiving goat milk-based formula and cow milk-based formula: A randomized, double-blind study. Food Nutr. Res. 2015, 59, 28613. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W.; Lawley, B.; Munro, K.; Gowri Pathmanathan, S.; Zhou, S.J.; Makrides, M.; Gibson, R.A.; Sullivan, T.; Prosser, C.G.; Lowry, D.; et al. Comparison of the Compositions of the Stool Microbiotas of Infants Fed Goat Milk Formula, Cow Milk-Based Formula, or Breast Milk. Appl. Environ. Microbiol. 2013, 79, 3040–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, M.; Holst, J.J.; Björck, I.M. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am. J. Clin. Nutr. 2007, 85, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.G.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fereday, A.; Gibson, N.R.; Cox, M.; Pacy, P.J.; Millward, D.J. Variation in the apparent sensitivity of the insulin-mediated inhibition of proteolysis to amino acid supply determines the efficiency of protein utilization. Clin. Sci. 1998, 95, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Nutrient | Unit | Whole Goat | Whole Cow | Partially Hydrolysed Cow † |
|---|---|---|---|---|
| Energy | kJ | 255 | 230 | 360 |
| Protein | g | 2.2 | 2.2 | 2.2 |
| Fat | g | 3.1 | 2.1 | 3.6 |
| Total carbohydrates | g | 6.1 | 7.0 | 11.0 |
| Lactose | g | 3.6 | 3.2 | 4.4 |
| Maltodextrin | g | 1.7 | 2.9 | 6.6 |
| Fructose | g | 0.6 | 0.7 | 0.0 |
| Fibre (inulin + oligofructose) | g | 0.2 | 0.3 | 0.0 |
| Sodium | mg | 23 | 19 | 35 |
| Vitamins | ||||
| Total vitamin A (RE) | mcg | 63 | 63 | 41 |
| Vitamin D3 | mcg | 1.0 | 1.0 | 1.0 |
| Vitamin E (TE) | mg | 1.6 | 1.6 | 1.4 |
| Vitamin C | mg | 9 | 9 | 9 |
| Thiamine | mcg | 62 | 69 | 116 |
| Riboflavin | mcg | 120 | 137 | 193 |
| Niacin | mg | 0.69 | 0.8 | 0.6 |
| Folic acid | mcg | 12 | 12 | 21 |
| Minerals | ||||
| Calcium | mg | 103 | 94 | 122 |
| Phosphorus | mg | 77 | 73 | 68 |
| Magnesium | mg | 10 | 6.7 | 32 |
| Iron | mg | 1.0 | 1.0 | 1.3 |
| Zinc | mg | 0.52 | 0.50 | 0.6 |
| Iodine | mcg | 10 | 9 | 14 |
| Other | ||||
| Inositol | mg | 7.4 | 8.3 | |
| Probiotic cultures | ||||
| Lactobacillus and Bifidobacterium | CFU (million) | 40 | 40 | 40 |
| Amino Acid † | Whole Goat | Whole Cow | Partially Hydrolysed Cow |
|---|---|---|---|
| mg | |||
| Glutamic Acid | 470 | 510 | 393 |
| Proline | 235 | 230 | 120 |
| Leucine | 214 | 232 | 249 |
| Lysine | 174 | 186 | 196 |
| Aspartic Acid | 161 | 181 | 247 |
| Valine | 153 | 151 | 126 |
| Serine | 123 | 111 | 94.1 |
| Threonine | 115 | 102 | 142 |
| Phenylalanine | 109 | 117 | 77.8 |
| lsoleucine | 103 | 123 | 125 |
| Tyrosine | 77.5 | 106 | 60.1 |
| Alanine | 69.6 | 88.8 | 110 |
| Arginine | 62.3 | 79.6 | 55.8 |
| Histidine | 61.9 | 66.3 | 43.4 |
| Methionine | 52.4 | 63.3 | 47.9 |
| Glycine | 38.9 | 45.6 | 43.2 |
| Tryptophan | 32.1 | 34.8 | 47.5 |
| Cysteine | 21.2 | 20.2 | 63.7 |
| Measure | Unit | Males (n = 15) | Females (n = 15) | ||
|---|---|---|---|---|---|
| Mean | SEM † | Mean | SEM ‡ | ||
| Age | years | 25.3 | 2.8 | 24.8 | 2.2 |
| Weight | kg | 74.8 | 10.1 | 58.3 | 7.7 *** |
| Height | cm | 175.0 | 4.0 | 164.0 | 4.4 *** |
| BMI | kg/m2 | 24.3 | 2.9 | 21.7 | 2.7 * |
| Glucose | mmol/L | 5.4 | 0.6 | 5.0 | 0.4 ** |
| Insulin | μU/mL | 7.3 | 0.8 | 7.9 | 0.4 |
| HOMA-IR | 1.8 | 1.3 | 1.7 | 0.6 | |
| Amino Acid † | Whole Goat | Whole Cow | Partially Hydrolysed Cow | p Value ‡ | Post Hoc |
|---|---|---|---|---|---|
| Alanine | 15392 ± 2104 | 19273 ± 2486 | 18960 ± 2475 | 0.261 | |
| Lysine | 8768 ± 807 | 7837 ± 852 | 5903 ± 786 | 0.036 | * |
| Proline | 15374 ± 848 | 16301 ± 1105 | 2613 ± 973 | <0.001 | ***^^^ |
| Leucine | 1771 ± 565 | 1344 ± 791 | −180 ± 547 | 0.03 | ** |
| Isoleucine | 46 ± 300 | 558 ± 554 | −304 ± 358 | 0.219 | |
| Valine | 6138 ± 725 | 1676 ± 905 | −6833 ± 795 | <0.001 | ***^^^### |
| Tyrosine | 489 ± 244 | 2237 ± 334 | −2395 ± 303 | <0.001 | ***^^^### |
| Methionine | 51 ± 115 | 255 ± 153 | −914 ± 126 | <0.001 | ***^^^ |
| Phenylalanine | −208 ± 198 | −77 ± 189 | −2388 ± 226 | <0.001 | ***^^^ |
| Asparagine | 1095 ± 197 | 1300 ± 207 | 384 ± 242 | 0.003 | *^ |
| Citrulline | 3204 ± 471 | 1377 ± 443 | 2609 ± 582 | 0.019 | ## |
| Glutamine | 9639 ± 1514 | 5988 ± 1758 | 1146 ± 1674 | <0.001 | ** |
| Ornithine | 1108 ± 332 | 791 ± 410 | −636 ± 305 | <0.001 | ***^ |
| Histidine | 1579 ± 398 | 1616 ± 453 | −1162 ± 322 | <0.001 | ***^^^ |
| Serine | 326 ± 371 | −38 ± 435 | −1646 ± 385 | 0.003 | ** |
| Taurine | −3644 ± 2665 | −1634 ± 1671 | −1025 ± 692 | 0.566 | |
| Aspartic Acid | −30 ± 92 | −59 ± 106 | −172 ± 38 | 0.211 | |
| Glutamic Acid | 518 ± 517 | 652 ± 922 | −1347 ± 617 | 0.027 | * |
| Glycine | −2423 ± 731 | −2665 ± 666 | −3877 ± 732 | 0.26 | |
| Hydroxy-proline | −208 ± 53 | −330 ± 85 | −292 ± 69 | 0.359 | |
| Arginine | 1250 ± 725 | 1167 ± 706 | −2431 ± 604 | <0.001 | **^^ |
| Threonine | −1108 ± 172 | −1299 ± 173 | −2160 ± 149 | <0.001 | ***^^^ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milan, A.M.; Hodgkinson, A.J.; Mitchell, S.M.; Prodhan, U.K.; Prosser, C.G.; Carpenter, E.A.; Fraser, K.; Cameron-Smith, D. Digestive Responses to Fortified Cow or Goat Dairy Drinks: A Randomised Controlled Trial. Nutrients 2018, 10, 1492. https://doi.org/10.3390/nu10101492
Milan AM, Hodgkinson AJ, Mitchell SM, Prodhan UK, Prosser CG, Carpenter EA, Fraser K, Cameron-Smith D. Digestive Responses to Fortified Cow or Goat Dairy Drinks: A Randomised Controlled Trial. Nutrients. 2018; 10(10):1492. https://doi.org/10.3390/nu10101492
Chicago/Turabian StyleMilan, Amber M., Alison J. Hodgkinson, Sarah M. Mitchell, Utpal K. Prodhan, Colin G. Prosser, Elizabeth A. Carpenter, Karl Fraser, and David Cameron-Smith. 2018. "Digestive Responses to Fortified Cow or Goat Dairy Drinks: A Randomised Controlled Trial" Nutrients 10, no. 10: 1492. https://doi.org/10.3390/nu10101492






