Bovine Milk Oligosaccharides with Sialyllactose for Preterm Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Procedures and Housing
2.2. Diets
2.3. Body Composition and Tissue Collection
2.4. Gut Structure
2.5. Gut Function
2.6. Microbiome and Microbial Metabolites
2.7. Hematology, Biochemistry and Systemic Immunity
2.8. Statistics
3. Results
3.1. Experiment 1
3.1.1. Clinical Condition, Body Growth and Composition
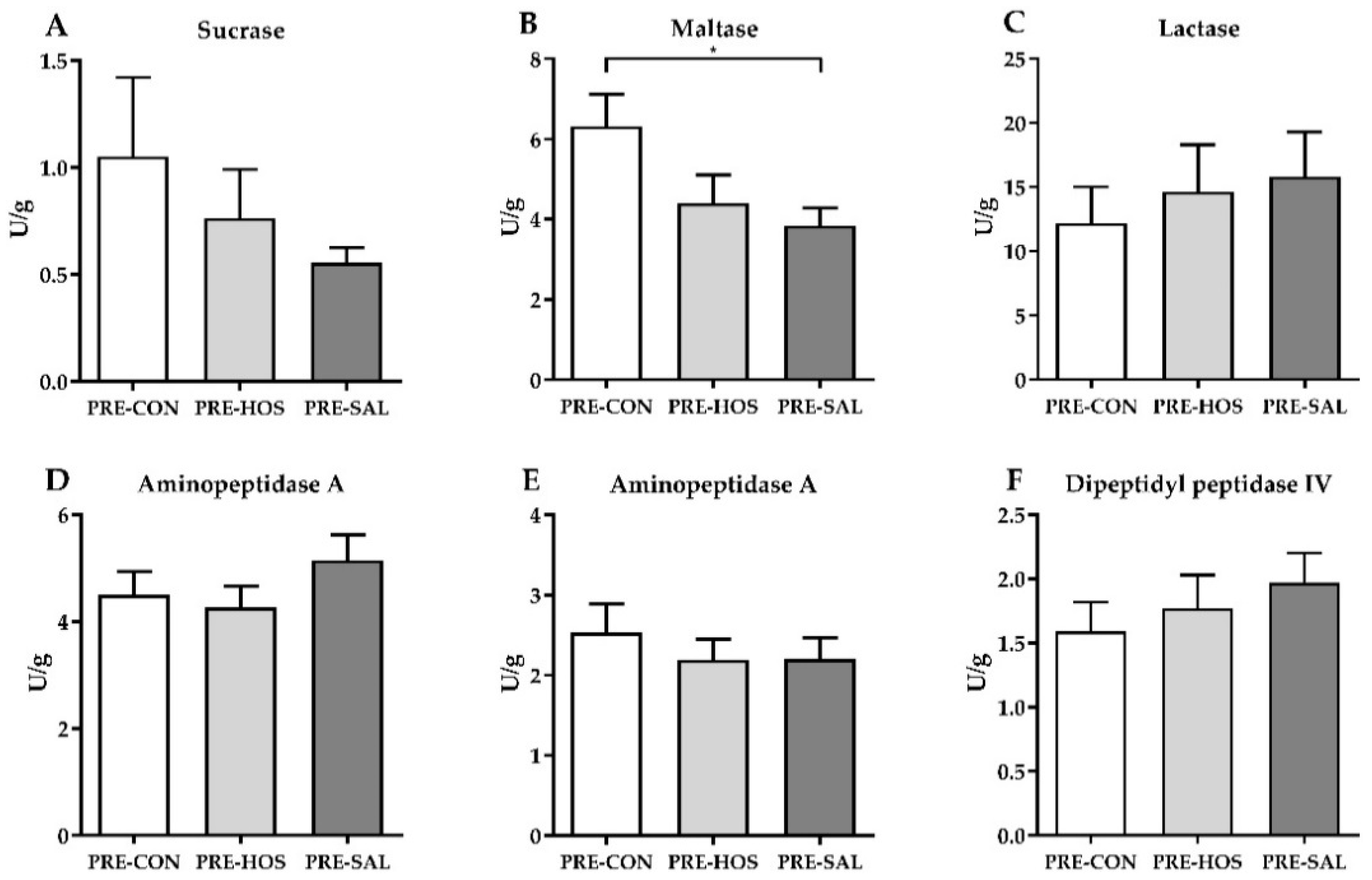
3.1.2. Gut Structure and Function
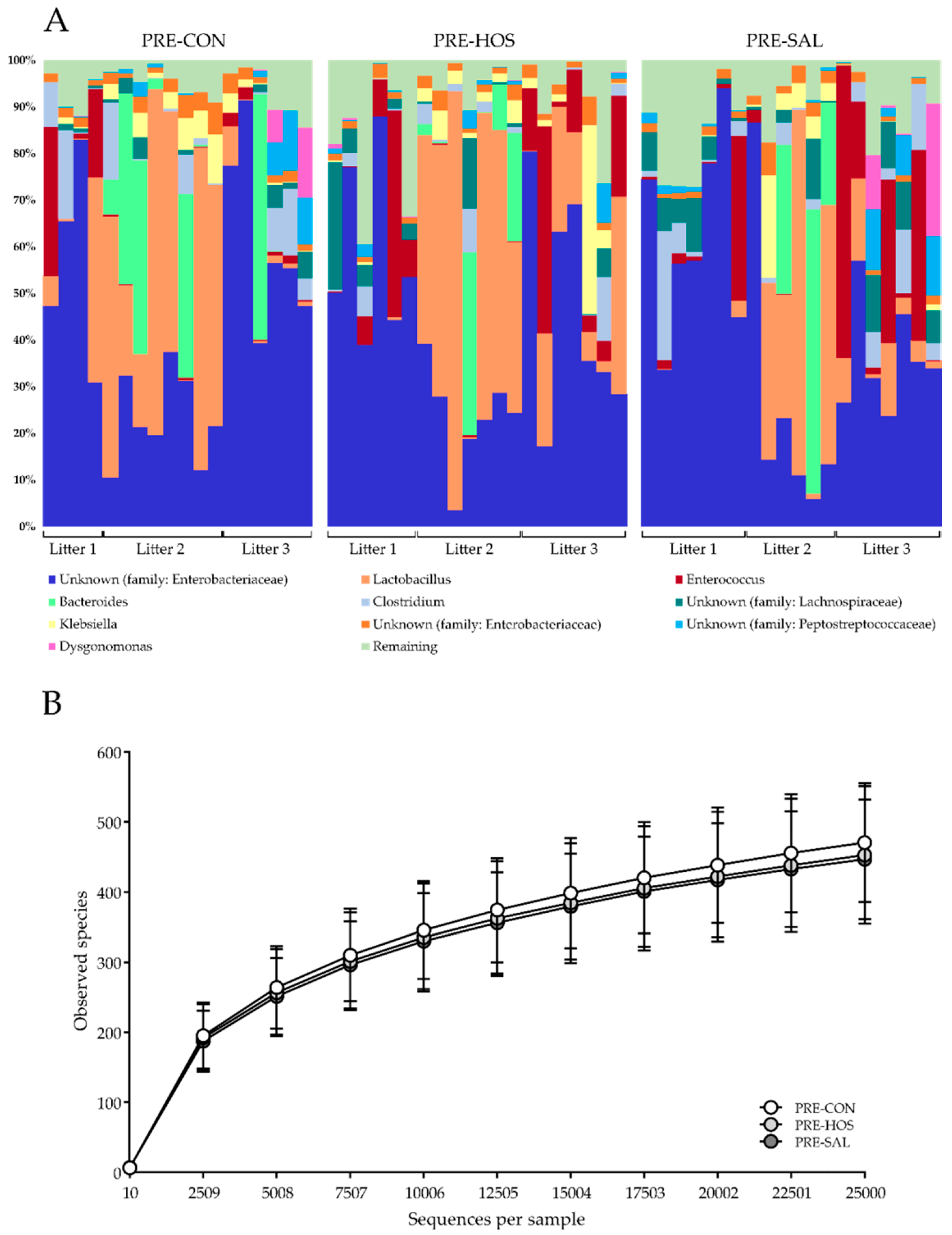
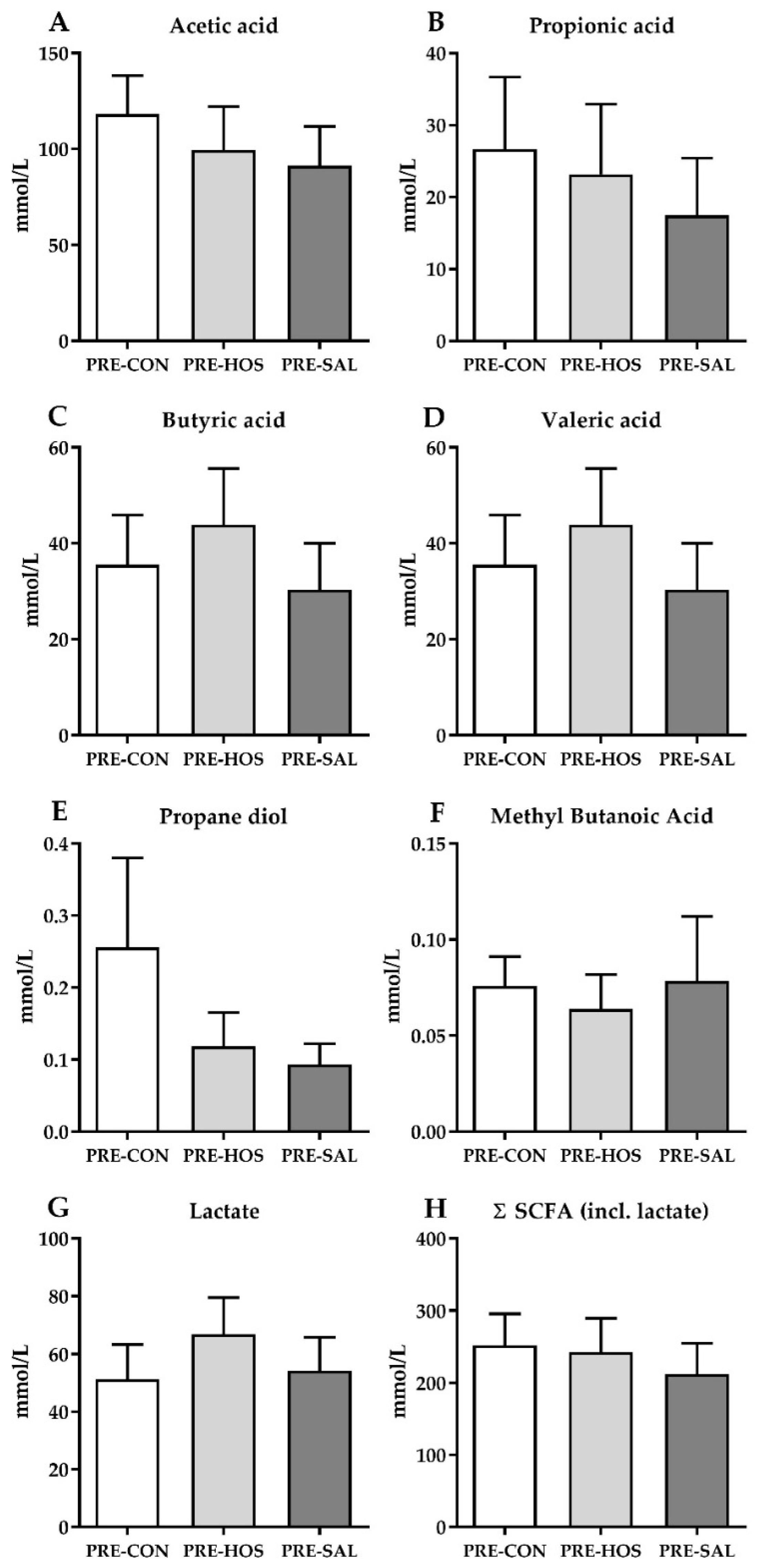
3.1.3. Gut Microbiota and Microbial Metabolites
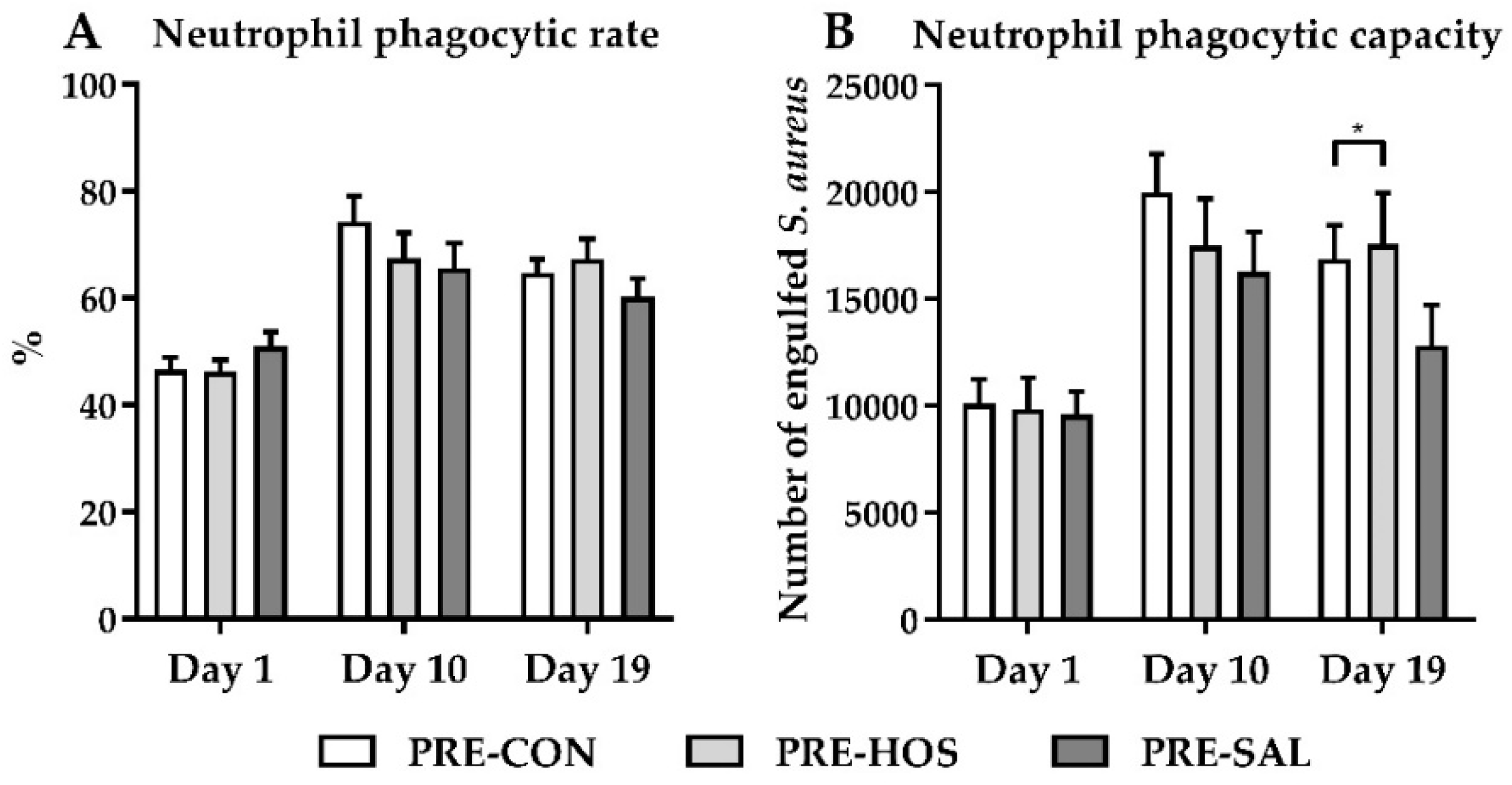
3.1.4. Hematology, Biochemistry and Systemic Immunity
3.2. Experiment 2
3.2.1. Clinical Condition, Body Growth and Composition
3.2.2. Gut Structure and Function
3.2.3. Gut Microbiota and Microbial Metabolites
3.2.4. Hematology, Biochemistry and Systemic Immunity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, R.K.; Singhal, A.; Vaidya, U.; Banerjee, S.; Anwar, F.; Rao, S. Optimizing Nutrition in Preterm Low Birth Weight Infants—Consensus Summary. Front. Nutr. 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, M.J.; Poroyko, V.; Caplan, M.; Alverdy, J.; Liu, D.C. Redefining the Role of Intestinal Microbes in the Pathogenesis of Necrotizing Enterocolitis. Pediatrics 2010, 125, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Van Saene, H.K.F.; Taylor, N.; Donnell, S.C.; Glynn, J.; Magnall, V.L.; Okada, Y.; Klein, N.J.; Pierro, A.; Lloyd, D.A. Gut overgrowth with abnormal flora: The missing link in parenteral nutrition-related sepsis in surgical neonates. Eur. J. Clin. Nutr. 2003, 57, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, Z.; Bolock, A.M.; Good, M. The Role of Mucosal Immunity in the Pathogenesis of Necrotizing Enterocolitis. Front. Pediatr. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Westerbeek, E.; van den Berg, A.; Lafeber, H.; Knol, J.; Fetter, W.; van Elburg, R. The intestinal bacterial colonisation in preterm infants: A review of the literature. Clin. Nutr. 2006, 25, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Suau, A.; Pochart, P.; Desjeux, J.-F. Fecal microbial community in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Butel, M.-J.; Suau, A.; Campeotto, F.; Magne, F.; Aires, J.; Ferraris, L.; Kalach, N.; Leroux, B.; Dupont, C. Conditions of bifidobacterial colonization in preterm infants: A prospective analysis. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.M.; Pane, C.A. Human Breast Milk: Current Concepts of Immunology and Infectious Diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2007, 37, 7–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Durkin, M.; Hinton, V.J.; Bellinger, D.; Kuhn, L. Influence of Breastfeeding on Cognitive Outcomes at Age 6-8 Years: Follow-up of Very Low Birth Weight Infants. Am. J. Epidemiol. 2003, 158, 1075–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, R.; Puppala, B.; Angst, D.; Coyle, B.W. Outcomes of early nutrition support in extremely low-birth-weight infants. Nutr. Clin. Pract. 2006, 21, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, P.; Warren, C.D.; Buescher, C.R.; Pickering, L.K.; Newburg, D.S. Survival of human milk oligosaccharides in the intestine of infants. Adv. Exp. Med. Biol. 2001, 501, 315–323. [Google Scholar] [PubMed]
- Moukarzel, S.; Bode, L. Human Milk Oligosaccharides and the Preterm Infant: A Journey in Sickness and in Health. Clin. Perinatol. 2017, 44, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Prebiotics and beyond. Nutr. Rev. 2009, 67, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Niñonuevo, M.R.; Lebrilla, C.B. Mass spectrometric methods for analysis of oligosaccharides in human milk. Nutr. Rev. 2009, 67, S216–S226. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.; Fleming, S.; Labhart, B.; Chichlowski, M.; Berg, B.; Donovan, S.; Dilger, R. Dietary Sialyllactose Influences Sialic Acid Concentrations in the Prefrontal Cortex and Magnetic Resonance Imaging Measures in Corpus Callosum of Young Pigs. Nutrients 2017, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.; Embleton, N.D.; Jacobs, S.E.; O’Connell, L.A.F.; Kuschel, C.A. Enteral feeding practices in very preterm infants: An international survey. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F56–F61. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sosa, S.; Martín, M.-J.; García-Pardo, L.-A.; Hueso, P. Sialyloligosaccharides in Human and Bovine Milk and in Infant Formulas: Variations with the Progression of Lactation. J. Dairy Sci. 2003, 86, 52–59. [Google Scholar] [CrossRef]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2018, 6, CD002971. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Sialic Acid Is an Essential Nutrient for Brain Development and Cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, M.; Tangvoranuntakul, P.; Takematsu, H.; Long, J.M.; Housley, G.D.; Kozutsumi, Y.; Suzuki, A.; Wynshaw-Boris, A.; Ryan, A.F.; Gallo, R.L.; et al. N-glycolylneuraminic acid deficiency in mice: Implications for human biology and evolution. Mol. Cell. Biol. 2007, 27, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Puente, R.; Hueso, P. Lactational changes in the N-glycoloylneuraminic acid content of bovine milk gangliosides. Biol. Chem. Hoppe Seyler 1993, 374, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Tangvoranuntakul, P.; Gagneux, P.; Diaz, S.; Bardor, M.; Varki, N.; Varki, A.; Muchmore, E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. USA 2003, 100, 12045–12050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Difilippo, E.; Pan, F.; Logtenberg, M.; Willems, R.; Braber, S.; Fink-Gremmels, J.; Schols, H.A.; Gruppen, H. Milk oligosaccharide variation in sow milk and their fermentation in piglet intestine. J. Agric. Food Chem. 2016, 64, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Salcedo, J.; Alexander, L.S.; Johnson, S.K.; Getty, C.M.; Chichlowski, M.; Berg, B.M.; Barile, D.; Dilger, R.N. Porcine Milk Oligosaccharides and Sialic Acid Concentrations Vary Throughout Lactation. Front. Nutr. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Ochonicky, K.L.; German, J.B.; Donovan, S.M.; Lebrilla, C.B. Structural determination and daily variations of porcine milk oligosaccharides. J. Agric. Food Chem. 2010, 58, 4653–4659. [Google Scholar] [CrossRef] [PubMed]
- Skórka, A.; Pieścik-Lech, M.; Kołodziej, M.; Szajewska, H. Infant formulae supplemented with prebiotics: Are they better than unsupplemented formulae? An updated systematic review. Br. J. Nutr. 2018, 119, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.O.; Martin, L.; Østergaard, M.V.; Rudloff, S.; Roggenbuck, M.; Nguyen, D.N.; Sangild, P.T.; Bering, S.B. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J. Nutr. Biochem. 2016, 40, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Puiman, P.J.; Stoll, B. Animal models to study neonatal nutrition in humans. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.J.; Birtles, M.J.; Cranwell, P.D.; Smith, W.C.; Pedraza, M. The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. World Rev. Nutr. Diet. 1992, 67, 40–113. [Google Scholar] [PubMed]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.D.; Sangild, P.T.; Munch, S.L.; van der Beek, E.M.; Renes, I.B.; Van Ginneken, C.; Greisen, G.O.; Thymann, T. Delayed growth, motor function and learning in preterm pigs during early postnatal life. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R481–R492. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jensen, M.L.; Chatterton, D.E.W.; Jensen, B.B.; Thymann, T.; Kvistgaard, A.S.; Sangild, P.T. Raw bovine milk improves gut responses to feeding relative to infant formula in preterm piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G81–G90. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.V.; Shen, R.L.; Støy, A.C.F.; Skovgaard, K.; Krych, Ł.; Leth, S.S.; Nielsen, D.S.; Hartmann, B.; Bering, S.B.; Schmidt, M.; et al. Provision of Amniotic Fluid During Parenteral Nutrition Increases Weight Gain With Limited Effects on Gut Structure, Function, Immunity, and Microbiology in Newborn Preterm Pigs. J. Parenter. Enter. Nutr. 2016, 40, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.V.; Bering, S.B.; Jensen, M.L.; Thymann, T.; Purup, S.; Diness, M.; Schmidt, M.; Sangild, P.T. Modulation of Intestinal Inflammation by Minimal Enteral Nutrition With Amniotic Fluid in Preterm Pigs. JPEN J. Parenter. Enter. Nutr. 2014, 38, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Krych, Ł.; Kot, W.; Bendtsen, K.M.B.; Hansen, A.K.; Vogensen, F.K.; Nielsen, D.S. Have you tried spermine? A rapid and cost-effective method to eliminate dextran sodium sulfate inhibition of PCR and RT-PCR. J. Microbiol. Methods 2018, 144, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kaminarides, S.; Stamou, P.; Massouras, T. Comparison of the characteristics of set type yoghurt made from ovine milk of different fat content. Int. J. Food Sci. Technol. 2007, 42, 1019–1028. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Jiang, P.; Frøkiær, H.; Heegaard, P.M.H.; Thymann, T.; Sangild, P.T. Delayed development of systemic immunity in preterm pigs as a model for preterm infants. Sci. Rep. 2016, 6, 36816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.S.H.; Baldwin, N.; Wagner, V.O.; Roy, S.; Rose, J.; Thorsrud, B.A.; Phothirath, P.; Röhrig, C.H. Safety evaluation of the human-identical milk monosaccharide sialic acid (N-acetyl-d-neuraminic acid) in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2014, 70, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, F.; Ikeuchi, Y.; Urashima, T.; Fujihara, M.; Ohtsuki, K.; Yanahira, S. Effects of Feeding Sialyllactose and Galactosylated N-Acetylneuraminic Acid on Swimming Learning Ability and Brain Lipid Composition in Adult Rats. J. Appl. Glycosci. 2006, 53, 249–254. [Google Scholar] [CrossRef]
- Fleming, S.; Chichlowski, M.; Berg, B.; Donovan, S.; Dilger, R. Dietary Sialyllactose Does Not Influence Measures of Recognition Memory or Diurnal Activity in the Young Pig. Nutrients 2018, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Sugawara, M.; Kawakami, H. Sialic acid in human milk: Composition and functions. Acta Paediatr. Taiwan 2001, 42, 11–17. [Google Scholar] [PubMed]
- Coppa, G.V.; Pierani, P.; Zampini, L.; Carloni, I.; Carlucci, A.; Gabrielli, O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. Suppl. 1999, 88, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.F.; Thymann, T.; Andersen, A.D.; Holst, J.J.; Hartmann, B.; Hilsted, L.; Langhorn, L.; Jelsing, J.; Sangild, P.T. Rapid gut growth but persistent delay in digestive function in the postnatal period of preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G550–G560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Q.; Tuohy, K.M.; Gibson, G.R.; Ward, R.E. In vitro measurement of the impact of human milk oligosaccharides on the faecal microbiota of weaned formula-fed infants compared to a mixture of prebiotic fructooligosaccharides and galactooligosaccharides. Lett. Appl. Microbiol. 2011, 52, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Kobayashi, T.; Songjinda, P.; Tateyama, A.; Tsubouchi, M.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K.; Nakayama, J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 2009, 56, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Szylit, O.; Maurage, C.; Gasqui, P.; Popot, F.; Favre, A.; Gold, F.; Borderon, J.C. Fecal Short-Chain Fatty Acids Predict Digestive Disorders in Premature Infants. J. Parenter. Enteral Nutr. 1998, 22, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernández-Barranco, A.; Margolles, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favre, A.; Szylit, O.; Popot, F.; Catala, I.; Rondeau, C.; Maurage, C.; Gold, F.; Borderon, J.C.; Butel, M.J. Diet, length of gestation, and fecal short chain fatty acids in healthy premature neonates. JPEN J. Parenter. Enteral Nutr. 2002, 26, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wang, F.; Chen, Y.; Yang, C.; Zhang, P.; Zhang, X.; Troy, F.A.; Wang, B. Developmental changes in the level of free and conjugated sialic acids, Neu5Ac, Neu5Gc and KDN in different organs of pig: A LC-MS/MS quantitative analyses. Glycoconj. J. 2017, 34, 21–30. [Google Scholar] [CrossRef] [PubMed]


| Serum | PRE-CON | PRE-HOS | PRE-SAL |
| Albumin (g/L) | 15.62 ± 2.14 | 15.62 ± 3.01 | 16.24 ± 3.32 |
| Total protein (g/L) | 26.93 ± 2.95 | 27.13 ± 4.82 | 26.96 ± 4.52 |
| Alkaline phosphatase (U/L) | 728.9 ± 298.8 | 974.7 ± 454.7 ∆ | 763.6 ± 284.7 |
| Alanine aminotransferase (U/L) | 28.85 ± 7.42 | 25.72 ± 5.80 | 25.78 ± 5.43 |
| Total bilirubin (µmol/L) | 2.15 ± 1.93 | 1.74 ± 0.99 | 1.98 ± 1.16 |
| Cholesterol (mmol/L) | 2.35 ± 0.46 | 2.42 ± 0.42 | 2.30 ± 0.56 |
| Creatinine (µmol/L) | 63.35 ± 34.37 | 59.56 ± 14.30 | 59.67 ± 30.64 |
| Creatine kinase (U/L) | 138.4 ± 85.0 | 123.9 ± 39.3 | 140.8 ± 76.5 |
| Iron (µmol/L) | 11.52 ± 3.96 | 12.74 ± 6.05 | 11.32 ± 5.65 |
| Phosphate (mmol/L) | 2.36 ± 0.43 | 2.22 ± 0.40 | 2.33 ± 0.38 |
| Aspartate aminotransferase (U/L) | 35.05 ± 19.09 | 26.67 ± 6.15 ∆ | 29.78 ± 5.43 |
| Blood urea nitrogen (mmol/L) | 8.74 ± 5.37 | 6.86 ± 4.30 | 6.23 ± 4.91 |
| Gamma-glutamyl transferase (U/L) | 26.05 ± 6.13 | 23.78 ± 11.24 | 21.94 ± 8.43 |
| Calcium (mmol/L) | 2.37 ± 0.25 | 2.44 ± 0.23 | 2.37 ± 0.24 |
| Magnesium (mmol/L) | 0.81 ± 0.13 | 0.86 ± 0.16 | 0.86 ± 0.15 |
| Sodium (mmol/L) | 140.4 ± 6.8 | 140.8 ± 8.0 | 139.0 ± 10.1 |
| Potassium (mmol/L) | 3.71 ± 0.67 | 3.82 ± 0.41 | 3.84 ± 0.47 |
| Lactate (mmol/L) | 1.56 ± 0.96 | 2.26 ± 2.38 | 2.27 ± 1.60 |
| Glucose (mmol/L) | 4.18 ± 1.58 | 5.32 ± 2.48 ∆ | 5.17 ± 2.77 |
| CSF | |||
| Albumin (mg/L) | 12.89 ± 3.71 | 19.60 ± 26.11 | 18.57 ± 13.32 |
| Total protein (mg/L) | 177.3 ± 51.2 | 294.0 ± 455.1 | 260.0 ± 217.9 |
| Lactate (mmol/L) | 1.71 ± 0.78 | 1.86 ± 0.56 | 1.82 ± 0.66 |
| Glucose (mmol/L) | 2.28 ± 0.97 | 2.78 ± 0.70 ∆ | 2.74 ± 1.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obelitz-Ryom, K.; Rendboe, A.K.; Nguyen, D.N.; Rudloff, S.; Brandt, A.B.; Nielsen, D.S.; Heckmann, A.B.; Chichlowski, M.; Sangild, P.T.; Thymann, T.; et al. Bovine Milk Oligosaccharides with Sialyllactose for Preterm Piglets. Nutrients 2018, 10, 1489. https://doi.org/10.3390/nu10101489
Obelitz-Ryom K, Rendboe AK, Nguyen DN, Rudloff S, Brandt AB, Nielsen DS, Heckmann AB, Chichlowski M, Sangild PT, Thymann T, et al. Bovine Milk Oligosaccharides with Sialyllactose for Preterm Piglets. Nutrients. 2018; 10(10):1489. https://doi.org/10.3390/nu10101489
Chicago/Turabian StyleObelitz-Ryom, Karina, Amalie Katrine Rendboe, Duc Ninh Nguyen, Silvia Rudloff, Anne Bladt Brandt, Dennis Sandris Nielsen, Anne Birgitte Heckmann, Maciej Chichlowski, Per Torp Sangild, Thomas Thymann, and et al. 2018. "Bovine Milk Oligosaccharides with Sialyllactose for Preterm Piglets" Nutrients 10, no. 10: 1489. https://doi.org/10.3390/nu10101489





