Family History of Premature Coronary Artery Disease (P-CAD)—A Non-Modifiable Risk Factor? Dietary Patterns of Young Healthy Offspring of P-CAD Patients: A Case-Control Study (MAGNETIC Project)
Abstract
:1. Introduction
2. Materials and Methods
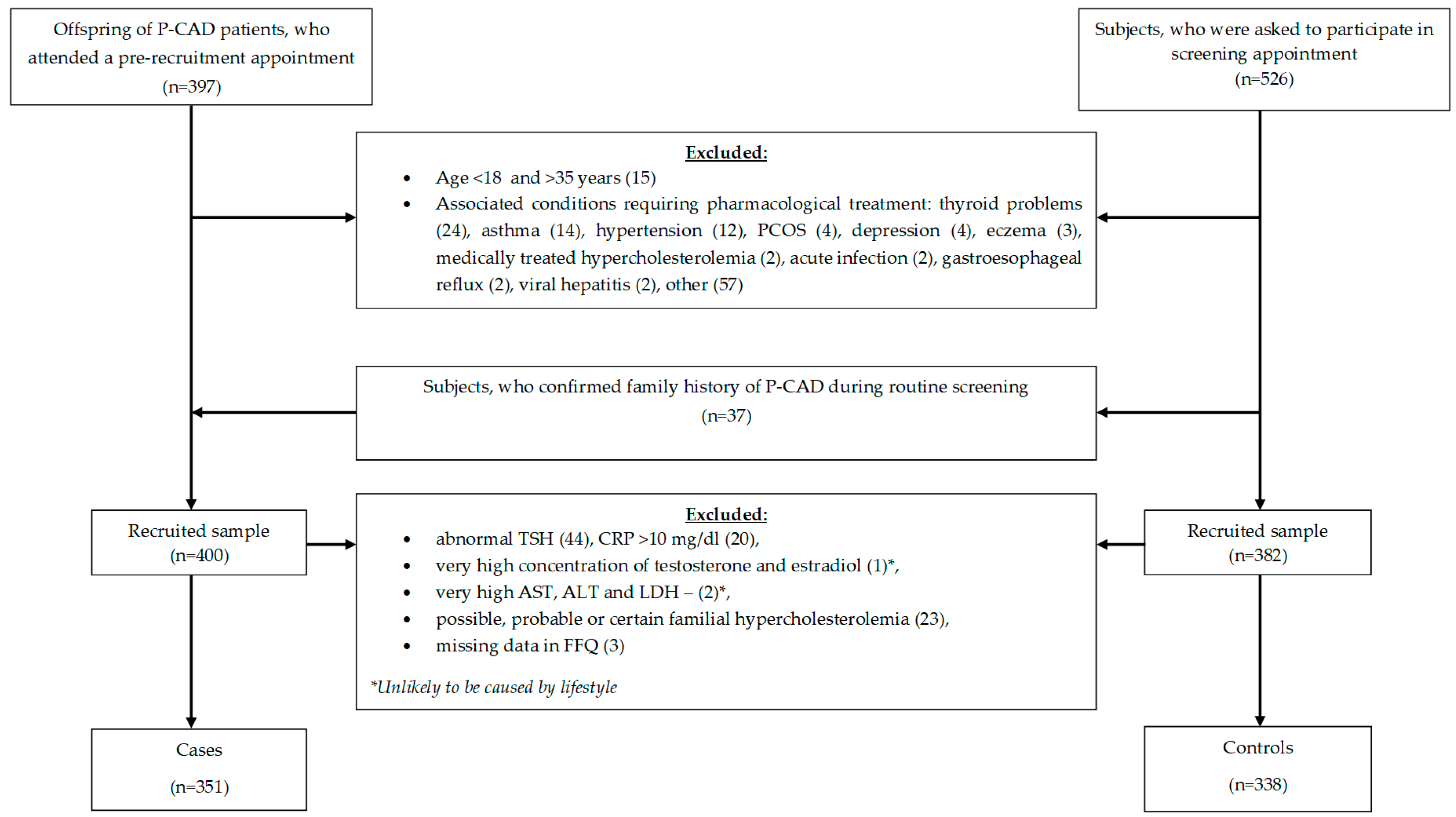
2.1. Study Design and Sample
2.2. Ethical Approval
2.3. Dietary Data Collection
2.4. Confounding Factors
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
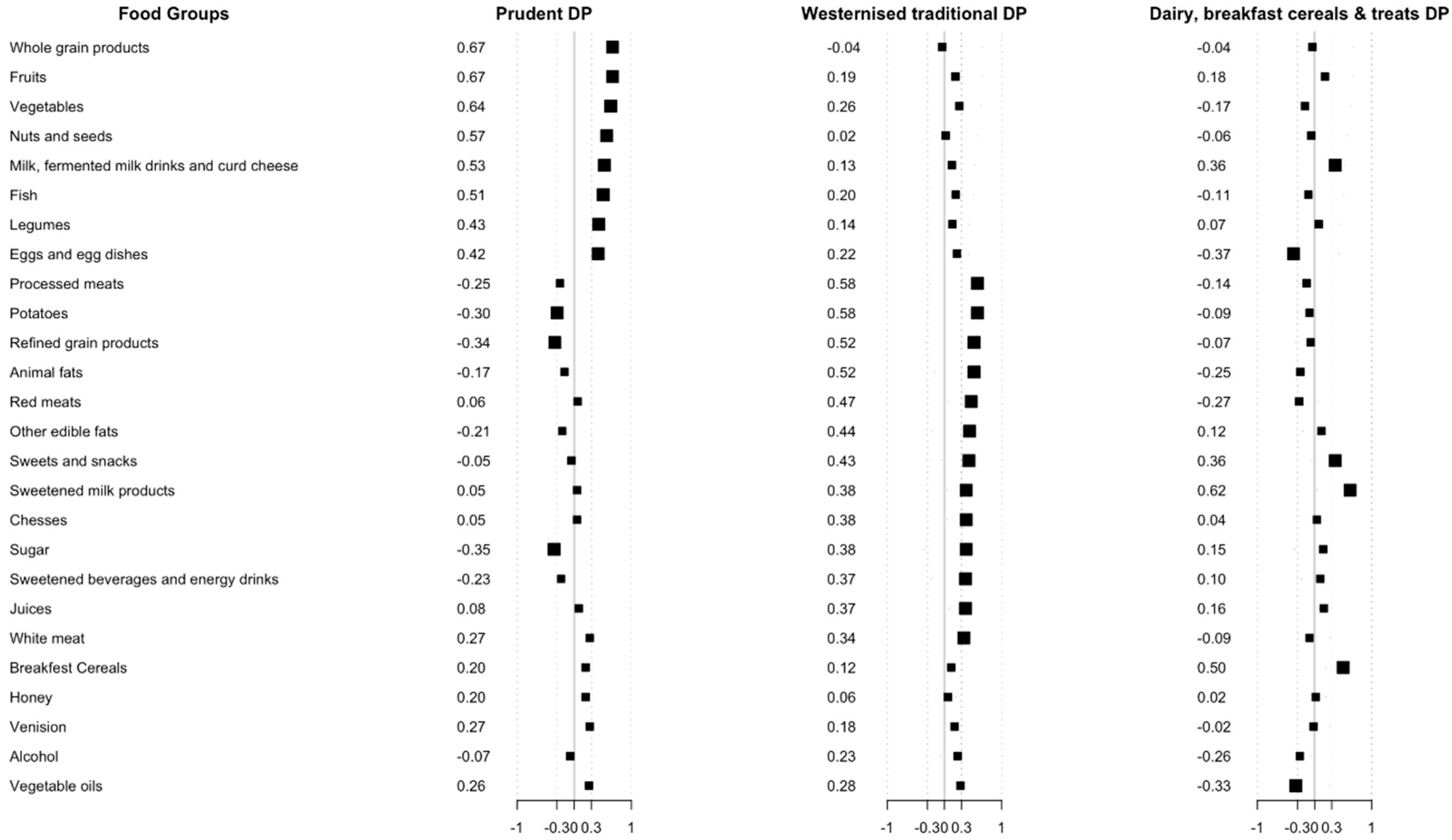
3.2. Dietary Patterns
3.3. Dietary Patterns and a Family History of P-CAD
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lloyd-Jones, D.M.; Nam, B.H.; D’Agostino, R.B.; Levy, D.; Murabito, J.M.; Wang, T.J.; Wilson, P.W.F.; O’Donnell, C.J. Parental Cardiovascular Disease as a Risk Factor for Cardiovascular Disease in Middle-aged Adults. JAMA 2004, 291, 2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivapalaratnam, S.; Boekholdt, S.M.; Trip, M.D.; Sandhu, M.S.; Luben, R.; Kastelein, J.J.; Wareham, N.J.; Khaw, K.T. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart 2010, 96, 1985–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulders, T.A.; Sivapalaratnam, S.; Stroes, E.S.G.; Kastelein, J.J.; Guerci, A.D.; Pinto-Sietsma, S.J. Asymptomatic Individuals With a Positive Family History for Premature Coronary Artery Disease and Elevated Coronary Calcium Scores Benefit From Statin Treatment: A Post Hoc Analysis From the St. Francis Heart Study. JACC Cardiovasc. Imaging 2012, 5, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, C.; Wietlisbach, V.; Jotterand, V.; Volet, M.; Lenain, V.; Nicod, P.; Darioli, R.; Paccaud, F.; Waeber, G.; Mooser, V. High prevalence of major cardiovascular risk factors in first-degree relatives of individuals with familial premature coronary artery disease—The GENECARD project. Atherosclerosis 2007, 194, 253–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Superko, H.R.; Roberts, R.; Agatston, A.; Frohwein, S.; Reingold, J.S.; White, T.J.; Sninsky, J.J.; Margolis, B.; Momary, K.M.; Garrett, B.C.; et al. Genetic Testing for Early Detection of Individuals at Risk of Coronary Heart Disease and Monitoring Response to Therapy: Challenges and Promises. Curr. Atheroscler. Rep. 2011, 13, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeemon, P.; Harikrishnan, S.; Sanjay, G.; Sivasubramonian, S.; Lekha, T.R.; Padmanabhan, S.; Tandon, N.; Prabhakaran, D. A PROgramme of Lifestyle Intervention in Families for Cardiovascular risk reduction (PROLIFIC Study): Design and rationale of a family based randomized controlled trial in individuals with family history of premature coronary heart disease. BMC Public Health 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Engelfriet, P.; Hoekstra, J.; Hoogenveen, R.; Büchner, F.; van Rossum, C.; Verschuren, M. Food and vessels: The importance of a healthy diet to prevent cardiovascular disease. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Pan, A.; Hou, T.; Rexrode, K.M.; Willett, W.C.; Hu, F.B. Food quality score and the risk of coronary artery disease: A prospective analysis in 3 cohorts. Am. J. Clin. Nutr. 2016, 104, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, A.A.; Shah, S. Risk factors, clinical features, angiographic characteristics and treatment outcomes of young myocardial infarction patients. JICC 2015, 5, 203–208. [Google Scholar] [CrossRef]
- Noble, N.; Paul, C.; Turon, H.; Oldmeadow, C. Which modifiable health risk behaviours are related? A systematic review of the clustering of Smoking, Nutrition, Alcohol and Physical activity (‘SNAP’) health risk factors. Prev. Med. 2015, 81, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Mansur Ade, P.; Mattar, A.P.; Rolim, A.L.; Yoshi, F.R.; Marin, J.F.; César, L.A.; Ramires, J.A. Distribution of risk factors in parents and siblings of patients with early coronary artery disease. Arq. Bras. Cardiol. 2003, 80, 582–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Department of Agriculture (USDA). A Series of Systematic Reviews on the Relationship between Dietary Patterns and Health Outcome. Available online: https://www.cnpp.usda.gov/ (accessed on 4 July 2018).
- Osadnik, T.; Osadnik, K.; Pawlas, N.; Strzelczyk, J.K.; Kasperczyk, J.; Gąsior, M. Metabolic and genetic profiling of young adults with and without family history of premature coronary heart disease (MAGNETIC). Study design and methodology. Arch. Med. Sci. 2018. [Google Scholar] [CrossRef]
- Wadolowska, L.; Niedzwiedzka, E.; Kowalkowska, J. Kwestionariusz Częstotliwości Spożycia Zywności FFQ-6 [Food Frequency Questionnaire FFQ-6]. Available online: http://www.uwm.edu.pl/edu/lidiawadolowska/ (accessed on 4 July 2018).
- Masson, L.F.; McNeill, G.; Tomany, J.O.; Simpson, J.A.; Peace, H.S.; Wei, L.; Grubb, D.A.; Bolton-Smith, C. Statistical approaches for assessing the relative validity of a food-frequency questionnaire: Use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003, 6, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Drabinska, N.; Jarocka-Cyrta, E.; Markiewicz, L.H.; Krupa-Kozak, U. The Effect of Oligofructose-Enriched Inulin on Faecal Bacterial Counts and Microbiota-Associated Characteristics in Celiac Disease Children Following a Gluten-Free Diet: Results of a Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Ryterska, K.; Maciejewska, D.; Banaszczak, M.; Milkiewicz, P.; Milkiewicz, M.; Gutowska, I.; Ossowski, P.; Kaczorowska, M.; Jamioł-Milc, D.; et al. Nutritional Strategies for the Individualized Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) Based on the Nutrient-Induced Insulin Output Ratio (NIOR). Int. J. Mol. Sci. 2016, 17, 1192. [Google Scholar] [CrossRef] [PubMed]
- Krusinska, B.; Hawrysz, I.; Wadolowska, L.; Slowinska, M.A.; Biernacki, M.; Czerwinska, A.; Golota, J.J. Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study. Nutrients 2018, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Jezewska-Zychowicz, M.; Gawecki, J.; Wadolowska, L.; Czarnocinska, J.; Galinski, G.; Kollajtis-Dolowy, A.; Roszkowski, W.; Wawrzyniak, A.; Przybylowicz, K.; Krusinska, B.; et al. Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing of Nutritional Data. The Committee of Human Nutrition, Polish Academy of Sciences. Available online: http://www.knozc.pan.pl/ (accessed on 4 July 2018).
- Kowalkowska, J.; Wadolowska, L.; Czarnocinska, J.; Czlapka-Matyasik, M.; Galinski, G.; Jezewska-Zychowicz, M.; Bronkowska, M.; Dlugosz, A.; Laboda, D.; Wyka, J. Analiza Zgodności Wewnętrznej Kwestionariusza do Badania Poglądów I Zwyczajów Żywieniowych (KomPAN) [Reproducibility of a Questionnaire for Dietary Habits, Lifestyle and Nutrition Knowledge Assessment (KomPAN)]. Available online: http://www.knozc.pan.pl/ (accessed on 4 July 2018).
- Stekhoven, D.J.; Buhlmann, P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 4 July 2018).
- Melaku, Y.A.; Gill, T.K.; Taylor, A.W.; Adams, R.; Shi, Z. A comparison of principal component analysis, partial least-squares and reduced-rank regressions in the identification of dietary patterns associated with bone mass in ageing Australians. Eur. J. Nutr. 2017, 57, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Duan, Y.; Grady, J. Unconditional or Conditional Logistic Regression Model for Age-Matched Case–Control Data? Front. Public Health 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Gasior, M.; Gierlotka, M.; Pyka, L.; Zdrojewski, T.; Wojtyniak, B.; Chlebus, K.; Rozentryt, P.; Niedziela, J.; Jankowski, P.; Nessler, J.; et al. Temporal Trends in Secondary Prevention in myocardial infarction patients discharged with left ventricular dysfunction in Poland. Eur. J. Prev. Cardiol. 2018, 25, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Gierlotka, M.; Gasior, M.; Tajstra, M.; Hawranek, M.; Osadnik, T.; Wilczek, K.; Kalarus, Z.; Lekston, A.; Zembala, M.; Poloński, L. Outcomes of invasive treatment in very elderly Polish patients with non-ST-segment-elevation myocardial infarction from 2003–2009 (from the PL-ACS registry). Cardiol. J. 2013, 20, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Dlugosz, A. Dietary Patterns, Adverse Health Outcomes, Socioeconomic Situation and Lifestyle of Adolescents from Less Urbanised Regions of Poland [Dissertations and Monographs]; Wydawnictwo UWM: Olsztyn, Poland, 2017; ISBN 978-83-8100-076-5. [Google Scholar]
- Waskiewicz, A.; Szczesniewska, D.; Szostak-Wegierek, D.; Kwaśniewska, M.; Pająk, A.; Stepaniak, U.; Kozakiewicz, K.; Tykarski, A.; Zdrojewski, T.; Zujko, M.E.; et al. Are dietary habits of the Polish population consistent with the recommendations for prevention of cardiovascular disease?—WOBASZ II project. Kardiol. Pol. 2016, 74, 969–977. [Google Scholar] [PubMed]
- Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Psaltopoulou, T.; Georgiopoulos, G.G.; Siasos, G.; Kokkou, E.; Antonopoulos, A.; Vogiatzi, G.; Tsalamandris, S.; Gennimata, V.; Papanikolaou, A.; et al. Western Dietary Pattern is Associated with Severe Coronary Artery Disease. Angiology 2018, 69, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, C.; Schulze, M.B.; Franco, O.H.; van Dam, R.M.; Mantzoros, C.S.; Hu, F.B. Dietary Patterns and Risk of Mortality from Cardiovascular Disease, Cancer, and All-Causes in a Prospective Cohort of Women: Heidemann-Dietary Patterns and Mortality. Circulation 2008, 118, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rimm, E.B.; Spiegelman, D.; Rifai, N.; Tofler, G.H.; Willet, W.C.; Hu, F.B. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 2001, 73, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, X.; Li, Y.; Liu, A.; Zhang, Q.; Hu, X.; Du, S.; Ma, J.; Xu, G.; Li, Y.; Guo, H.; et al. Dietary Pattern and Its Association with the Prevalence of Obesity and Related Cardiometabolic Risk Factors among Chinese Children. PLoS ONE 2012, 7, e43183. [Google Scholar] [CrossRef] [PubMed]
- Dickens, E.; Ogden, J. The role of parental control and modelling in predicting a child’s diet and relationship with food after they leave home. A prospective study. Appetite 2014, 76, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Mikkilä, V.; Räsänen, L.; Raitakari, O.T.; Pietinen, P.; Viikari, J. Consistent dietary patterns identified from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. Br. J. Nutr. 2005, 93, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Wadolowska, L.; Ulewicz, N.; Sobas, K.; Wuenstel, J.W.; Slowinska, M.A.; Niedzwiedzka, E.; Czlapka-Matyasik, M. Dairy-Related Dietary Patterns, Dietary Calcium, Body Weight and Composition: A Study of Obesity in Polish Mothers and Daughters, the MODAF Project. Nutrients 2018, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Zazpe, I.; Razquin, C.; Sánchez-Tainta, A.; Corella, D.; Salas-Salvadó, J.; Toledo, E.; Ros, E.; Muñoz, M.Á.; Recondo, J.; Gómez-Gracia, E.; et al. Empirically-derived food patterns and the risk of total mortality and cardiovascular events in the PREDIMED study. Clin. Nutr. 2015, 34, 859–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.; Rodríguez-Artalejo, F.; Gaio, G.; Santos, A.C.; Ramos, E.; Lopes, C. Major Habitual Dietary Patterns Are Associated with Acute Myocardial Infarction and Cardiovascular Risk Markers in a Southern European Population. J. Am. Diet. Assoc. 2011, 111, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X.; Ding, H. Is coronary artery disease a multifactorial inherited disorder with a sex-influenced trait? Med. Hypotheses 2008, 71, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Srinivasan, S.R.; Valdez, R.; Greenlund, K.J.; Wattigney, W.A.; Berenson, G.S. Longitudinal Changes in Cardiovascular Risk from Childhood to Young Adulthood in Offspring of Parents with Coronary Artery Disease the Bogalusa Heart Study. JAMA 1997, 278, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Masala, G.; Palli, D.; Panico, S.; Vineis, P.; Sacerdote, C.; Mattiello, A.; Galasso, R.; Salvini, S.; et al. Associations between dietary pattern and lifestyle, anthropometry and other health indicators in the elderly participants of the EPIC-Italy cohort. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Masala, G.; Ceroti, M.; Pala, V.; Krogh, V.; Vineis, P.; Sacerdote, C.; Saieva, C.; Salvini, S.; Sieri, S.; Berrino, F.; et al. A dietary pattern rich in olive oil and raw vegetables is associated with lower mortality in Italian elderly subjects. Br. J. Nutr. 2007, 98, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, H.M.; Yaxley, A.; Thomas, J.; Delaney, C.; Koczwara, B.; Luszcz, M.; Miller, M. Do dietary patterns in older age influence the development of cancer and cardiovascular disease: A longitudinal study of ageing. Clin. Nutr. 2016, 35, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.M.; Worcester, M.U.; Elliott, P.C.; Le Grande, M.R.; Higgins, R.O.; Goble, A.J. Change in women’s dietary fat intake following an acute cardiac event: Extent, predictors and comparison with noncardiac Australian women and older adults. Eur. J. Cardiovasc. Nurs. 2006, 5, 206–213. [Google Scholar] [CrossRef] [PubMed]
- White, H.R.; Johnson, V.; Buyske, S. Parental modeling and parenting behavior effects on offspring alcohol and cigarette use. A growth curve analysis. J. Subst. Abuse 2000, 12, 287–310. [Google Scholar] [CrossRef]
- McCann, S.E.; Marshall, J.R.; Brasure, J.R.; Graham, S.; Freudenheim, J.L. Analysis of patterns of food intake in nutritional epidemiology (Food classification in principal components analysis and the subsequent impact on estimates for endometrial cancer). Public Health Nutr. 2001, 4, 989–997. [Google Scholar] [CrossRef] [PubMed]
| Food Group | Questionnaire Item(s) | Remarks |
|---|---|---|
| Sugar | Sugar | Sugar added to beverages, such as tea, coffee, etc. |
| Honey | Honey | Honey added to dishes and added to beverages |
| Sweets and snacks | Bakers’ confectionery | Biscuits, cream cakes, sponge cakes, cheesecakes, doughnuts, poppy-seed cakes, croissants etc. |
| Ice creams and custard | Ice creams and custard | |
| Chocolates | Chocolate, chocolate sweets and chocolate bars | |
| Sugar confectionery | Boiled sweets, hard caramels, jellied sweets, fudge, etc. | |
| Savoury snacks | Crisps, crackers, pretzels | |
| Milk, fermented milk drinks, and curd cheese | Milk and milk beverages—natural | Milk and natural milk beverages (yoghurt, kefir, buttermilk), porridge, etc. |
| Cheese curds | Cheese curd, natural cottage cheese, soft cheese, mozzarella, cottage cheese with herbs, etc. | |
| Sweetened milk products | Milk beverages—sweetened | Fruit yoghurts, yoghurts with chocolate flakes, flavoured buttermilk, hot chocolate, etc. |
| Flavoured cheese curds | Flavored curds (with fruit, chocolate, vanilla), etc. | |
| Cheeses | Cheese | Hard cheese, blue cheese, processed cheese, cheese spreads, etc. |
| Eggs and egg dishes | Eggs and egg dishes | Scrambled eggs, omelette, egg salad, cooked eggs |
| Breakfast cereals | Breakfast cereals | Muesli, cornflakes, other cereals—sweetened or unsweetened, etc. |
| Wholegrain products | Wholemeal cereals | Wholemeal wheat or rye bread, seeded loafs, pumpernickel, wholemeal cracker bread, etc. |
| Coarse groats | Buckwheat groats, barley, brown rice, wholemeal pasta, etc. | |
| Refined grain products | Refined cereals | White bread, rye, wheat-rye bread, toast bread, white bread rolls, brioche, bagels, etc. |
| Fine groats | Semolina, milled barley, pasta, white rice, rice flakes, etc. | |
| Animal fats | Butter | Butter |
| Cream | Single, double, sour, used as an ingredient or added to beverages | |
| Other animal fats | Lard, pork fat, etc. | |
| Red meats | Red meat | Pork, beef, veal, etc. |
| Venison | Venison | Wild boar, venison, quail, mallard, hare, etc. |
| Processed meats | Sausages, bacon, reconstituted meat | Sausages, meat loaf, hot-dogs, smoked sausages, bacon, etc. |
| High quality cured meats | Ham, poultry and pork-beef good quality cold meats, etc. | |
| Offal products | Liver, blood sausage, sweetbread, liver pate, etc. | |
| Vegetables | All kind of vegetables (cruciferous, root, yellow-orange, leafy green, tomatoes, gourds, and squashes) | Cabbages, Brussel sprouts, cauliflower, broccoli, kale, carrots, peppers, spinach, chicory, lettuce, rocket, leek, celery, parsley, tomatoes, fresh cucumber, marrow, courgettes, pumpkins, aubergines, Parsnip, beetroots, onion, garlic, celeriac, radishes, turnip, salads, mixed vegetables |
| Potatoes | Potatoes | Boiled, baked, French fries, potato rosti, gnocchi, etc. |
| Vegetable oils | Vegetable based oil | |
| Other edible fats | Margarine | Margarine for baking, frying, spreading |
| Mayonnaise | Mayonnaise and salad dressings | |
| White meat | Poultry and rabbit | |
| Fish | Lean fish | Pollock, cod, perch, hake, carp to 1 kg, tuna, panga, trout etc. |
| Oily fish | Salmon, sardines, herring, mackerel, eel, large carp etc. | |
| Fruit | All kind of fruits (stone fruits, kiwi and citrus fruits, tropical fruits, berries, bananas, apples, and pears) | Apricots, cherries, nectarines, peaches, plums, grapes, kiwi, oranges, mandarins, grapefruit, lemons, pomelos, pineapples, watermelon, melons, fresh dates and figs, strawberries, raspberries, blackberries, blueberries, redcurrants, blackcurrants, bananas, apples, pears |
| Nuts and seeds | Nuts and nut spreads | Peanuts, hazelnuts, walnuts, cashews, coconuts, chestnuts, etc. |
| Seeds and bran | Pumpkin seeds, sesame seeds, sunflower seeds, wheat germs, wheat bran, etc. | |
| Legumes | Fresh and tinned legumes | Corn, green peas, green beans, etc. |
| Dry and processed pulses | Beans (fava, butter kidney, broad, French, green), soya, peas, chickpea, and processed pulses (baked beans, hummus, other bread spreads) | |
| Juices | Fruit juices and nectars | Mixed fruit juice, orange, grapefruit, apple, pear, grape, blackcurrant, cherry juice |
| Vegetable and vegetable-fruit juices | Mixed vegetable juice, tomato, carrot and carrot-fruit juice | |
| Sweetened beverages and energy drinks | Sweetened beverages | |
| Energy drinks | ||
| Alcohol | Beer | Beer |
| Wine and cocktails | Wine and cocktails | |
| Spirits | Vodka and other spirits |
| Variable | Total Sample | Family History of P-CAD | p | |
|---|---|---|---|---|
| With | Without | |||
| N (%) | 689 (100.0) | 351 (50.9) | 338 (49.1) | |
| Age (years) | 28.0 (SD 4.5) | 28.6 (SD 4.6) | 27.3 (SD 4.2) | 0.0001 |
| Female | 299 (43.4) | 142 (40.5) | 157 (46.4) | 0.11 |
| Current smoking (vs. past smoker or non-smoker) | 154 (22.4) | 96 (27.4) | 58(17.2) | 0.001 |
| Place of residence—city >20,000 inhabitants (vs. city <20,000) | 514 (74.6) | 252 (71.8) | 262 (77.5) | 0.08 |
| Financial situation above average (vs. average or below average) | 173 (25.1) | 74 (21.1) | 99 (29.2) | 0.01 |
| Higher education (vs. secondary or primary) | 389 (56.4) | 195 (55.6) | 194 (57.4) | 0.63 |
| Physical activity at leisure time | ||||
| Low | 171 (24.8) | 90 (25.6) | 81 (24.0) | 0.78 |
| Moderate | 328 (47.6) | 168 (47.9) | 160 (47.3) | |
| High | 190 (27.6) | 93 (26.5) | 97 (28.7) | |
| Dietary Patterns | Family History of P-CAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| With | Without | With | |||||||
| % | p for Trend | OR | ORcrude | 95% CI | p | ORadj * | 95% CI | p | |
| ‘Prudent’ | |||||||||
| Bottom tertile (ref.) | 56.1 | 0.02 | 1.0 (ref.) | 1.0 (ref.) | ----------- | ---- | 1.0 (ref.) | -------- | ------- |
| Middle tertile | 51.3 | 1.0 | 0.82 | 0.57–1.19 | 0.30 | 0.94 | 0.64–1.39 | 0.75 | |
| Upper tertile | 45.4 | 1.0 | 0.65 | 0.45–0.94 | 0.02 | 0.72 | 0.48–1.09 | 0.11 | |
| Per 1 unit of SD of dietary pattern score | ----- | ------ | 1.0 | 0.82 | 0.70–0.95 | 0.01 | 0.85 | 0.72–1.01 | 0.07 |
| ‘Westernized traditional’ | |||||||||
| Bottom tertile (ref.) | 43.5 | 0.0007 | 1.0 | 1.0 (ref.) | ----------- | ------- | 1.0 (ref.) | ------------ | ------- |
| Middle tertile | 50.0 | 1.0 | 1.30 | 0.90–1.88 | 0.16 | 1.24 | 0.85–1.82 | 0.26 | |
| Upper tertile | 59.4 | 1.0 | 1.90 | 1.31–2.76 | 0.0007 | 1.72 | 1.16–2.57 | 0.007 | |
| Per 1 unit of SD of dietary pattern score | ----- | ------ | 1.0 | 1.31 | 1.12–1.53 | 0.0007 | 1.25 | 1.06–1.48 | 0.007 |
| ‘Dairy, breakfast cereals, and treats’ | |||||||||
| Bottom tertile (ref.) | 44.3 | 0.02 | 1.0 | 1.0 (ref.) | ------------ | -------- | 1.0 (ref.) | ----------- | ----- |
| Middle tertile | 53.5 | 1.0 | 1.44 | 1.00–2.09 | 0.05 | 1.76 | 1.20–2.61 | 0.004 | |
| Upper tertile | 55.0 | 1.0 | 1.54 | 1.06–2.21 | 0.02 | 1.75 | 1.19–2.58 | 0.005 | |
| Per 1 unit of SD of dietary pattern score | ------ | ------ | 1.0 | 1.11 | 0.95–1.29 | 0.19 | 1.16 | 0.99–1.36 | 0.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osadnik, T.; Pawlas, N.; Lonnie, M.; Osadnik, K.; Lejawa, M.; Wądołowska, L.; Bujak, K.; Fronczek, M.; Reguła, R.; Gawlita, M.; et al. Family History of Premature Coronary Artery Disease (P-CAD)—A Non-Modifiable Risk Factor? Dietary Patterns of Young Healthy Offspring of P-CAD Patients: A Case-Control Study (MAGNETIC Project). Nutrients 2018, 10, 1488. https://doi.org/10.3390/nu10101488
Osadnik T, Pawlas N, Lonnie M, Osadnik K, Lejawa M, Wądołowska L, Bujak K, Fronczek M, Reguła R, Gawlita M, et al. Family History of Premature Coronary Artery Disease (P-CAD)—A Non-Modifiable Risk Factor? Dietary Patterns of Young Healthy Offspring of P-CAD Patients: A Case-Control Study (MAGNETIC Project). Nutrients. 2018; 10(10):1488. https://doi.org/10.3390/nu10101488
Chicago/Turabian StyleOsadnik, Tadeusz, Natalia Pawlas, Marta Lonnie, Kamila Osadnik, Mateusz Lejawa, Lidia Wądołowska, Kamil Bujak, Martyna Fronczek, Rafał Reguła, Marcin Gawlita, and et al. 2018. "Family History of Premature Coronary Artery Disease (P-CAD)—A Non-Modifiable Risk Factor? Dietary Patterns of Young Healthy Offspring of P-CAD Patients: A Case-Control Study (MAGNETIC Project)" Nutrients 10, no. 10: 1488. https://doi.org/10.3390/nu10101488
APA StyleOsadnik, T., Pawlas, N., Lonnie, M., Osadnik, K., Lejawa, M., Wądołowska, L., Bujak, K., Fronczek, M., Reguła, R., Gawlita, M., Strzelczyk, J. K., Góral, M., Gierlotka, M., Poloński, L., & Gąsior, M. (2018). Family History of Premature Coronary Artery Disease (P-CAD)—A Non-Modifiable Risk Factor? Dietary Patterns of Young Healthy Offspring of P-CAD Patients: A Case-Control Study (MAGNETIC Project). Nutrients, 10(10), 1488. https://doi.org/10.3390/nu10101488








