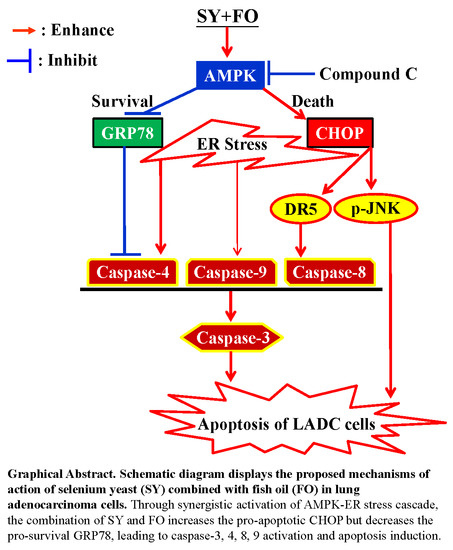
Opposite Regulation of CHOP and GRP78 and Synergistic Apoptosis Induction by Selenium Yeast and Fish Oil via AMPK Activation in Lung Adenocarcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents and Chemicals
2.3. Measurement of Cell Viability
2.4. Photograph of the Cells
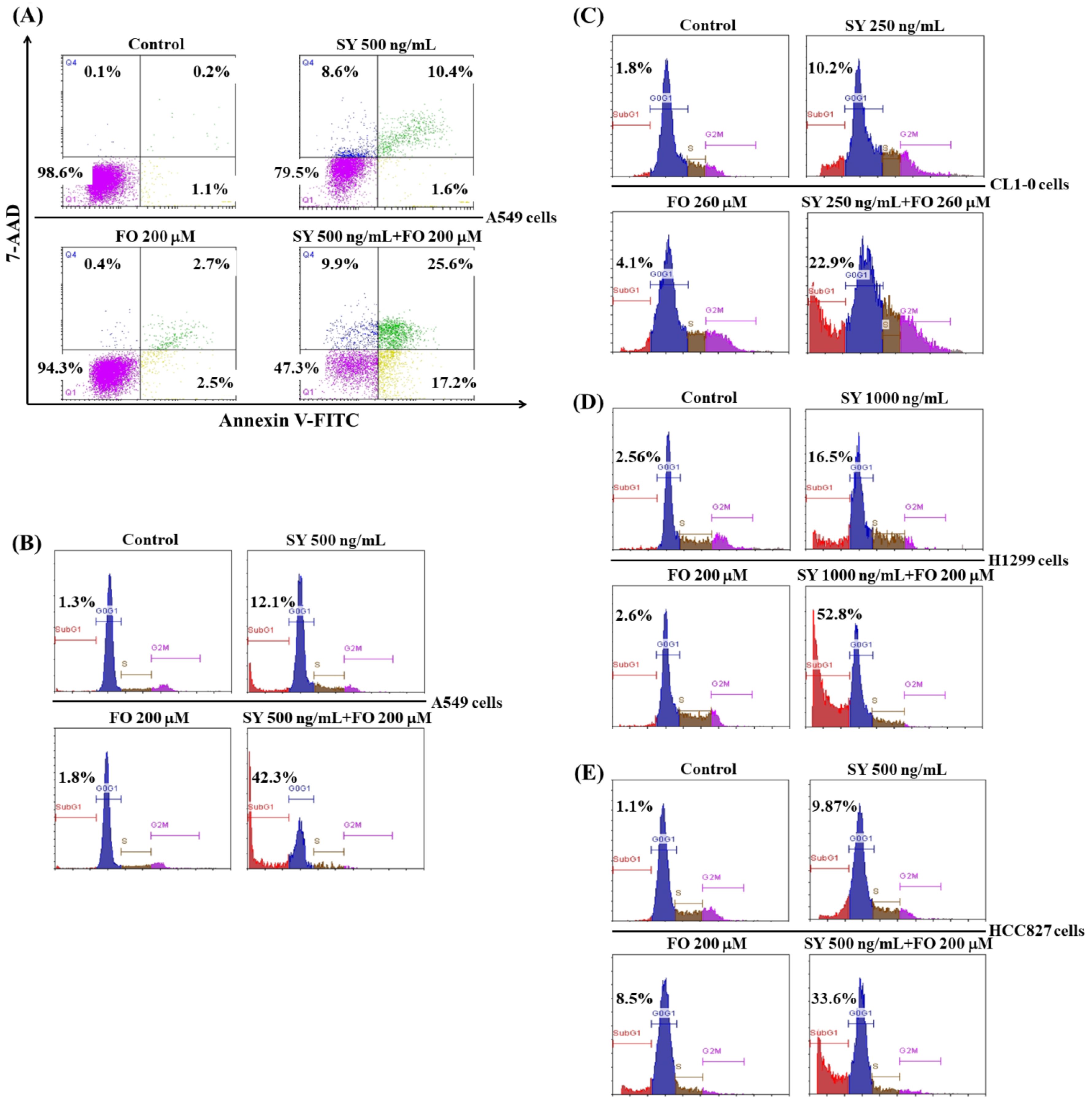
2.5. Analysis of Apoptotic and Necrotic Cell Death by Annexin V/7-Amino-Actinomycin D Staining
2.6. Analysis of Apoptotic Sub-G1 Fraction by Propidium Iodide Staining
2.7. Western Blot
2.8. Antibodies
2.9. Analysis of Synergistic Combination Effect
2.10. Statistical Analysis
3. Results
3.1. SY and FO Act Synergistically to Inhibit the Growth of LADC Cells
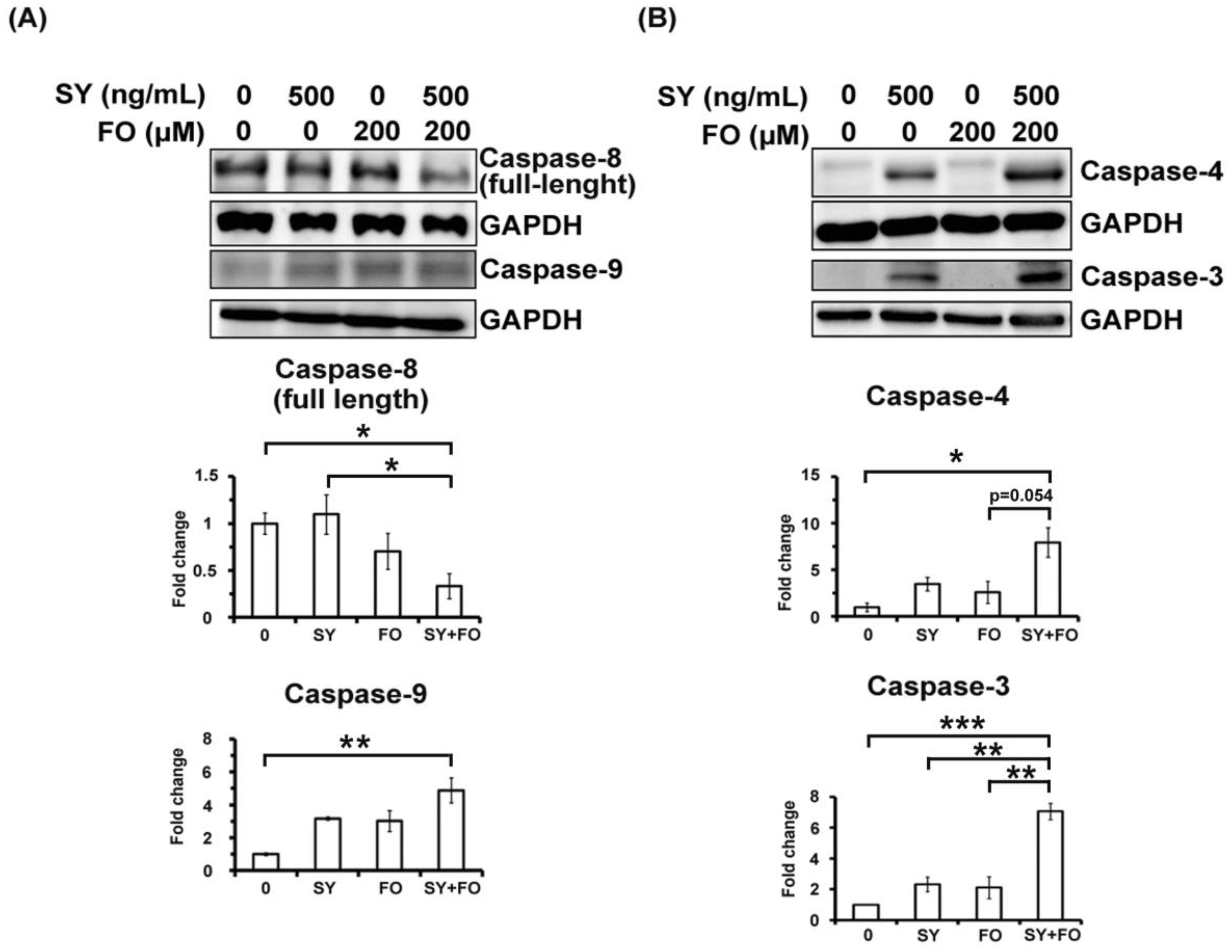
3.2. SY and FO Act Synergistically to Induce Apoptosis of LADC Cells
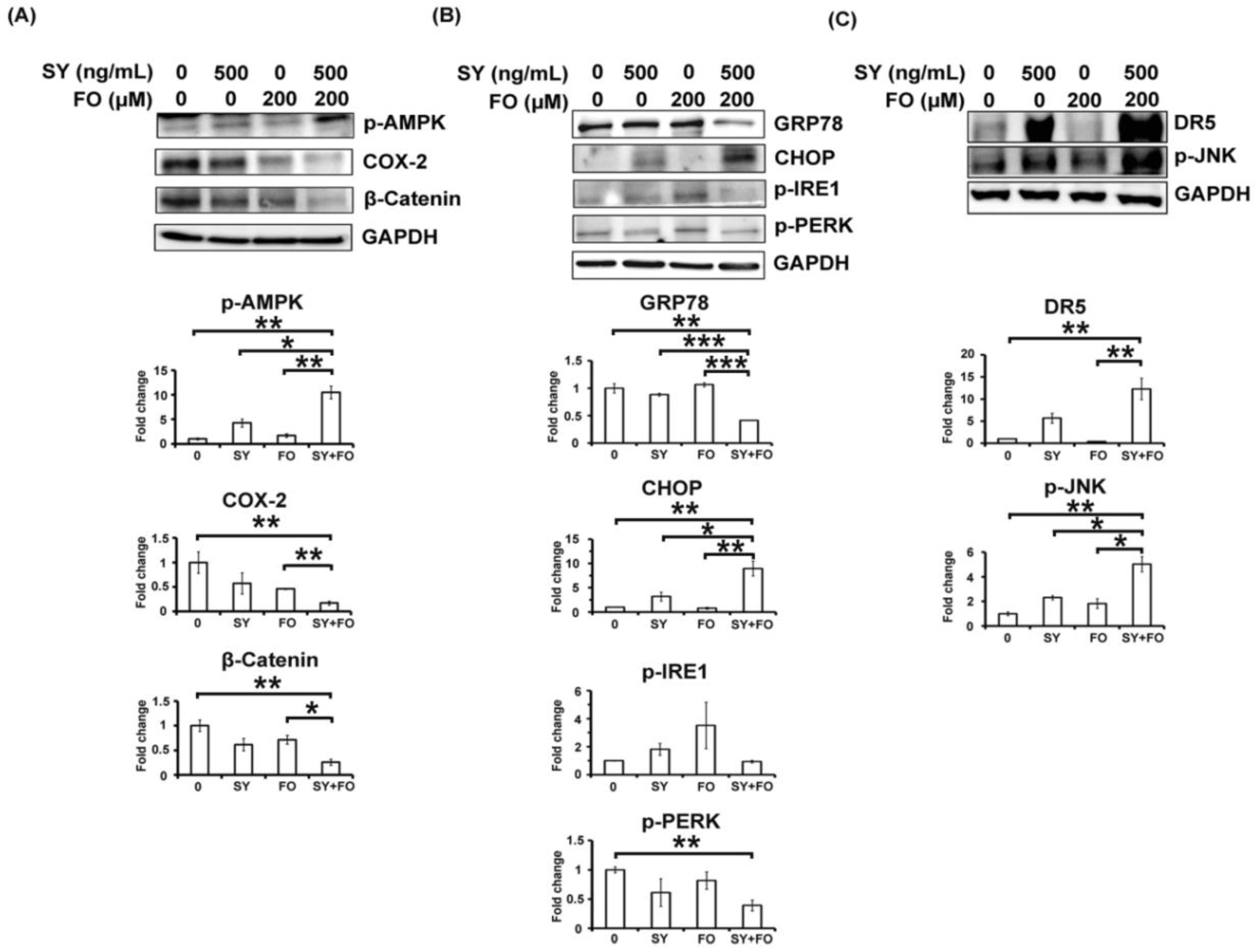
3.3. Synergistic Activation of AMPK by SY and FO in A549 LADC Cells
3.4. Combination of SY and FO Elevates CHOP and Reduces GRP78 in A549 LADC Cells
3.5. AMPK Inhibition Diminishes the Synergistic Apoptosis Induction and Caspase-4 Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| CC | compound C |
| CHOP | CCAAT/enhancer-binding protein homologous protein |
| DR5 | death receptor-5 |
| EGFR | epidermal growth factor receptor |
| ER | endoplasmic reticulum |
| FO | fish oil |
| GRP78 | glucose-regulated protein 78 |
| p-IRE1 | phospho-inositol-requiring enzyme 1 |
| LADC | Lung adenocarcinoma |
| SEM | standard error of the mean |
| SY | selenium yeast |
| p-JNK | phospho-Jun amino-terminal kinase |
| p-PERK | phospho-protein kinase-like endoplasmic reticulum kinase |
| UPR | unfolded protein response |
References
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, T.V.; Budkevich, I.N.; Zhivotovsky, B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising selenium for modulation of cancer treatments. Anticancer Res. 2017, 37, 6497–6509. [Google Scholar] [PubMed]
- Lu, J.; Zhang, J.; Jiang, C.; Deng, Y.; Ozten, N.; Bosland, M.C. Cancer chemoprevention research with selenium in the post-select era: Promises and challenges. Nutr. Cancer 2016, 68, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Kennedy, D.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, A.; Sagar, S.; Wong, R.; Seely, D. Selenium and lung cancer: A systematic review and meta analysis. PLoS ONE 2011, 6, e26259. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium exposure and cancer risk: An updated meta-analysis and meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef] [PubMed]
- Karp, D.D.; Lee, S.J.; Keller, S.M.; Wright, G.S.; Aisner, S.; Belinsky, S.A.; Johnson, D.H.; Johnston, M.R.; Goodman, G.; Clamon, G.; et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: Ecog 5597. J. Clin. Oncol. 2013, 31, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug Targets 2004, 4, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, F.; Bai, Y.; Chen, T.; Zheng, W. Selenium nanoparticles inhibit the growth of hela and mda-mb-231 cells through induction of s phase arrest. Colloids Surf. B Biointerfaces 2012, 94, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, Y.K.; Kim, H.J.; Park, O.J.; Kim, Y.M. Ampk interacts with beta-catenin in the regulation of hepatocellular carcinoma cell proliferation and survival with selenium treatment. Oncol. Rep. 2016, 35, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Kim, Y.M.; Surh, Y.J.; Baik, H.W.; Lee, S.K.; Ha, J.; Park, O.J. Selenium regulates cyclooxygenase-2 and extracellular signal-regulated kinase signaling pathways by activating amp-activated protein kinase in colon cancer cells. Cancer Res. 2006, 66, 10057–10063. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, S.Y.; Kim, Y.M.; Kim, D.C.; Lee, W.S.; Surh, Y.J.; Park, O.J. Suppression of mTOR via akt-dependent and -independent mechanisms in selenium-treated colon cancer cells: Involvement of ampkalpha1. Carcinogenesis 2010, 31, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Shigemi, Z.; Manabe, K.; Hara, N.; Baba, Y.; Hosokawa, K.; Kagawa, H.; Watanabe, T.; Fujimuro, M. Methylseleninic acid and sodium selenite induce severe ER stress and subsequent apoptosis through UPR activation in PEL cells. Chem. Biol. Interact. 2017, 266, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Ip, C. Synergy between selenium and vitamin e in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Res. 2003, 63, 6988–6995. [Google Scholar] [PubMed]
- Wu, Y.; Zhang, H.; Dong, Y.; Park, Y.M.; Ip, C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005, 65, 9073–9079. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Velotti, F. Omega-3 fatty acids and cancer cell cytotoxicity: Implications for multi-targeted cancer therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M. Fish oil omega-3 fatty acids and cardio-metabolic health, alone or with statins. Eur. J. Clin. Nutr. 2013, 67, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jeong, S.; Jing, K.; Shin, S.; Kim, S.; Heo, J.Y.; Kweon, G.R.; Park, S.K.; Wu, T.; Park, J.I.; et al. Docosahexaenoic acid induces cell death in human non-small cell lung cancer cells by repressing mTOR via AMPK activation and PI3K/Akt inhibition. BioMed Res. Int. 2015, 2015, 239764. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sui, C.; Meng, F.; Ma, P.; Jiang, Y. The omega-3 polyunsaturated fatty acid docosahexaenoic acid inhibits proliferation and progression of non-small cell lung cancer cells through the reactive oxygen species-mediated inactivation of the PI3k /Akt pathway. Lipids Health Dis. 2017, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer 2011, 117, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Song, K.S.; Shin, S.; Kim, N.; Jeong, S.; Oh, H.R.; Park, J.H.; Seo, K.S.; Heo, J.Y.; Han, J.; et al. Docosahexaenoic acid induces autophagy through p53/ampk/mtor signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy 2011, 7, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jing, K.; Shin, S.; Jeong, S.; Han, S.H.; Oh, H.; Yoo, Y.S.; Han, J.; Jeon, Y.J.; Heo, J.Y.; et al. Omega3-polyunsaturated fatty acids induce cell death through apoptosis and autophagy in glioblastoma cells: In vitro and in vivo. Oncol. Rep. 2018, 39, 239–246. [Google Scholar] [PubMed]
- Jakobsen, C.H.; Storvold, G.L.; Bremseth, H.; Follestad, T.; Sand, K.; Mack, M.; Olsen, K.S.; Lundemo, A.G.; Iversen, J.G.; Krokan, H.E.; et al. DHA induces ER stress and growth arrest in human colon cancer cells: Associations with cholesterol and calcium homeostasis. J. Lipid Res. 2008, 49, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of grp78 in colon cancer cell lines. Biochim. Biophys. Acta 2012, 1822, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenium yeast: Composition, quality, analysis, and safety. Pure Appl. Chem. 2006, 78, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.; Meyer, N. Annexin v/7-aad staining in keratinocytes. Methods Mol. Biol. 2011, 740, 57–63. [Google Scholar] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.E.; Stratton, M.S.; Lillico, A.J.; Fakih, M.; Natarajan, R.; Clark, L.C.; Marshall, J.R. A report of high-dose selenium supplementation: Response and toxicities. J. Trace Elem. Med. Biol. 2004, 18, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.; Mukohara, T.; Hansen, M.; Meyerson, M.; Johnson, B.E.; Janne, P.A. Gefitinib induces apoptosis in the egfrl858r non-small-cell lung cancer cell line h3255. Cancer Res. 2004, 64, 7241–7244. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lawrence, D.A.; Marsters, S.; Acosta-Alvear, D.; Kimmig, P.; Mendez, A.S.; Paton, A.W.; Paton, J.C.; Walter, P.; Ashkenazi, A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 2014, 345, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, K.; Bihani, T.; Lin, A.; Park, Y.M.; Mori, K.; Ip, C. Enhanced selenium effect on growth arrest by bip/grp78 knockdown in p53-null human prostate cancer cells. Oncogene 2006, 25, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.H.; Qiao, Y.; Ozdelen, E.; Schubert, R.; Sevinc, S.; Harbinski, F.; Grubissich, L.; Singer, S.; Halperin, J.A. Small-molecule targeting of translation initiation for cancer therapy. Oncotarget 2013, 4, 1606–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonthal, A.H. Endoplasmic reticulum stress and autophagy as targets for cancer therapy. Cancer Lett. 2009, 275, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lizardo, M.M.; Morrow, J.J.; Miller, T.E.; Hong, E.S.; Ren, L.; Mendoza, A.; Halsey, C.H.; Scacheri, P.C.; Helman, L.J.; Khanna, C. Upregulation of glucose-regulated protein 78 in metastatic cancer cells is necessary for lung metastasis progression. Neoplasia 2016, 18, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, G.M.; Leclerc, G.J.; Kuznetsov, J.N.; DeSalvo, J.; Barredo, J.C. Metformin induces apoptosis through AMPK-dependent inhibition of UPR signaling in all lymphoblasts. PLoS ONE 2013, 8, e74420. [Google Scholar] [CrossRef] [PubMed]
- Kajbaf, F.; De Broe, M.E.; Lalau, J.D. Therapeutic concentrations of metformin: A systematic review. Clin. Pharmacokinet. 2016, 55, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, K.; Nagaya, T.; Tokudome, Y.; Imaeda, N.; Fujiwara, N.; Sato, J.; Goto, C.; Ikeda, M.; Maki, S.; Tajima, K.; et al. Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: A cross-sectional study. J. Nutr. 2003, 133, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Murdolo, G.; Bartolini, D.; Tortoioli, C.; Piroddi, M.; Torquato, P.; Galli, F. Selenium and cancer stem cells. Adv. Cancer Res. 2017, 136, 235–257. [Google Scholar] [PubMed]
- Hu, Y.; McIntosh, G.H.; Le Leu, R.K.; Nyskohus, L.S.; Woodman, R.J.; Young, G.P. Combination of selenium and green tea improves the efficacy of chemoprevention in a rat colorectal cancer model by modulating genetic and epigenetic biomarkers. PLoS ONE 2013, 8, e64362. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hou, L.; Song, H.; Xu, P.; Sun, Y.; Wu, K. Akt/AMPK/mTOR pathway was involved in the autophagy induced by vitamin E succinate in human gastric cancer SGC-7901 cells. Mol. Cell. Biochem. 2017, 424, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghoshal, S.; Porter, T.D. Phosphorylation of hepatic amp-activated protein kinase and liver kinase b1 is increased after a single oral dose of green tea extract to mice. Nutr. Res. 2012, 32, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Z.; Jia, L.; Zhao, Y.; Zhang, X.; Wu, K. Endoplasmic reticulum stress contributes to vitamin e succinate-induced apoptosis in human gastric cancer SGC-7901 cells. Cancer Lett. 2010, 296, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chan, Y.L.; Li, T.L.; Bauer, B.A.; Hsia, S.; Wang, C.H.; Huang, J.S.; Wang, H.M.; Yeh, K.Y.; Huang, T.H.; et al. Reduction of splenic immunosuppressive cells and enhancement of anti-tumor immunity by synergy of fish oil and selenium yeast. PLoS ONE 2013, 8, e52912. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cao, Y.; Luo, Y.; Hu, H.; Ling, H. Signalling mechanism(s) of epithelial-mesenchymal transition and cancer stem cells in tumour therapeutic resistance. Clin. Chim. Acta 2018, 483, 156–163. [Google Scholar] [CrossRef] [PubMed]

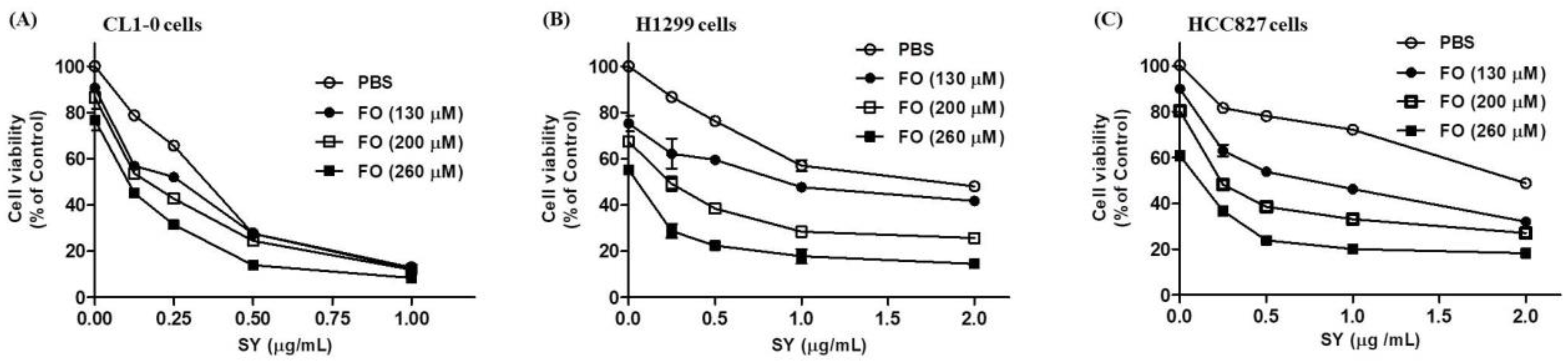

| SY (ng/mL) | FO (μM) | FA | CI |
|---|---|---|---|
| 250 | 130 | 0.29 | 0.945 |
| 250 | 200 | 0.36 | 0.968 |
| 250 | 260 | 0.44 | 0.983 |
| 500 | 130 | 0.43 | 0.840 |
| 500 | 200 | 0.65 | 0.576 |
| 500 | 260 | 0.70 | 0.631 |
| 1000 | 130 | 0.51 | 0.969 |
| 1000 | 200 | 0.69 | 0.650 |
| 1000 | 260 | 0.72 | 0.711 |
| 2000 | 130 | 0.63 | 0.999 |
| 2000 | 200 | 0.72 | 0.813 |
| 2000 | 260 | 0.80 | 0.683 |
| SY (ng/mL) | FO (μM) | FA | CI |
|---|---|---|---|
| 125 | 130 | 0.43 | 0.740 |
| 125 | 200 | 0.46 | 0.565 |
| 125 | 260 | 0.55 | 0.738 |
| 250 | 130 | 0.52 | 0.974 |
| 250 | 200 | 0.57 | 0.950 |
| 250 | 260 | 0.68 | 0.775 |
| 500 | 130 | 0.73 | 0.990 |
| 500 | 200 | 0.76 | 0.950 |
| 500 | 260 | 0.86 | 0.664 |
| 1000 | 130 | 0.88 | 1.009 |
| 1000 | 200 | 0.88 | 1.040 |
| 1000 | 260 | 0.92 | 0.810 |
| SY (ng/mL) | FO (μM) | FA | CI |
|---|---|---|---|
| 250 | 130 | 0.38 | 0.856 |
| 250 | 200 | 0.51 | 0.347 |
| 250 | 260 | 0.71 | 0.468 |
| 500 | 130 | 0.62 | 0.607 |
| 500 | 200 | 0.78 | 0.389 |
| 500 | 260 | 0.41 | 0.993 |
| 1000 | 130 | 0.52 | 0.955 |
| 1000 | 200 | 0.72 | 0.529 |
| 1000 | 260 | 0.82 | 0.381 |
| 2000 | 130 | 0.58 | 1.208 |
| 2000 | 200 | 0.74 | 0.697 |
| 2000 | 260 | 0.85 | 0.422 |
| SY (ng/mL) | FO (μM) | FA | CI |
|---|---|---|---|
| 250 | 130 | 0.37 | 0.706 |
| 250 | 200 | 0.52 | 0.675 |
| 250 | 260 | 0.63 | 0.700 |
| 500 | 130 | 0.46 | 0.677 |
| 500 | 200 | 0.61 | 0.612 |
| 500 | 260 | 0.76 | 0.549 |
| 1000 | 130 | 0.54 | 0.694 |
| 1000 | 200 | 0.67 | 0.603 |
| 1000 | 260 | 0.8 | 0.521 |
| 2000 | 130 | 0.68 | 0.575 |
| 2000 | 200 | 0.73 | 0.604 |
| 2000 | 260 | 0.82 | 0.535 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, R.-H.; Lai, G.-M.; Chow, J.-M.; Liao, C.-H.; Zheng, Y.-M.; Tsai, W.-L.; Hsia, S.; Lai, I.-C.; Lee, H.-L.; Chuang, S.-E.; et al. Opposite Regulation of CHOP and GRP78 and Synergistic Apoptosis Induction by Selenium Yeast and Fish Oil via AMPK Activation in Lung Adenocarcinoma Cells. Nutrients 2018, 10, 1458. https://doi.org/10.3390/nu10101458
Kao R-H, Lai G-M, Chow J-M, Liao C-H, Zheng Y-M, Tsai W-L, Hsia S, Lai I-C, Lee H-L, Chuang S-E, et al. Opposite Regulation of CHOP and GRP78 and Synergistic Apoptosis Induction by Selenium Yeast and Fish Oil via AMPK Activation in Lung Adenocarcinoma Cells. Nutrients. 2018; 10(10):1458. https://doi.org/10.3390/nu10101458
Chicago/Turabian StyleKao, Ruey-Ho, Gi-Ming Lai, Jyh-Ming Chow, Chien-Huang Liao, Yu-Mei Zheng, Wei-Lun Tsai, Simon Hsia, I-Chun Lai, Hsin-Lun Lee, Shuang-En Chuang, and et al. 2018. "Opposite Regulation of CHOP and GRP78 and Synergistic Apoptosis Induction by Selenium Yeast and Fish Oil via AMPK Activation in Lung Adenocarcinoma Cells" Nutrients 10, no. 10: 1458. https://doi.org/10.3390/nu10101458
APA StyleKao, R.-H., Lai, G.-M., Chow, J.-M., Liao, C.-H., Zheng, Y.-M., Tsai, W.-L., Hsia, S., Lai, I.-C., Lee, H.-L., Chuang, S.-E., Whang-Peng, J., & Yao, C.-J. (2018). Opposite Regulation of CHOP and GRP78 and Synergistic Apoptosis Induction by Selenium Yeast and Fish Oil via AMPK Activation in Lung Adenocarcinoma Cells. Nutrients, 10(10), 1458. https://doi.org/10.3390/nu10101458