Achacha (Garcinia humilis) Rind Improves Cardiovascular Function in Rats with Diet-Induced Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
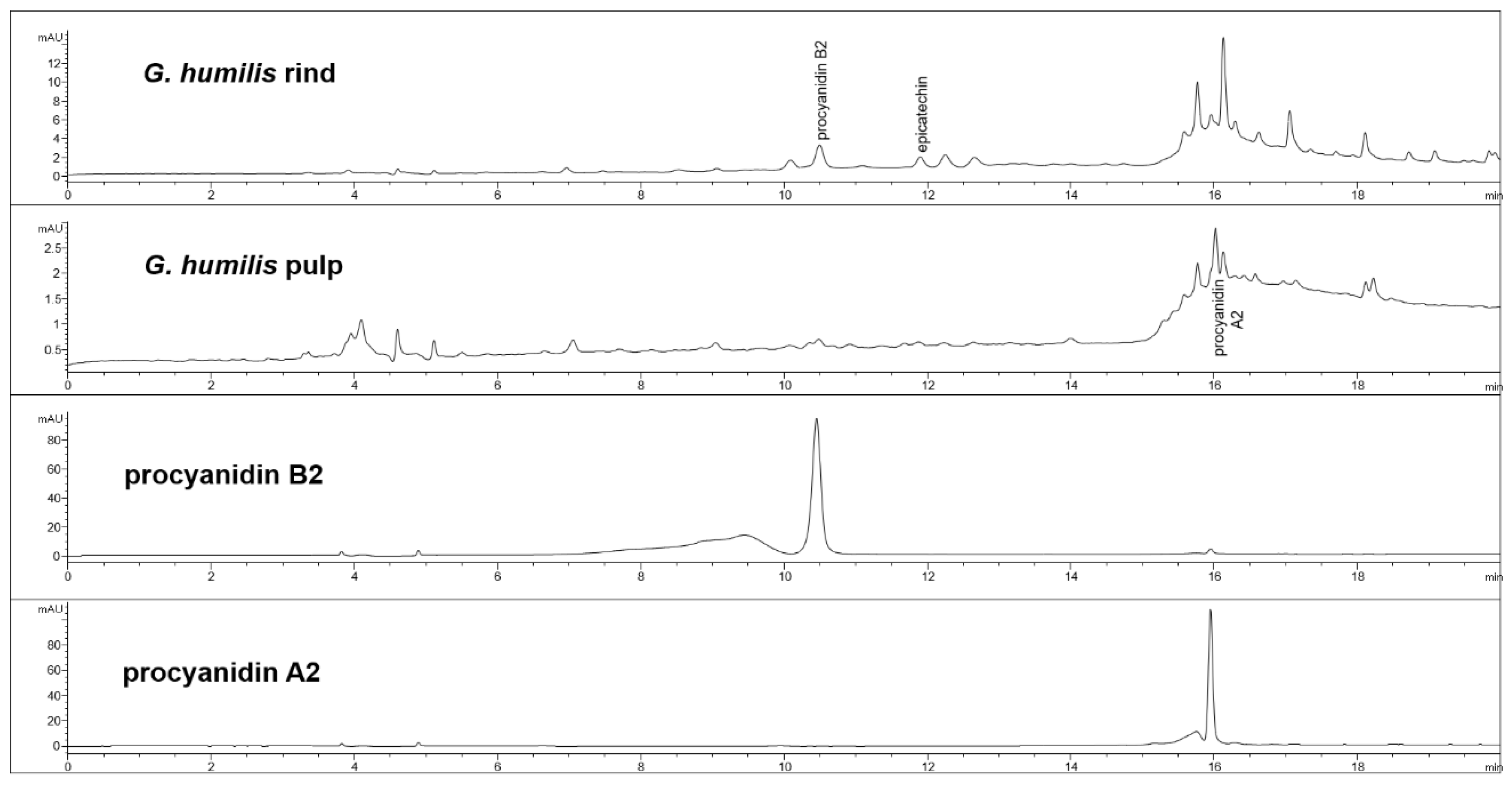
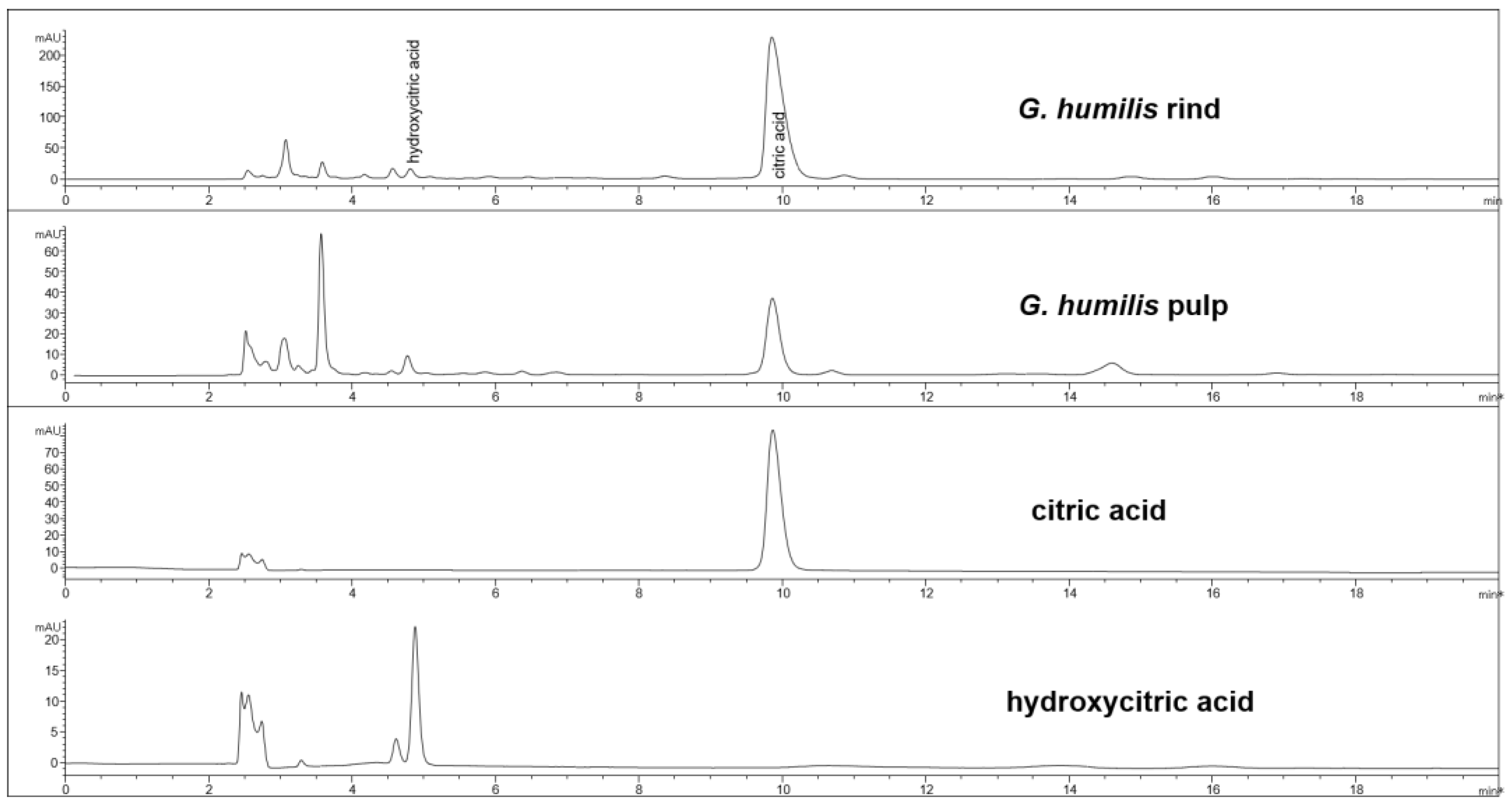
2.1. G. humilis Rind and Pulp Powder Preparation and Analyses
2.2. Rats and Diets
2.3. Rat Measurements
2.4. Statistical Analysis
3. Results
3.1. Weight and Phytochemical Analysis of G. humilis
3.2. Diet Intake and Body Composition
3.3. Plasma Biochemistry and Oral Glucose Tolerance
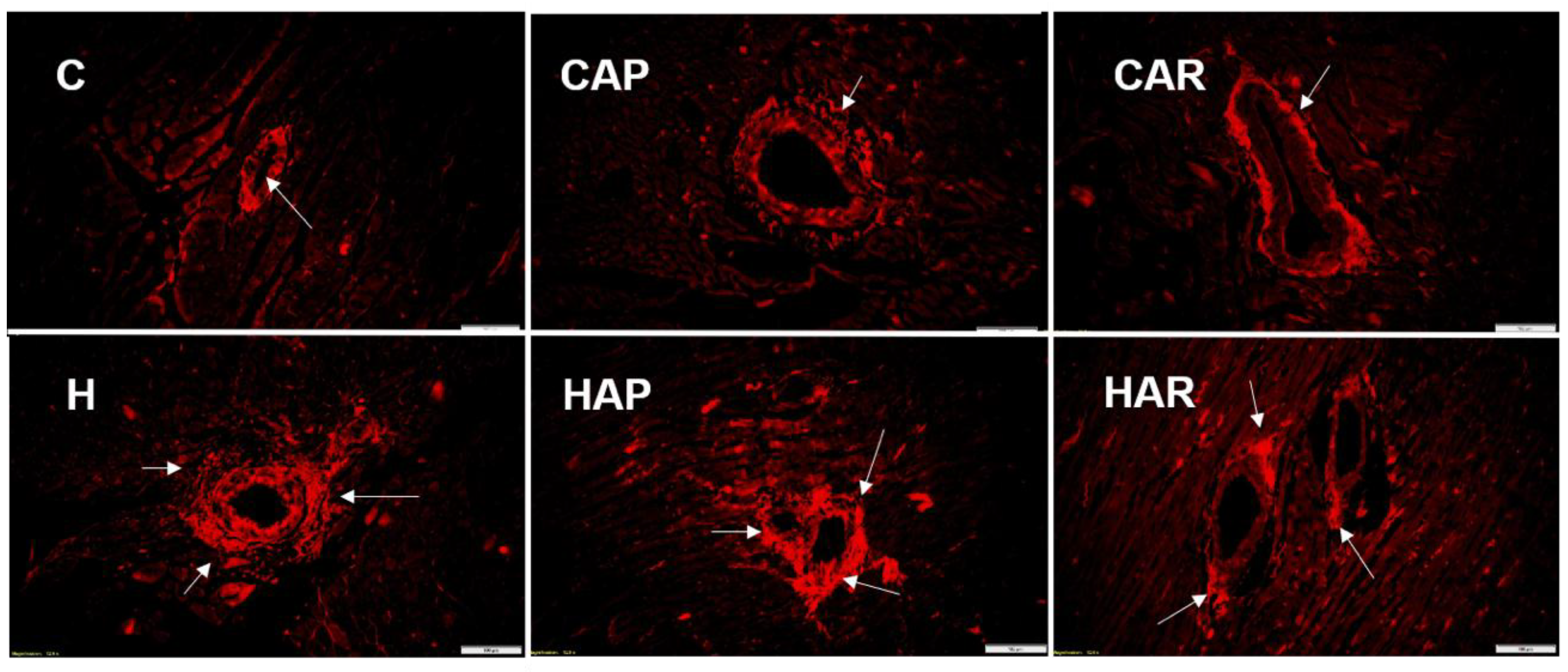
3.4. Cardiovascular Structure and Function
3.5. Liver Structure and Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hemshekhar, M.; Sunitha, K.; Santhosh, M.S.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.S.; Niranjana, S.R.; Girish, K.S. An overview on genus Garcinia: Phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351. [Google Scholar] [CrossRef]
- John, O.D.; Brown, L.; Panchal, S.K. Garcinia fruits: Their potential to combat metabolic syndrome. In Nutraceuticals and Natural Product Derivatives: Disease Prevention & Drug Discovery, 1st ed.; Ullah, M., Ahmad, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 39–80. [Google Scholar]
- Lim, T.K. Garcinia humilis. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; Volume 2, pp. 59–61. [Google Scholar]
- Duarte, O. Achachairú (Garcinia humilis (Vahl) C. D. Adam). In Postharvest Biology and Technology of Tropical and Subtropical Fruits: Açai to Citrus, 1st ed.; Yahia, E., Ed.; Elsevier: New York, NY, USA, 2011; pp. 48–54e. [Google Scholar]
- Terrazas, P.M.; de Souza Marques, E.; Mariano, L.N.; Cechinel-Filho, V.; Niero, R.; Andrade, S.F.; Maistro, E.L. Benzophenone guttiferone A from Garcinia achachairu Rusby (Clusiaceae) presents genotoxic effects in different cells of mice. PLoS ONE 2013, 8, e76485. [Google Scholar] [CrossRef] [PubMed]
- About the Achacha. Available online: http://achacha.com.au/about/ (accessed on 9 October 2017).
- Niero, R.; Dal Molin, M.M.; Silva, S.; Damian, N.S.; Maia, L.O.; Delle Monache, F.; Cechinel Filho, V.; de Andrade, S.F. Gastroprotective effects of extracts and guttiferone A isolated from Garcinia achachairu Rusby (Clusiaceae) against experimentally induced gastric lesions in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Mariano, L.N.B.; da Silva, L.M.; de Souza, P.; Boeing, T.; Somensi, L.B.; Bonomini, T.J.; Delle Monache, F.; Cechinel Filho, V.; de Andrade, S.F.; Niero, R. Gastroprotective xanthones isolated from Garcinia achachairu: Study on mucosal defensive factors and H+, K+-ATPase activity. Chem. Biol. Interact. 2016, 258, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Filho, V.C.; Meyre-Silva, C.; Niero, R.; Bolda Mariano, L.N.; Gomes do Nascimento, F.; Vicente Farias, I.; Gazoni, V.F.; Dos Santos Silva, B.; Gimenez, A.; Gutierrez-Yapu, D.; et al. Evaluation of antileishmanial activity of selected Brazilian plants and identification of the active principles. Evid. Based Complement. Alternat. Med. 2013, 2013, 265025. [Google Scholar] [CrossRef] [PubMed]
- Mariano, L.N.B.; Vendramini-Costa, D.B.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Corrêa, R.; Cechinel Filho, V.; Delle Monache, F.; Niero, R. In vitro antiproliferative activity of uncommon xanthones from branches of Garcinia achachairu. Pharm. Biol. 2016, 54, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Igho, O.; Kang, H.S.; Rachel, P.; Barbara, W.; Edzard, E. The use of Garcinia extract (hydroxycitric acid) as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. J. Obes. 2011, 2011, 509038. [Google Scholar]
- Kumar, S.; Sharma, S.; Chattopadhyay, S.K. The potential health benefit of polyisoprenylated benzophenones from Garcinia and related genera: Ethnobotanical and therapeutic importance. Fitoterapia 2013, 89, 86–125. [Google Scholar] [CrossRef] [PubMed]
- Beerhues, L. Benzophenone synthase from cultured cells of Centaurium erythraea. FEBS Lett. 1996, 383, 264–266. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Pratt, C.A. Metabolic syndrome and its association with diet and physical activity in US adolescents. J. Am. Diet. Assoc. 2008, 108, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Sweazea, K. Compounding evidence implicating Western diets in the development of metabolic syndrome. Acta Physiol. 2014, 211, 471–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome: The atherosclerosis risk in communities study. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Skoumas, Y.; Stefanadis, C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA study. J. Am. Diet. Assoc. 2007, 107, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.E.; Frederiksen, H.; Struntze Krogholm, K.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Loo, A.E.K.; Chia, F.P.P.; Huang, D. Oligomeric proanthocyanidins from mangosteen pericarps. J. Agric. Food Chem. 2007, 55, 7689–7694. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Ninomiya, K.; Tagashira, Y.; Maejima, K.; Yoshida, T.; Amakura, Y. Polyphenolic constituents of the pericarp of mangosteen (Garcinia mangostana L.). J. Agric. Food Chem. 2015, 63, 7670–7674. [Google Scholar] [CrossRef] [PubMed]
- Saroni Arwa, P.; Zeraik, M.L.; Ximenes, V.F.; da Fonseca, L.M.; Bolzani, V.d.S.; Siqueira Silva, D.H. Redox-active biflavonoids from Garcinia brasiliensis as inhibitors of neutrophil oxidative burst and human erythrocyte membrane damage. J. Ethnopharmacol. 2015, 174, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Du, F.; Fang, G. Two new proanthocyanidins from the leaves of Garcinia multiflora. Nat. Prod. Res. 2014, 28, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-H.; Park, S.K.; Choi, G.-Y.; Park, J.-H.; Lee, T.-H.; Jung, H.-N.; Kim, D.-O. Anti-hypertensive effect of grape seed extract in male spontaneously hypertensive rats. Food Sci. Biotechnol. 2015, 24, 2229–2233. [Google Scholar] [CrossRef]
- Magos, G.A.; Mateos, J.C.; Páez, E.; Fernández, G.; Lobato, C.; Márquez, C.; Enríquez, R.G. Hypotensive and vasorelaxant effects of the procyanidin fraction from Guazuma ulmifolia bark in normotensive and hypertensive rats. J. Ethnopharmacol. 2008, 117, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.S.; Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Chemistry and biochemistry of (−)-hydroxycitric acid from Garcinia. J. Agric. Food Chem. 2002, 50, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Lewis, Y.; Neelakantan, S. (−)-Hydroxycitric acid-the principal acid in the fruits of Garcinia cambogia desr. Phytochemistry 1965, 4, 619–625. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Stoupi, S.; Williamson, G.; Drynan, J.W.; Barron, D.; Clifford, M.N. A comparison of the in vitro biotransformation of (–)-epicatechin and procyanidin B2 by human faecal microbiota. Mol. Nutr. Food Res. 2010, 54, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.-P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.-P.; Gruppen, H.; Hollman, P.C. Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 (epicatechin-(4β-8)-epicatechin) in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Cienfuegos-Jovellanos, E.; del Mar Quiñones, M.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J. Agric. Food Chem. 2009, 57, 6156–6162. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, X.; Li, J.; Li, Z.; Niu, Y.; Ren, W.; Tan, J.; Yin, S. Role of NADPH oxidase pathway in renal protection induced by procyanidin B2: In L-NAME induced rat hypertension model. J. Funct. Foods 2018, 47, 405–415. [Google Scholar] [CrossRef]
- Rabadán-Chávez, G.; Reyes-Maldonado, E.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Jaramillo-Flores, M. The prothrombotic state associated with obesity-induced hypertension is reduced by cocoa and its main flavanols. Food Funct. 2016, 7, 4880–4888. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.Q.; Patel, B.; Kang, S.S.; Dhariwal, S.K.; Husain, F.; Wood, E.G.; Pothecary, M.R.; Corder, R. Regulation of vascular endothelial function by red wine procyanidins: Implications for cardiovascular health. Tetrahedron 2015, 71, 3059–3065. [Google Scholar] [CrossRef]
- Cai, Q.; Li, B.-Y.; Gao, H.-Q.; Zhang, J.-H.; Wang, J.-F.; Yu, F.; Yin, M.; Zhang, Z. Grape seed procyanidin B2 inhibits human aortic smooth muscle cell proliferation and migration induced by advanced glycation end products. Biosci. Biotechnol. Biochem. 2011, 75, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Savickas, A.; Vetchý, D.; Masteikova, R.; Kasauskas, A.; Bernatoniene, J. Direct effects of (−)-epicatechin and procyanidin B2 on the respiration of rat heart mitochondria. Biomed. Res. Int. 2015, 2015, 232836. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-Y.; Zhang, X.-Y.; Guan, S.-W.; Hua, Z.-C. Protective effect of procyanidin B2 against CCl4-induced acute liver injury in mice. Molecules 2015, 20, 12250–12265. [Google Scholar] [CrossRef] [PubMed]
- Muthenna, P.; Raghu, G.; Akileshwari, C.; Sinha, S.N.; Suryanarayana, P.; Reddy, G.B. Inhibition of protein glycation by procyanidin-B2 enriched fraction of cinnamon: Delay of diabetic cataract in rats. IUBMB Life 2013, 65, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yue, Y.; Li, J.; Li, Z.; Li, X.; Niu, Y.; Xiang, J.; Ding, H. Procyanidin B2 attenuates neurological deficits and blood-brain barrier disruption in a rat model of cerebral ischemia. Mol. Nutr. Food Res. 2015, 59, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, P.; Yu, F.; Zhang, Z.; Cai, Q.; Lu, W.; Li, B.; Qin, W.; Cheng, M.; Wang, H. Grape seed procyanidin B2 ameliorates hepatic lipid metabolism disorders in db/db mice. Mol. Med. Rep. 2017, 16, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, B.; Li, X.; Yu, F.; Cai, Q.; Zhang, Z.; Cheng, M.; Gao, H. Anti-inflammatory effects of grape seed procyanidin B2 on a diabetic pancreas. Food Funct. 2015, 6, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M.Á. Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. J. Nutr. Biochem. 2011, 22, 1186–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M.Á. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur. J. Nutr. 2012, 51, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Zhang, Z.; Li, Y. Grape seed procyanidin B2 ameliorates mitochondrial dysfunction and inhibits apoptosis via the AMP-activated protein kinase–silent mating type information regulation 2 homologue 1–PPARγ co-activator-1α axis in rat meningeal cells under high-dose glucosamine. Br. J. Nutr. 2015, 113, 35–44. [Google Scholar] [PubMed]
- Zhang, W.-Y.; Liu, H.-Q.; Xie, K.-Q.; Yin, L.-L.; Li, Y.; Kwik-Uribe, C.L.; Zhu, X.-Z. Procyanidin dimer B2 (epicatechin-(4β-8)-epicatechin) suppresses the expression of cyclooxygenase-2 in endotoxin-treated monocytic cells. Biochem. Biophys. Res. Commun. 2006, 345, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, L.; Yuan, Y.; Luo, X.; Jiang, M.; Ni, J.; Wang, N. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem. Pharmacol. 2014, 92, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Micaelo, N.; González-Abuín, N.; Pinent, M.; Ardévol, A.; Blay, M. Procyanidin B2 inhibits inflammasome-mediated IL-1β production in lipopolysaccharide-stimulated macrophages. Mol. Nutr. Food Res. 2015, 59, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-M.; Cai, X.; Kwik-Uribe, C.L.; Zeng, R.; Zhu, X.-Z. Inhibitory effects of procyanidin B2 dimer on lipid-laden macrophage formation. J. Cardiovasc. Pharmacol. 2006, 48, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Pérez-Vendrell, A.M.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats. Eur. J. Nutr. 2017, 56, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Ginés, I.; Gil-Cardoso, K.; Serrano, J.; Casanova-Martí, À.; Blay, M.; Pinent, M.; Ardévol, A.; Terra, X. Effects of an intermittent grape-seed proanthocyanidin (GSPE) treatment on a cafeteria diet obesogenic challenge in rats. Nutrients 2018, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Rabadan-Chávez, G.; Quevedo-Corona, L.; Garcia, A.M.; Reyes-Maldonado, E.; Jaramillo-Flores, M.E. Cocoa powder, cocoa extract and epicatechin attenuate hypercaloric diet-induced obesity through enhanced β-oxidation and energy expenditure in white adipose tissue. J. Funct. Foods 2016, 20, 54–67. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, M.; Naz, A.; Siddiqui, H.; Ahmad, S.I.; Faizi, S. Hypertensive and toxicological study of citric acid and other constituents from Tagetes patula roots. Arch. Pharm. Res. 2004, 27, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Domoto, T.; Hiramitsu, M.; Katagiri, T.; Sato, K.; Miyake, Y.; Aoi, S.; Ishihara, K.; Ikeda, H.; Umei, N. Effect on blood pressure of daily lemon ingestion and walking. J. Nutr. MeTable 2014, 2014, 912684. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C.; Harper, K.J. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hypertension. J. Clin. Hypertens. 2008, 10, 3–11. [Google Scholar] [CrossRef]
- Nii, Y.; Fukuta, K.; Sakai, K.; Yamamoto, S. Japanese citrus fruit (Sudachi) juice is associated with increased bioavailability of calcium from whole small fish and suppressed bone resorption in rats. J. Nutr. Sci. Vitaminol. 2004, 50, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Nii, Y.; Osawa, T.; Kunii, D.; Fukuta, K.; Sakai, K.; Kondo, M.; Yamamoto, S. Effect of citrus fruit (Sudachi) juice on absorption of calcium from whole small fish in healthy young men. Food Sci. Technol. Res. 2006, 12, 27–30. [Google Scholar] [CrossRef]
- Leonhardt, M.; Hrupka, B.; Langhans, W. Effect of hydroxycitrate on food intake and body weight regain after a period of restrictive feeding in male rats. Physiol. Behav. 2001, 74, 191–196. [Google Scholar] [CrossRef]
- Rao, R.N.; Sakariah, K. Lipid-lowering and antiobesity effect of (−) hydroxycitric acid. Nutr. Res. 1988, 8, 209–212. [Google Scholar] [CrossRef]


| Variables | C | CAP | CAR | H | HAP | HAR | p value | ||
|---|---|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||||
| Initial body weight (0 week), g | 340 ± 3 a | 341 ± 1 a | 339 ± 1 a | 338 ± 1 a | 338 ± 2 a | 339 ± 1 a | 0.09 | 0.86 | 0.44 |
| Midpoint body weight (8 week), g | 361 ± 9 b | 347 ± 5 b | 363 ± 6 b | 437 ± 9 a | 414 ± 7 a | 438 ± 8 a | <0.0001 | 0.02 | 0.82 |
| Final body weight (16 week), g | 403 ± 10 b | 380 ± 7 b | 385 ± 7 b | 524 ± 14 a | 504 ± 8 a | 512 ± 12 a | <0.0001 | 0.11 | 0.97 |
| Food intake, g/day | 38.7 ± 2.1 a | 35.1 ± 2.0 ab | 37.6 ± 1.9 a | 27.8 ± 2.5 b | 27.2 ± 2.2 b | 28.6 ± 2.8 b | <0.0001 | 0.59 | 0.80 |
| Water intake, g/day | 33.6 ± 2.5 a | 27.3 ± 2.9 a | 30.1 ± 2.7 a | 29.5 ± 2.1 a | 28.1 ± 2.2 a | 31.4 ± 2.1 a | 0.77 | 0.36 | 0.57 |
| Flavonoids intake, mg/kg/day | - | 4.5 ± 0.2 c | 67.6 ± 3.0 a | - | 3.5 ± 0.6 c | 51.4 ± 2.9 b | 0.0002 | <0.0001 | <0.0001 |
| Procyanidin B2 intake, mg/kg/day | - | 3.8 ± 0.2 c | 55.2 ± 2.5 a | - | 2.9 ± 0.5 c | 42.0 ± 2.4 b | 0.0003 | <0.0001 | <0.0001 |
| Citric acid intake, mg/kg/day | - | 20.2 ± 1.0 c | 136.9 ± 6.1 a | - | 15.6 ± 2.7 c | 104. 2 ± 5.9 b | 0.0001 | <0.0001 | <0.0001 |
| Energy intake, kJ/day | 434 ± 41 b | 401 ± 28 b | 423 ± 21 b | 636 ± 47 a | 595.8 ± 43 a | 642 ± 50 a | <0.0001 | 0.59 | 0.95 |
| Feed conversion efficiency, g/kJ | 0.09 ± 0.01 bc | 0.09 ± 0.01 bc | 0.06 ± 0.00 c | 0.14 ± 0. 01 a | 0.15 ± 0.01 a | 0.11 ± 0.01 b | <0.0001 | 0.0004 | 0.19 |
| Body weight gained (8–16 week), % | 11.8 ± 0.9 c | 9.63 ± 0.9 cd | 6.77 ± 0.7 d | 19.8 ± 1.3 a | 22.0 ± 1.1 a | 16.8 ± 1.4 b | <0.0001 | 0.0004 | 0.14 |
| Abdominal circumference, cm | 19.0 ± 0.3 b | 18.5 ± 0.1 b | 18.9 ± 0.2 b | 21.6 ± 0.3 a | 21.4 ± 0.4 a | 21.0 ± 0.3 a | <0.0001 | 0.37 | 0.22 |
| Body mass index, kg/m2 | 6.28 ± 0.13 b | 6.32 ± 0.21 b | 6.51 ± 0.13 b | 8.04 ± 0.21 a | 7.61 ± 0.18 a | 7.93 ± 0.21 a | <0.0001 | 0.36 | 0.43 |
| Retroperitoneal fat, mg/mm * | 276 ± 24 b | 252 ± 11 b | 234 ± 16 b | 565 ± 47 a | 517± 44 a | 525 ± 36 a | <0.0001 | 0.37 | 0.90 |
| Epididymal fat, mg/mm * | 99 ± 9 b | 71± 8 b | 75 ± 9 b | 180 ± 41 a | 193 ± 19 a | 143 ± 16 a | <0.0001 | 0.13 | 0.21 |
| Omental fat, mg/mm * | 160 ± 12 b | 122 ± 10 b | 118 ± 9 b | 255 ± 21 a | 256 ± 10 a | 231 ± 15 a | <0.0001 | 0.058 | 0.35 |
| Total abdominal fat, mg/mm * | 536 ± 39 b | 445 ± 24 b | 427 ± 26 b | 1000 ± 85 a | 965 ± 70 a | 902 ± 62 a | <0.0001 | 0.18 | 0.87 |
| Visceral adiposity index, % | 6.23 ± 0.38 b | 4.92 ± 0.22 b | 5.21 ± 0.29 b | 9.11 ± 0.47 a | 8.81 ± 0.53 a | 8.76 ± 0.40 a | <0.0001 | 0.09 | 0.43 |
| Liver weight, mg/mm * | 234 ± 8 cd | 246 ± 7 c | 214 ± 4 d | 355 ± 12 a | 357 ± 10 a | 320 ± 10 b | <0.0001 | 0.0008 | 0.68 |
| Plasma biochemistry | |||||||||
| Basal blood glucose (16 week), mmol/L | 3.9 ± 0.2 b | 3.8 ± 0.2 b | 3.9 ± 0.1 b | 4.7 ± 0.1 a | 4.6 ± 0.2 a | 4.7 ± 0.1 a | <0.0001 | 0.76 | 0.99 |
| Area under the curve (16 week), mmol/L·minute | 647 ± 26 b | 652 ± 18 b | 623 ± 18 b | 768 ± 30 a | 752 ± 15 a | 743 ± 17 a | <0.0001 | 0.80 | 0.81 |
| ALT, U/L | 29.6 ± 2.6 b | 31.3 ± 2.9 bc | 31.0 ± 1.0 bc | 44.0 ± 3.4 a | 45.5 ± 3.8 a | 36.1 ± 2.0 b | <0.0001 | 0.18 | 0.14 |
| AST, U/L | 73.6 ± 3.4 b | 81.4± 6.1 b | 74.0 ± 2.0 b | 104.6 ± 6.2 a | 92.3 ± 5.9 ab | 85.3 ± 3.0 b | <0.0001 | 0.10 | 0.046 |
| Total cholesterol, mmol/L | 1.40 ± 0.1 a | 1.53 ± 0.05 a | 1.45 ± 0.1 a | 1.60 ± 0.1 a | 1.59 ± 0.05 a | 1.60 ± 0.05 a | 0.0409 | 0.75 | 0.68 |
| Triglyceride, mmol/L | 0.62 ± 0.1 d | 0.74 ± 0.1 dc | 0.5 ± 0.1 d | 2.00 ± 0.2 a | 1.87 ± 0.28 a | 1.27 ± 0.2 bc | <0.0001 | 0.028 | 0.23 |
| NEFA, mmol/L | 1.27 ± 0.2 b | 1.23 ± 0.18 b | 1.5 ± 0.2 b | 3.74 ± 0.4 a | 3.73 ± 0.25 a | 3.99 ± 0.3 a | <0.0001 | 0.55 | 0.98 |
| Catalase activity, kU/L | 43.7 ± 7.6 b | 46.7 ± 6.7 b | 36.9 ± 2.3 b | 51.7 ± 5.58 a | 53.6 ± 7.7 a | 60.0 ± 5.86 a | 0.0166 | 0.9224 | 0.361 |
| Cardiovascular variables | |||||||||
| Systolic blood pressure (16 week), mmHg | 127 ± 2 b | 126 ± 3 b | 129 ± 2 b | 143 ± 3 a | 141 ± 5 a | 130 ± 3 b | <0.0001 | 0.16 | 0.029 |
| LV + Septum, mg/mm * | 22.8 ± 1.0 a | 22.3 ± 1.1 a | 21.9 ± 0.6 a | 23.0 ± 0.9 a | 23.4 ± 0.6 a | 22.8 ± 0.6 a | 0.28 | 0.76 | 0.85 |
| Right ventricle, mg/mm * | 4.73 ± 0.31 a | 5.01 ± 0.24 a | 4.95 ± 0.31 a | 5.57 ± 0.39 a | 5.61 ± 0.56 a | 4.76 ± 0.32 a | 0.17 | 0.46 | 0.35 |
| Diastolic stiffness constant (κ) | 22.1 ± 0.4 c | 21.6 ± 0.8 c | 20.9 ± 0.3 c | 27.0 ± 0.8 a | 24.7 ± 0.9 b | 22.6 ± 0.6 c | <0.0001 | 0.0004 | 0.034 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, O.D.; Wanyonyi, S.; Mouatt, P.; Panchal, S.K.; Brown, L. Achacha (Garcinia humilis) Rind Improves Cardiovascular Function in Rats with Diet-Induced Metabolic Syndrome. Nutrients 2018, 10, 1425. https://doi.org/10.3390/nu10101425
John OD, Wanyonyi S, Mouatt P, Panchal SK, Brown L. Achacha (Garcinia humilis) Rind Improves Cardiovascular Function in Rats with Diet-Induced Metabolic Syndrome. Nutrients. 2018; 10(10):1425. https://doi.org/10.3390/nu10101425
Chicago/Turabian StyleJohn, Oliver D., Stephen Wanyonyi, Peter Mouatt, Sunil K. Panchal, and Lindsay Brown. 2018. "Achacha (Garcinia humilis) Rind Improves Cardiovascular Function in Rats with Diet-Induced Metabolic Syndrome" Nutrients 10, no. 10: 1425. https://doi.org/10.3390/nu10101425





