The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
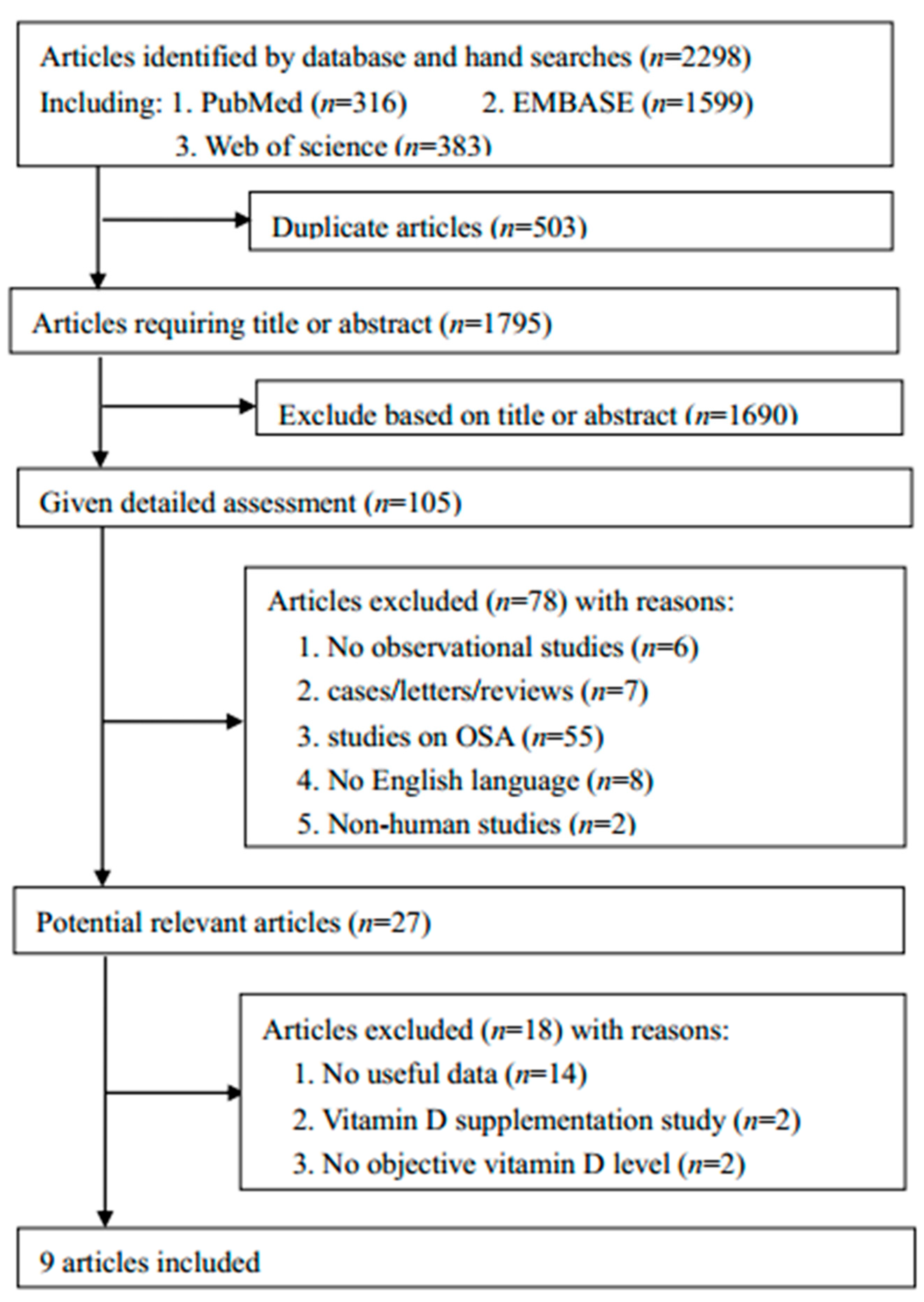
3.1. Literature Search and Study Characteristics
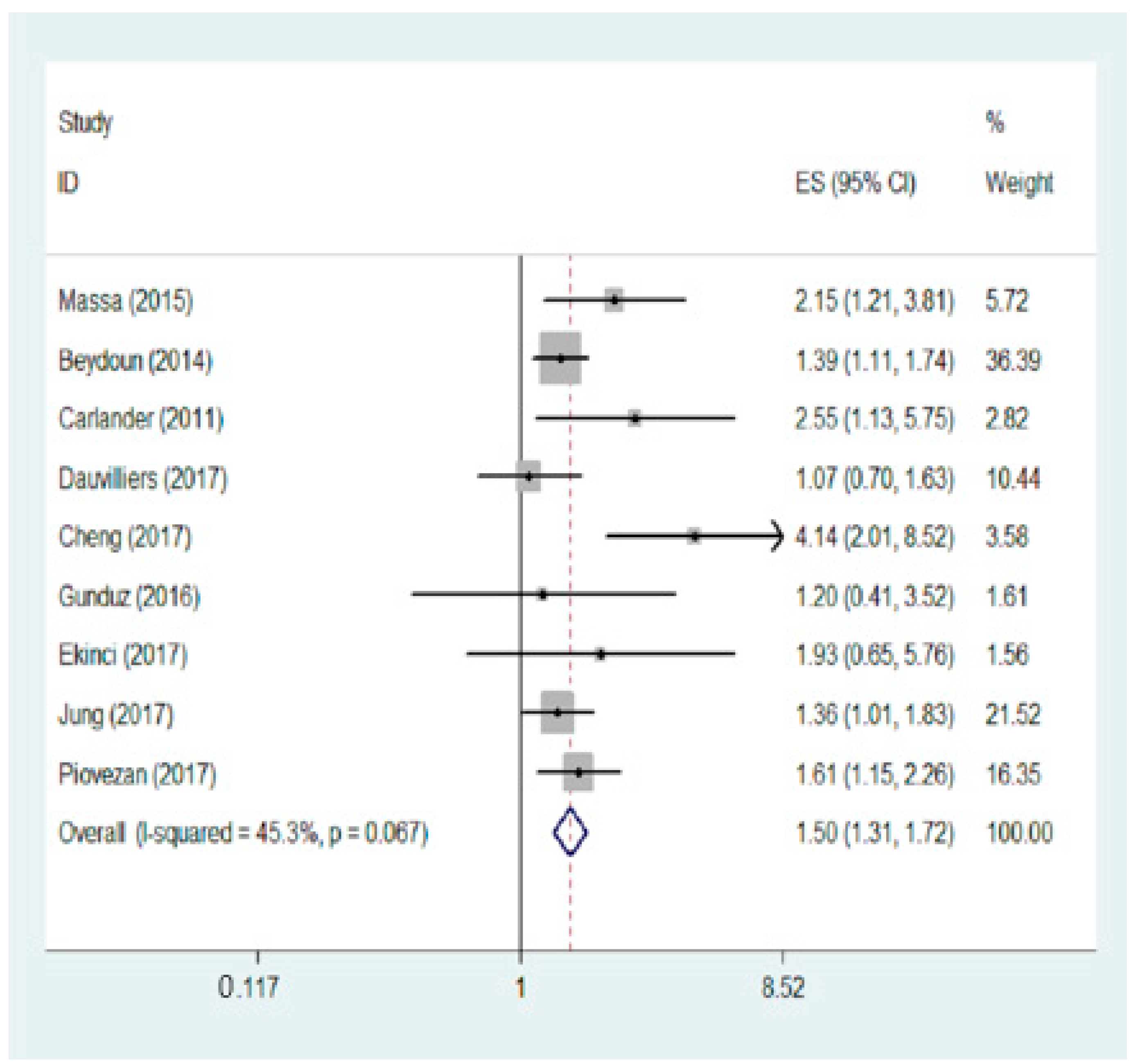
3.2. Meta-Analysis Result
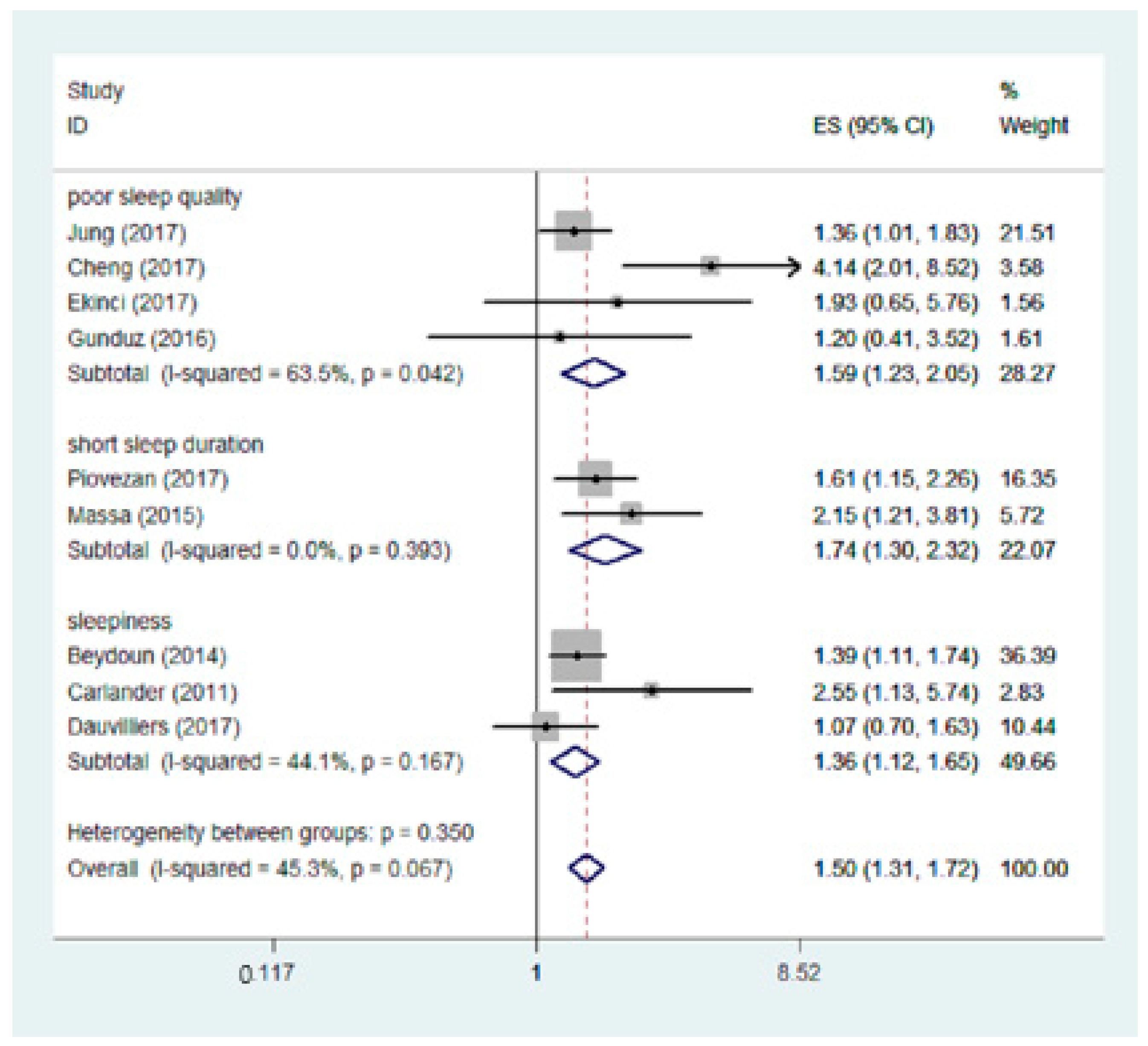
3.3. Subgroup Analysis
3.4. Sensitivity Analysis
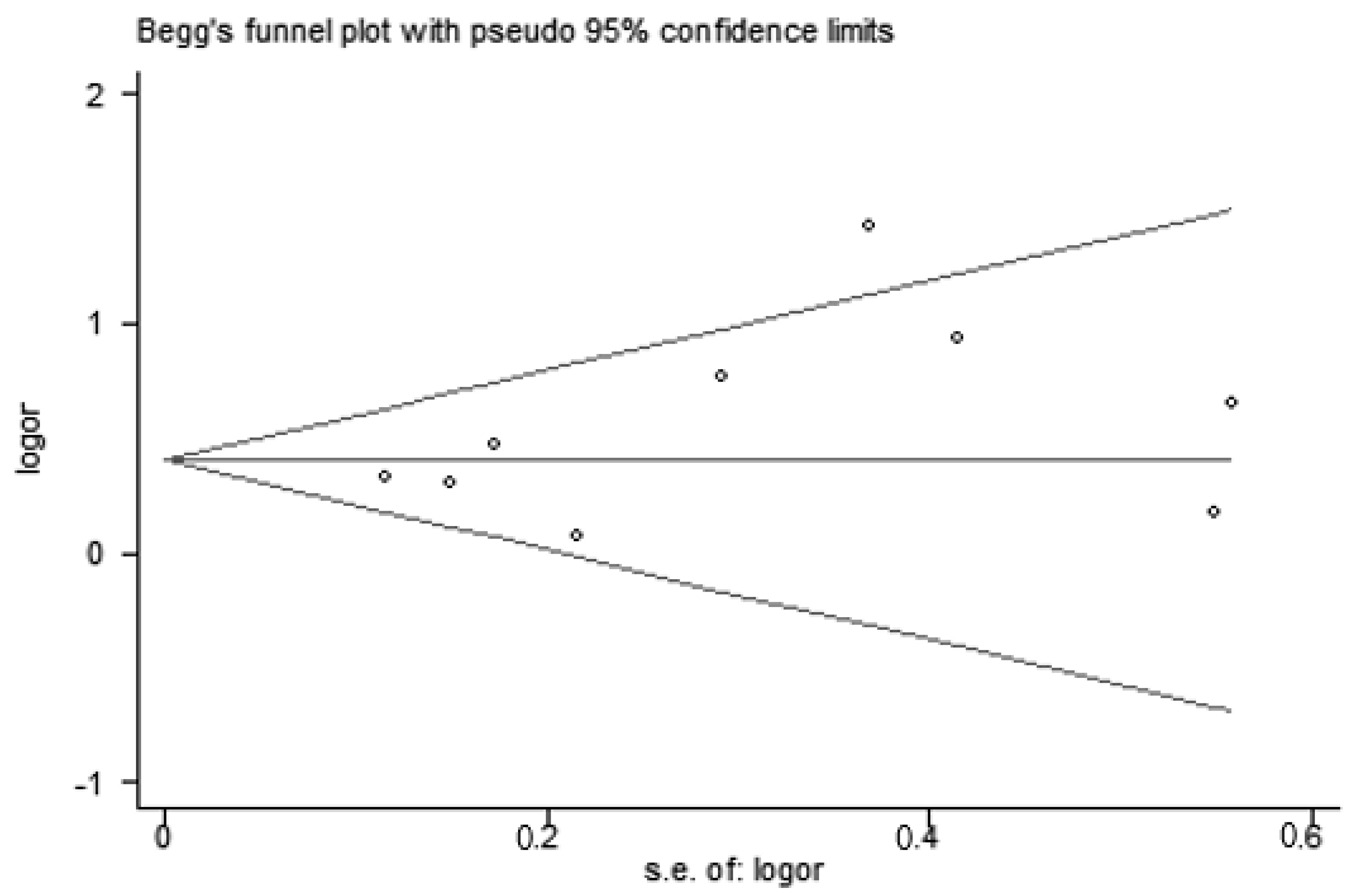
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dahl, R.E. The regulation of sleep and arousal: Development and psychopathology. Dev. Psychopathol. 1996, 8, 3–27. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; Doncarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L. National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Vitaterna, M.H.; Takahashi, J.S.; Turek, F.W. Overview of circadian rhythms. Alcohol. Res. Health 2001, 25, 85–93. [Google Scholar] [PubMed]
- Jones, B.E. Neurobiology of waking and sleeping. Handb. Clin. Neurol. 2011, 98, 131–149. [Google Scholar] [PubMed]
- Bhaskar, S.; Hemavathy, D.; Prasad, S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J. Fam. Med. Prim. Care 2016, 5, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Hublin, C.; Kaprio, J.; Partinen, M.; Koskenvuo, M. The epidemiology of narcolepsy in Finland. Duodecim Lääketieteellinen Aikakauskirja 2007, 30, 1141–1147. (In Finnish) [Google Scholar]
- Kerkhof, G.A. Epidemiology of sleep and sleep disorders in The Netherlands. Sleep Med. 2017, 30, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Colten, H.R.; Altevogt, B.M. Sleep disorders and sleep deprivation: An unmet public health problem. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 473–474. [Google Scholar]
- Aurora, R.N.; Kim, J.S.; Crainiceanu, C.; O’Hearn, D.; Punjabi, N.M. Habitual sleep duration and all-cause mortality in a general community sample. Sleep 2016, 39, 1903. [Google Scholar] [CrossRef] [PubMed]
- Avidan, A.Y.; Fries, B.E.; James, M.L.; Szafara, K.L.; Wright, G.T.; Chervin, R.D. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J. Am. Geriatr. Soc. 2005, 53, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Laberge, L.; Gagnon, C.; Dauvilliers, Y. Daytime sleepiness and myotonic dystrophy. Curr. Neurol. Neurosci. Rep. 2013, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Bulathsinghala, P.; Syrigos, K.N.; Saif, M.W. Role of Vitamin D in the Prevention of Pancreatic Cancer. J. Nutr. Metab. 2010, 2010, 721365. [Google Scholar] [CrossRef] [PubMed]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. From vitamin D to hormone d: Fundamentals of the vitamin d endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S. [Google Scholar] [CrossRef] [PubMed]
- Mozos, I.; Marginean, O. Links between vitamin d deficiency and cardiovascular diseases. BioMed Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin d deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, D.E.; Chesson, A.L., Jr.; Jain, S.K.; Marino, A.A. The link between vitamin d metabolism and sleep medicine. Sleep Med. Rev. 2014, 18, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Liu, P.Y.; Josh, P.; Cui, X. Intracellular distribution of the vitamin d receptor in the brain: Comparison with classic target tissues and redistribution with development. Neuroscience 2014, 268, 1. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, W.E.; O’Brien, L.P. 1,25(OH) 2 vitamin D 3 sites of action in the brain. Histochemistry 1987, 87, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Massa, J.; Stone, K.L.; Wei, E.K.; Harrison, S.L.; Barrett-Connor, E.; Lane, N.E.; Paudel, M.; Redline, S.; Ancoli-Israel, S.; Orwoll, E.; et al. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: The mros sleep study. Sleep 2015, 38, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, D.E.; Reddy, A.; Keigley, Q.; Kim, P.Y.; Marino, A.A. Vitamin D, race, and excessive daytime sleepiness. J. Clin. Sleep Med. 2012, 8, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Smyth, C. The epworth sleepiness scale (ESS). Medsurg. Nurs. 2009, 18, 134. [Google Scholar] [PubMed]
- Sato-Mito, N.; Shibata, S.; Sasaki, S.; Sato, K. Dietary intake is associated with human chronotype as assessed by both morningness-eveningness score and preferred midpoint of sleep in young Japanese women. Int. J. Food Sci. Nutr. 2011, 62, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep symptoms associated with intake of specific dietary nutrients. J. Sleep Res. 2014, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013, 64, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, S.; Kosger, H.; Aldemir, S.; Akcal, B.; Tevrizci, H.; Hizli, D.; Celik, H.T. Sleep deprivation in the last trimester of pregnancy and inadequate vitamin D: Is there a relationship? J. Chin. Med. Assoc. 2016, 79, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P. Bias in meta-analysis detected by a simple, graphical test. Prospectively identified trials could be used for comparison with meta-analyses. BMJ 1998, 316, 471. [Google Scholar] [PubMed]
- Beydoun, M.A.; Gamaldo, A.A.; Canas, J.A.; Beydoun, H.A.; Shah, M.T.; McNeely, J.M.; Zonderman, A.B. Serum nutritional biomarkers and their associations with sleep among us adults in recent national surveys. PLoS ONE 2014, 9, e103490. [Google Scholar] [CrossRef] [PubMed]
- Carlander, B.; Puech-Cathala, A.M.; Jaussent, I.; Scholz, S.; Bayard, S.; Cochen, V.; Dauvilliers, Y. Low vitamin D in narcolepsy with cataplexy. PLoS ONE 2011, 6, e20433. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.S.; Loy, S.L.; Cheung, Y.B.; Cai, S.; Colega, M.T.; Godfrey, K.M.; Chong, Y.; Tan, K.H.; Shek, L.P.; Lee, Y.S.; et al. Plasma vitamin d deficiency is associated with poor sleep quality and night-time eating at mid-pregnancy in Singapore. Nutrients 2017, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Dauvilliers, Y.; Evangelista, E.; Lopez, R.; Barateau, L.; Scholz, S.; Crastes de Paulet, B.; Carlander, B.; Jaussent, I. Vitamin D deficiency in type 1 narcolepsy: A reappraisal. Sleep Med. 2017, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, R.M.K.; Balci, S.; Serbes, M.; Dogruel, D.; Altintas, D.U.; Yilmaz, M. Decreased serum vitamin b12 and vitamin D levels affect sleep quality in children with familial Mediterranean fever. Rheumatol. Int. 2017, 38, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Chae, C.H.; Kim, Y.O.; Son, J.S.; Kim, C.W.; Park, H.O.; Lee, J.H.; Shin, Y.H.; Kwak, H.S. The relationship between serum vitamin D levels and sleep quality in fixed day indoor field workers in the electronics manufacturing industry in Korea. Ann. Occup. Environ. Med. 2017, 29, 25. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, R.D.; Hirotsu, C.; Feres, M.C.; Cintra, F.D.; Andersen, M.L.; Tufik, S.; Poyares, D. Obstructive sleep apnea and objective short sleep duration are independently associated with the risk of serum vitamin D deficiency. PLoS ONE 2017, 12, e0180901. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Dietary reference intakes for calcium and vitamin d. Pediatrics 2010, 130, 1427. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin d deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K. Vitamin d effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin d functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Stumpf, W.E.; Sar, M.; Clark, S.A.; Deluca, H.F. Brain target sites for 1,25-dihydroxyvitamin d. Science 1982, 215, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Musiol, I.; Stumpf, W.; Bidmon, H.; Heiss, C.; Mayerhofer, A.; Bartke, A. Vitamin d nuclear binding to neurons of the septal, substriatal and amygdaloid area in the Siberian hamster (Phodopus sungorus) brain. Neuroscience 1992, 48, 841–848. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin d deficiency. Nederlands Tijdschrift Voor Geneeskunde 2007, 150, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Hashemipour, S.; Hajmanuchehri, F.; Kazemifar, A.M. Is vitamin d deficiency associated with non specific musculoskeletal pain? Glob. J. Health Sci. 2013, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.; Wilson, A.; Stocks, N.; Moulding, N. Muscle pain as an indicator of vitamin d deficiency in an urban Australian aboriginal population. Med. J. Aust. 2006, 185, 76–77. [Google Scholar] [PubMed]
- Heath, K.M.; Elovic, E.P. Vitamin d deficiency: Implications in the rehabilitation setting. Am. J. Phys. Med. Rehabil. 2006, 85, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, A.; Abdelnasser, A.M.; Hamdy, A.; Omran, A.A.; Elrehany, M.A. Hypovitaminosis d in female patients with chronic low back pain. Clin. Rheumatol. 2007, 26, 1895. [Google Scholar] [CrossRef] [PubMed]
- Plotnikoff, G.A.; Quigley, J.M. Prevalence of severe hypovitaminosis d in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin. Proc. 2003, 78, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Comment on: Hypovitaminosis d among rheumatology outpatients in clinical practice: Reply. Rheumatology 2009, 48, 1348–1351. [Google Scholar] [CrossRef]
- Toujani, S.; Kaabachi, W.; Mjid, M.; Hamzaoui, K.; Cherif, J.; Beji, M. Vitamin d deficiency and interleukin-17 relationship in severe obstructive sleep apnea-hypopnea syndrome. Ann. Thorac. Med. 2017, 12, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Okura, K.; Lavigne, G.J.; Huynh, N.; Manzini, C.; Fillipini, D.; Montplaisir, J.Y. Comparison of sleep variables between chronic widespread musculoskeletal pain, insomnia, periodic leg movements syndrome and control subjects in a clinical sleep medicine practice. Sleep Med. 2008, 9, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007, 30, 1145. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, T. Elevated levels of c-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003, 107, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A. Vitamin d in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Shah, S.; Long, Q.; Crankshaw, A.K.; Tangpricha, V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin d supplementation. Clin. J. Pain 2013, 29, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.L.; Chonchol, M.; Pierce, G.L.; Walker, A.E.; Seals, D.R. 25-hydroxyvitamin d deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 2011, 57, 63. [Google Scholar] [CrossRef] [PubMed]
- David Feldman, M.D.; Krishnan, A.; Moreno, J.; Swami, S.; Peehl, D.M.; Sandy Srinivas, M.D. Vitamin d inhibition of the prostaglandin pathway as therapy for prostate cancer. Nutr. Rev. 2007, 65, 113–115. [Google Scholar] [CrossRef]
- Krueger, J.M.; Majde, J.A.; Rector, D.M. Cytokines in immune function and sleep regulation. Handb. Clin. Neurol. 2011, 98, 229. [Google Scholar] [PubMed]
- Barcelo, A.; de la Pena, M.; Barbe, F.; Pierola, J.; Bosch, M.; Agusti, A.G. Prostaglandin d synthase (beta trace) levels in sleep apnea patients with and without sleepiness. Sleep Med. 2007, 8, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Shahi, M.M.; Hosseini, S.A.; Helli, B.; Haghighyzade, M.H.; Abolfathi, M. The effect of vitamin d supplement on quality of sleep in adult people with sleep disorders. Tehran. Univ. Med. J. 2017, 75, 443–448. [Google Scholar]
| Search Terms |
|---|
| #1 sleep [Mesh Terms] |
| #2 sleep duration * OR sleep quality * OR sleep disorders * OR short sleep OR hypersomnia OR sleep OR sleep time OR Short-term sleep restriction OR daytime sleepiness OR long sleepers OR short sleepers OR sleep initiation and maintenance disorders OR habitual short sleepers OR sleep deprivation OR nap OR napping OR sleep disturbance OR sleep disorders OR siesta OR sleep time OR drowse OR insomnia OR drowsiness OR 24-h sleep duration OR night time sleep duration OR short sleep duration OR long sleep duration |
| #3 #1 OR #2 |
| #4 vitamin D [Mesh Terms] |
| #5 vitamin D analogues OR doxercalciferol OR alfacalcidol OR vitamin D3 OR vitamin D2 OR activated vitamin D OR 1alpha-vitamin D OR calcitriol OR calcidiol OR 1,25dihydroxycholecalciferol OR 25-hydroxyvitamin D2 OR calcifediol OR 1,25OH2D OR dihydrotachysterol OR ergocalciferols OR 25OHD OR Vit D OR 25-hydroxy vitamin D2 OR VitD OR vitamin D-3 OR 25-hydroxycholecalciferol OR 25OHD OR 25-hydroxy-vitamin D OR ergocalciferol OR 1,25-dihydroxyvitamin D3 OR 25-OH vitamin D OR cholecalciferol OR 25-hydroxyvitamin D |
| #6 #4 OR #5 |
| #7 #3 and #6 |
| Author, Year, Country | Study Design | Sample Size (Age;% Female) | Sleep Measurement | 25(OH)D Cutoffs ng/mL | Sleep Characteristic | Vitamin D Measurement | Adjusted Variable | NOS |
|---|---|---|---|---|---|---|---|---|
| Cheng, 2017 [33], Singapore | cohort | 1152 (≥18, 100%) | PSQI | <20 | Sleep quality | ID-LC–MS/MS | ethnicity, age, early pregnancy BMI, education, household income, parity, night shift, status, physical activity, total EPDS score, and gestational weight gain per week. | 9 |
| 20–32 | ||||||||
| >32 | ||||||||
| Ekinci, 2017 [35], Turkey | cross-sectional | 63 (3–16; 47.6%) | PSQI | <20 | Sleep quality | HPLC | NO | 7 |
| ≥20 | ||||||||
| Gunduz, 2016 [26], Turkey | cross-sectional | 92 (18–45; 100%) | PSQI | <20 | Sleep quality | HPLC | NO | 6 |
| 20–32 | ||||||||
| >32 | ||||||||
| Jung, 2017 [36], Korea | cross-sectional | 1472 (19–39; 20%) | PSQI | <10 | Sleep quality | ECLIA | age, sex, marital status, level of education, BMI, smoking habits, alcohol consumption habits, regular exercise, employee tenure, occupational stress | 7 |
| >10 | ||||||||
| Massa, 2015 [20], USA | cross-sectional | 3048 (≥65; 0%) | wrist actigraphy | <20 | Sleep duration | LC-MS/MS | age, clinic, season, comorbidities, BMI, physical and cognitive function. | 7 |
| 20–30 | ||||||||
| 30–40 | ||||||||
| ≥40 | ||||||||
| Piovezan, 2017 [37], Brazil | cross-sectional | 657 (28–78; 56%) | polysomnography | <30 | Sleep duration | CMIA | age, gender, ethnicity, obesity, smoking, hypertension, diabetes, sedentary lifestyle, seasonality, creatinine serum levels | 7 |
| >30 | ||||||||
| Beydoun, 2014 [31], USA | cross-sectional | 2459 (20–80; 52%) | sleep questionnaire | <20 | Sleepiness | HPLC | age, sex, race/ethnicity, education, marital status, and family income | 6 |
| ≥20 | ||||||||
| Carlander, 2011 [32], France | case-control | 106 (16–65; 60%) | poly-somnography ESS | <30 | Sleepiness (NC) | RIA | age at onset, duration and severity of disease at baseline, treatment intake at time of study, season of blood sampling | 6 |
| >30 | ||||||||
| Dauvilliers, 2017 [34], France | case-control | 348 (6–68; 35%) | ESS; AESS | <30 | Sleepiness (NC) | RIA | age, BMI, and season of blood sampling | 8 |
| >30 |
| Group | Number | OR | 95% CI | p Value | I2 (%) | |
|---|---|---|---|---|---|---|
| All | 9 | 1.50 | 1.31, 1.72 | <0.001 | 45.3% | |
| Study design | cross-sectional | 6 | 1.47 | 1.27, 1.71 | <0.001 | 0.0% |
| cohort | 1 | 4.14 | 2.01, 8.52 | 0.02 | ||
| case-control | 2 | 1.29 | 0.89, 1.87 | 0.18 | 71.1% | |
| Sample size | <1000 | 5 | 1.46 | 1.15, 1.86 | 0.002 | 12.9% |
| ≥1000 | 4 | 1.52 | 1.29, 1.79 | <0.001 | 69.9% | |
| Sleep characteristic | Poor sleep quality | 4 | 1.59 | 1.23,2.05 | <0.001 | 63.5% |
| Short sleep duration | 2 | 1.74 | 1.30, 2.32 | <0.001 | 0.0% | |
| Sleepiness | 3 | 1.36 | 1.12, 1.65 | 0.002 | 44.1% | |
| Vitamin D cut off | 10 ng/mL | 1 | 1.36 | 1.01, 1.83 | 0.04 | |
| 20 ng/mL | 5 | 1.59 | 1.31, 1.94 | <0.001 | 58.2% | |
| 30 ng/mL | 4 | 1.46 | 1.13, 1.87 | 0.003 | 52.5% | |
| Geographic location | Asia | 4 | 1.59 | 1.23, 2.05 | <0.001 | 63.5% |
| European | 2 | 1.51 | 1.26, 1.81 | 0.18 | 71.0% | |
| America | 3 | 1.29 | 0.89, 1.87 | <0.001 | 5.8% |
| Group | Number | OR | 95% CI | p | I2 (%) |
|---|---|---|---|---|---|
| All | 9 | 1.50 | 1.31, 1.72 | <0.001 | 45.3% |
| Exclude unadjusted | 7 | 1.63 | 1.28, 2.07 | <0.001 | 57.9% |
| Exclude only men | 8 | 1.47 | 1.28, 1.69 | <0.001 | 46.2% |
| Exclude only women | 7 | 1.45 | 1.26, 1.67 | <0.001 | 9.4% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Kou, T.; Zhuang, B.; Ren, Y.; Dong, X.; Wang, Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1395. https://doi.org/10.3390/nu10101395
Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2018; 10(10):1395. https://doi.org/10.3390/nu10101395
Chicago/Turabian StyleGao, Qi, Tingyan Kou, Bin Zhuang, Yangyang Ren, Xue Dong, and Qiuzhen Wang. 2018. "The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis" Nutrients 10, no. 10: 1395. https://doi.org/10.3390/nu10101395
APA StyleGao, Q., Kou, T., Zhuang, B., Ren, Y., Dong, X., & Wang, Q. (2018). The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients, 10(10), 1395. https://doi.org/10.3390/nu10101395





