Endogenous Omega (n)-3 Fatty Acids in Fat-1 Mice Attenuated Depression-Like Behavior, Imbalance between Microglial M1 and M2 Phenotypes, and Dysfunction of Neurotrophins Induced by Lipopolysaccharide Administration
Abstract
1. Introduction
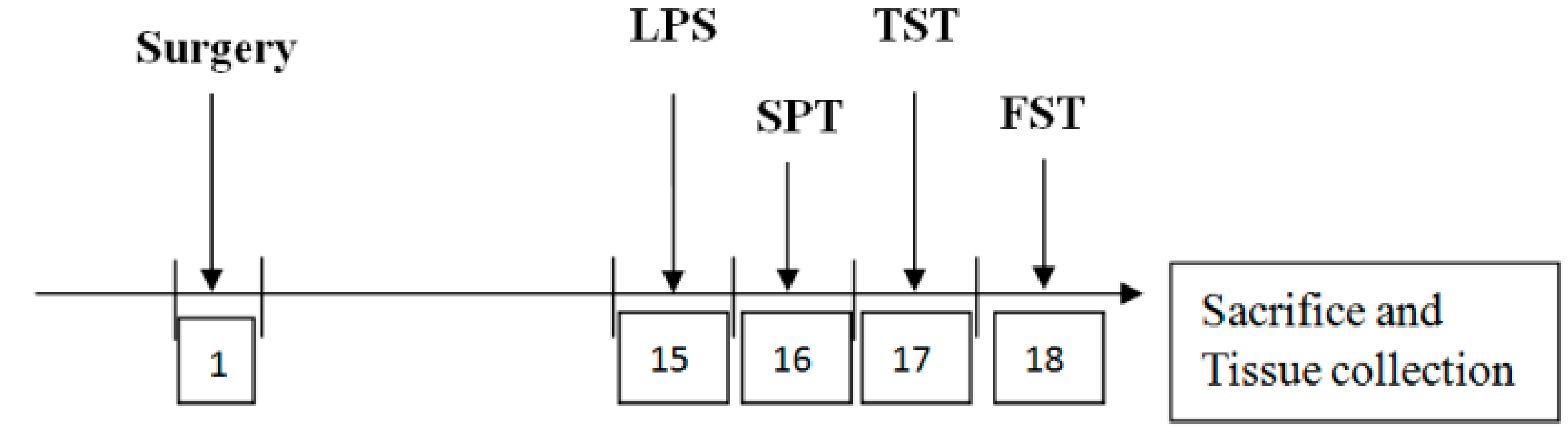
2. Materials and Methods
2.1. Animals
2.2. Genotyping
2.3. ICV Saline or LPS Injection
2.4. Behavioral Tests
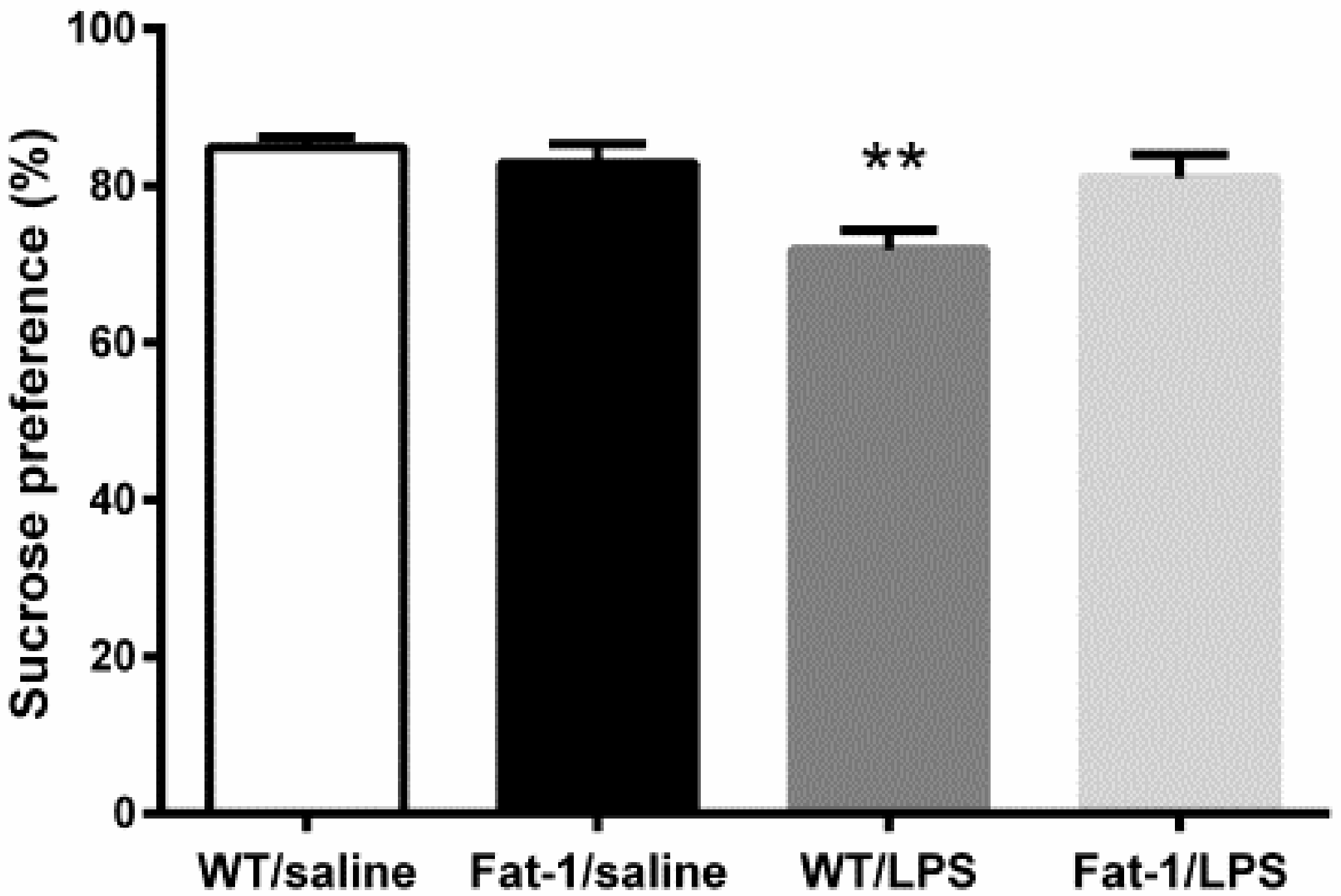
2.4.1. Sucrose Preference Test
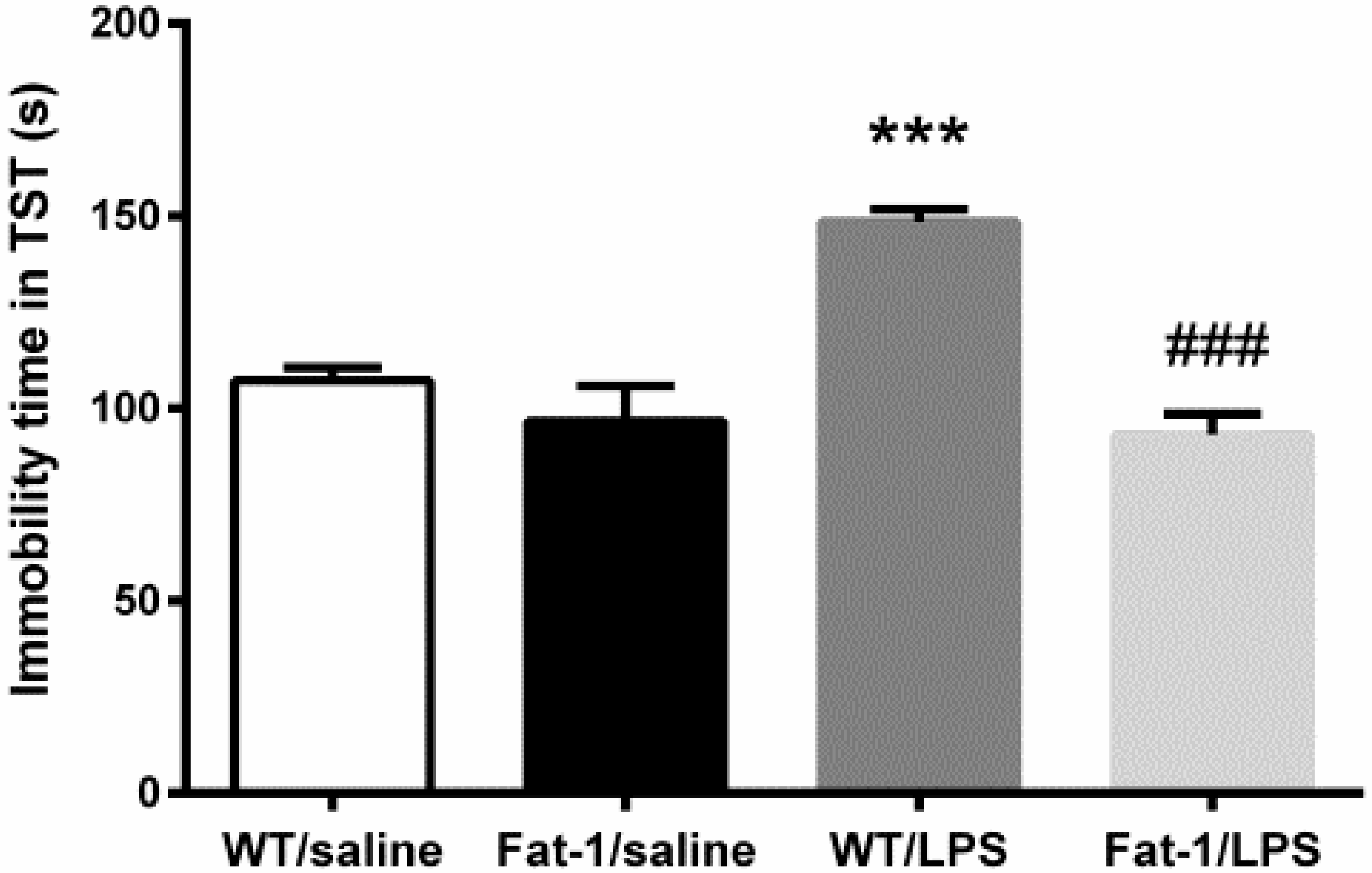
2.4.2. Tail Suspension Test
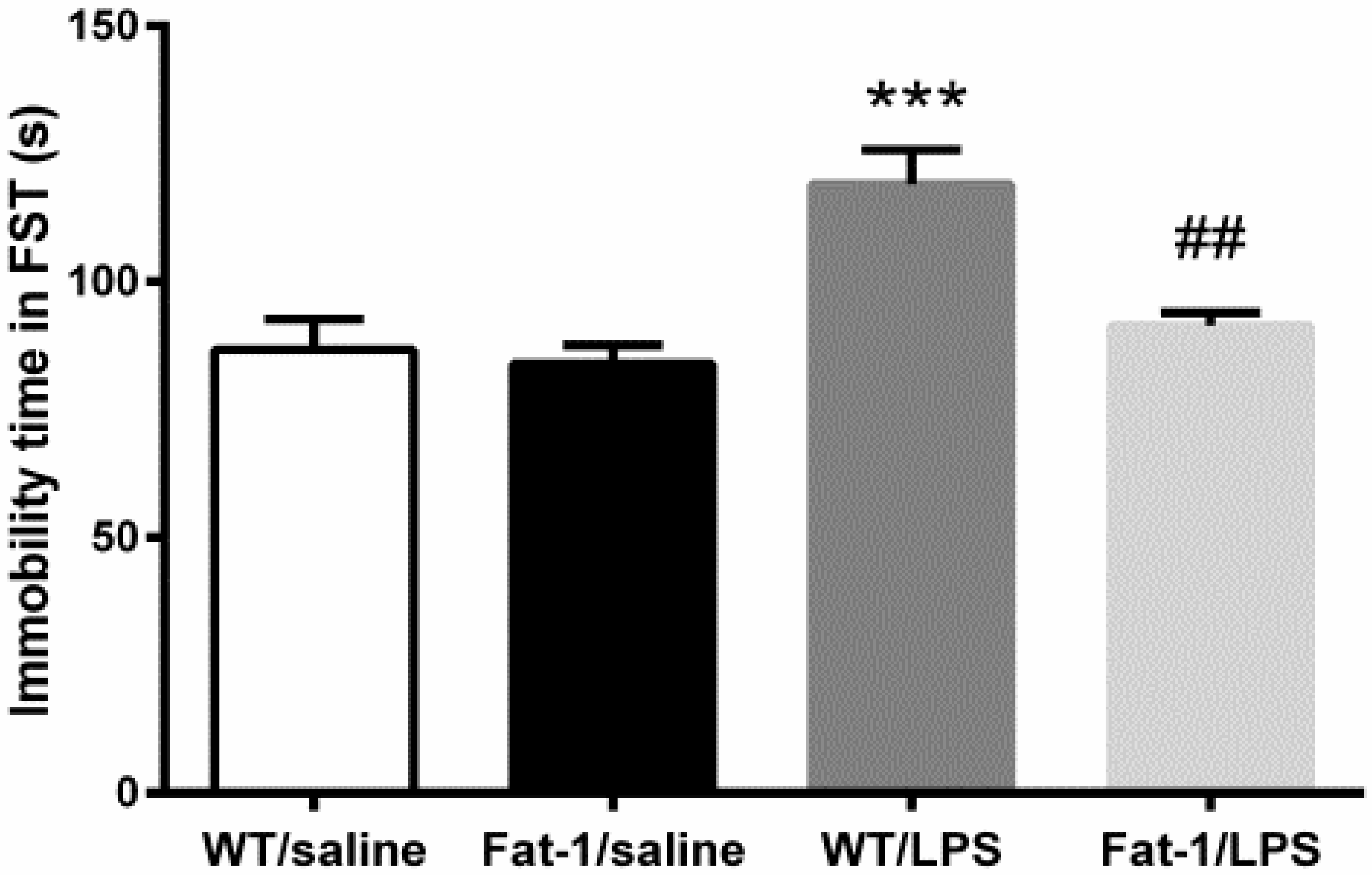
2.4.3. Forced Swimming Test
2.5. Fatty Acid Analysis in the Brain
2.6. ELISA Assays
2.7. Real-Time PCR
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. Fatty Acid Profile in the Brain
3.2. Depressive-Like Behaviors Induced by LPS Were Attenuated in the Fat-1 Mice
3.3. LPS-Induced M1 Polarization and Cytokine Changes in the Hippocampus
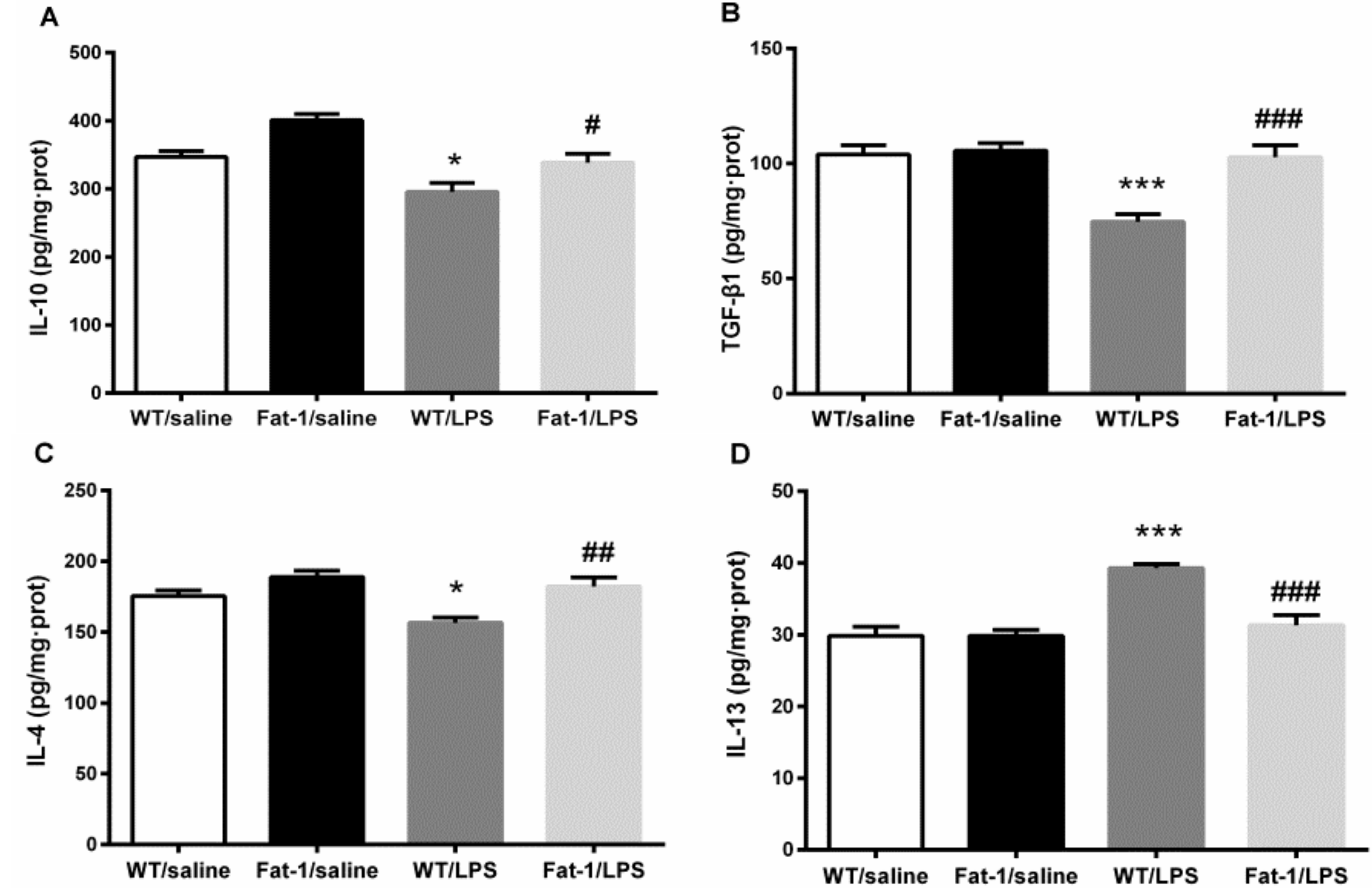
3.4. The Effect of LPS on M2 Polarization and Related Factors in the Hippocampus
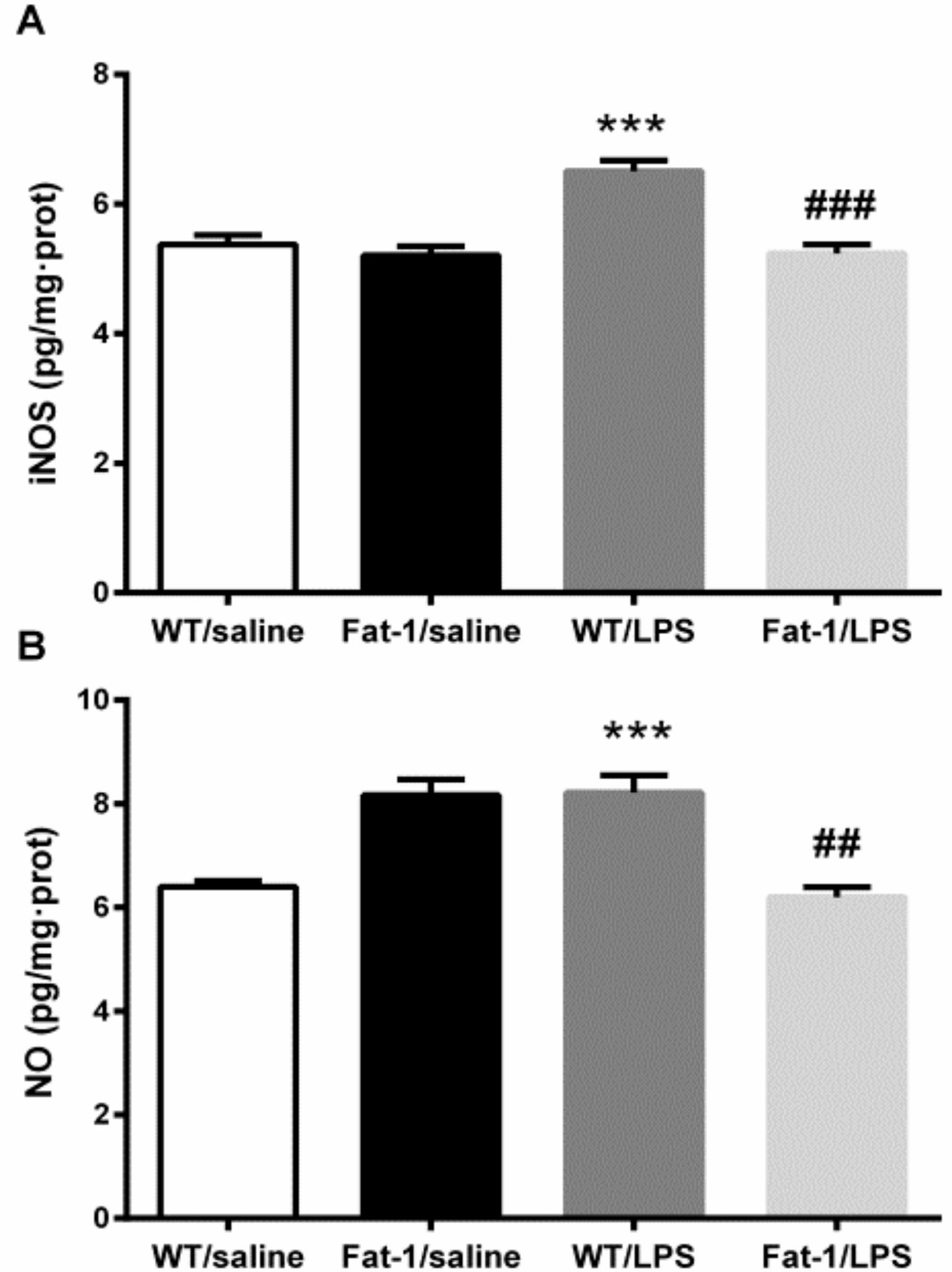
3.5. Oxidative Stress-Related Nitric Oxide Enzyme iNOS and NO Levels
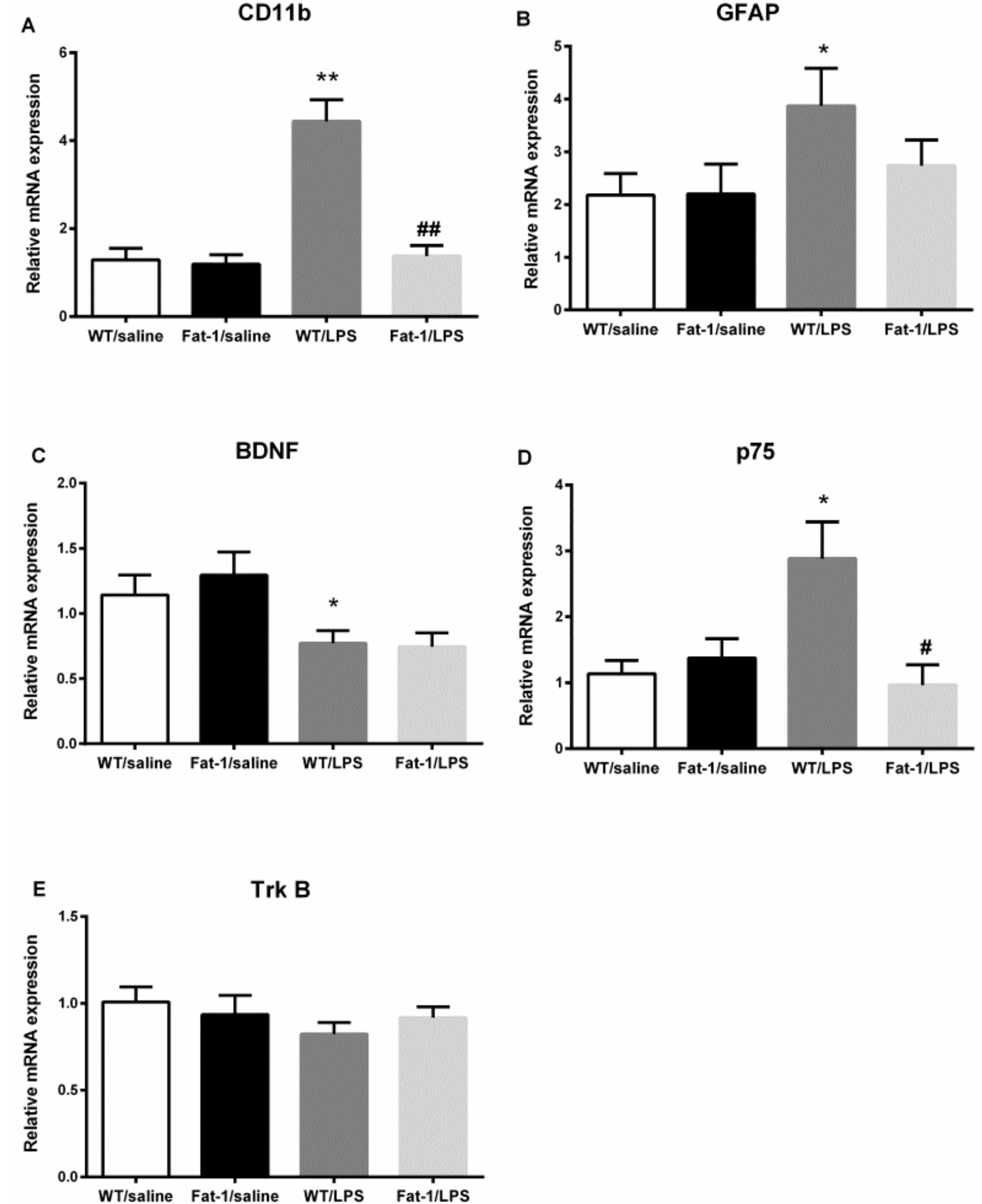
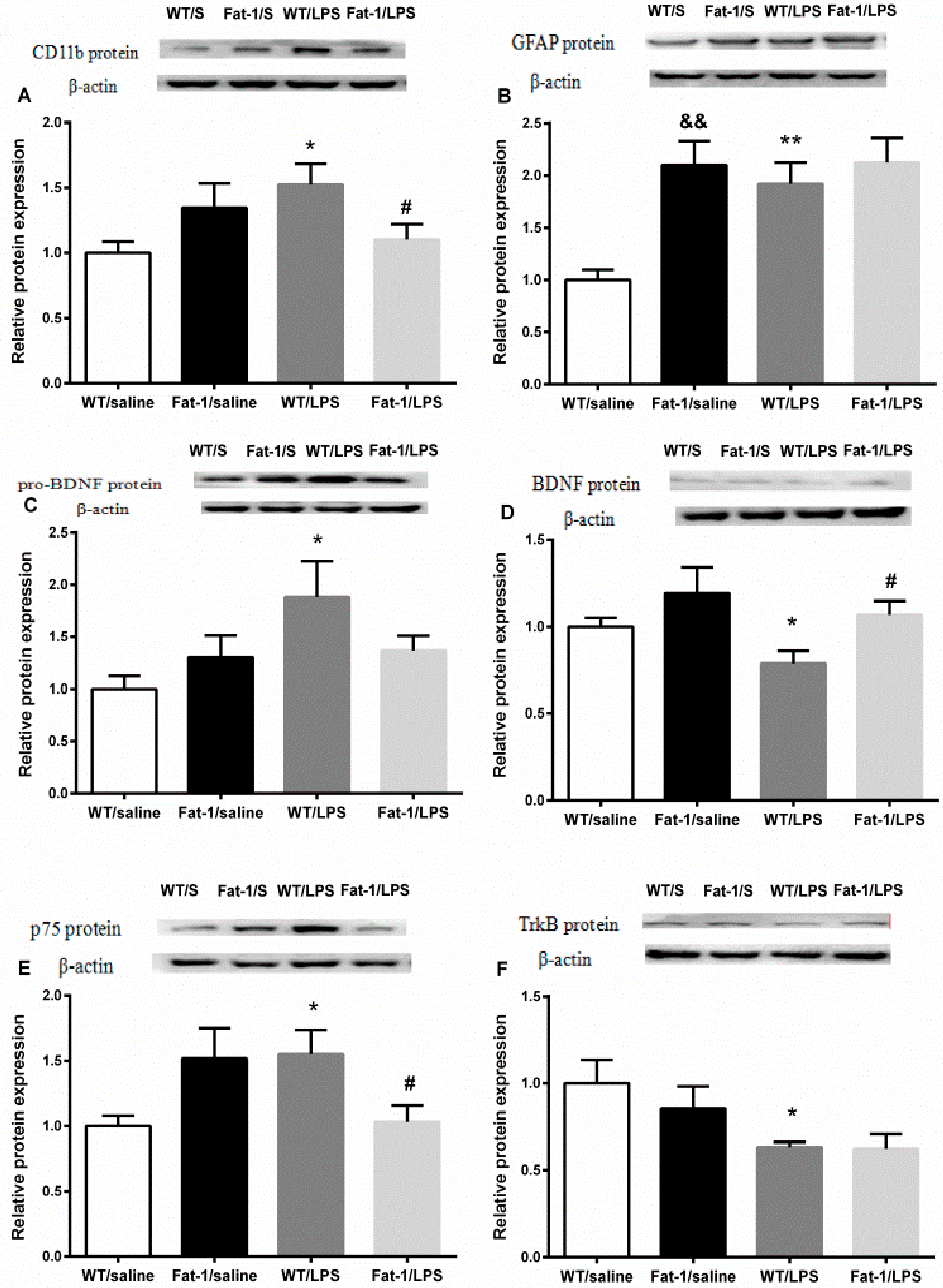
3.6. Expression of Neurotrophins and Their Receptors in the Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henry, C.J.; Yan, H.; Wynne, A.; Hanke, M.; Himler, J.; Bailey, M.T.; Sheridan, J.F.; Godbout, J.P. Minocycline attenuates lipopolysaccharide (lps)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflamm. 2008, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S. The macrophage theory of depression. Med. Hypotheses 1991, 35, 298–306. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, W.H.; Suk, K. Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem. Pharmacol. 2016, 103, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Baba, A.; Matsuda, T. Astrocyte apoptosis: Implications for neuroprotection. Prog. Neurobiol. 2004, 72, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Perez-Urrutia, N.; Mendoza, C.; Alvarez-Ricartes, N.; Oliveros-Matus, P.; Echeverria, F.; Grizzell, J.A.; Barreto, G.E.; Iarkov, A.; Echeverria, V. Intranasal cotinine improves memory, and reduces depressive-like behavior, and gfap+ cells loss induced by restraint stress in mice. Exp. Neurol. 2017, 295, 211. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Lawson, M.C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, T.; Ullrich, C.; Sperner-Unterweger, B.; Humpel, C. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience 2011, 184, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.; Keshavarz, S.A.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Amini, H.; Chamari, M.; Djazayery, A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010, 178, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kanada, S.; Takenaka, M.; Hamazaki, T. Dietary n-3 fatty acids selectively attenuate lps-induced behavioral depression in mice. Physiol. Behav. 2004, 81, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, M.; Gibrat, C.; Saint-Pierre, M.; Julien, C.; Calon, F.; Cicchetti, F. Modulation of brain-derived neurotrophic factor as a potential neuroprotective mechanism of action of omega-3 fatty acids in a parkinsonian animal model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Langenbach, R.; Bosetti, F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Boil. 2008, 22, 1491. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Wang, J.; Wu, L.; Kang, Z.B. Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004, 427, 504. [Google Scholar] [CrossRef] [PubMed]
- Linge, R.; Pazos, Á.; Díaz, Á. Social isolation differentially affects anxiety and depressive-like responses of bulbectomized mice. Behav. Brain Res. 2013, 245, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Mocaër, E.; Porsolt, R. Antidepressant-like activity of s 20098 (agomelatine) in the forced swimming test in rodents: Involvement of melatonin and serotonin receptors. J. Psychiatry Neurosci. Jpn. 2004, 29, 126–133. [Google Scholar] [PubMed]
- Meng, Q.; Luchtman, D.W.; El Bahh, B.; Zidichouski, J.A.; Yang, J.; Song, C. Ethyl-eicosapentaenoate modulates changes in neurochemistry and brain lipids induced by Parkinsonian neurotoxin 1-methyl-4-phenylpyridinium in mouse brain slices. Eur. J. Pharmacol. 2010, 649, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Taepavarapruk, P.; Song, C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: Effects of omega-3 fatty acid epa treatment. J. Neurochem. 2010, 112, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Pan, F. The effects of egb761 on lipopolysaccharide-induced depressive-like behaviour in c57bl/6j mice. Exp. Immunol. 2015, 40, 11–17. [Google Scholar] [CrossRef]
- Kalkman, H.O.; Feuerbach, D. Antidepressant therapies inhibit inflammation and microglial m1-polarization. Pharmacol. Ther. 2016, 163, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Yi, H.; Xu, L.; Zhang, Z. Fluoxetine and s-citalopram inhibit m1 activation and promote m2 activation of microglia in vitro. Neuroscience 2015, 294, 60. [Google Scholar] [CrossRef] [PubMed]
- Schiepers, O.J.; Wichers, M.C.; Maes, M. Cytokines and major depression. Prog. Neuro-Psychopharmacol. Boil. Psychiatry 2005, 29, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Bluthe, R.; Bristow, A.; Lestage, J.; Imbs, C.; Dantzer, R. Central injection of interleukin-13 potentiates lps-induced sickness behavior in rats. Neuroreport 2001, 12, 3979. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Ji, K.A.; Jeon, S.B.; Jin, B.K.; Kim, S.U.; Jou, I.; Joe, E. Interleukin-13 enhances cyclooxygenase-2 expression in activated rat brain microglia: Implications for death of activated microglia. J. Immunol. 2006, 177, 1323. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Jope, R.S.; Beurel, E. A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress. BMC Neurosci. 2015, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Lichtblau, N.; Minkwitz, J.; Chittka, T.; Thormann, J.; Kirkby, K.C.; Sander, C.; Mergl, R.; Faßhauer, M.; Stumvoll, M. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J. Psychiatr. Res. 2014, 55, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Chibana, K.; Trudeau, J.B.; Mustovich, A.T.; Hu, H.; Zhao, J.; Balzar, S.; Chu, H.W.; Wenzel, S.E. Il-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin. Exp. Allergy 2008, 38, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, J.; Mostert, J.P.; Koch, M.W. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J. Neurol. Sci. 2008, 267, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Friedman, W.J.; Dreyfus, C.F. Differential regulation of neurotrophin expression in basal forebrain astrocytes by neuronal signals. J. Neurosci. Res. 2004, 76, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Dantzer, R.; Kelley, K.W.; Mccusker, R.H. Central administration of insulin-like growth factor-i decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflamm. 2011, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ide, M.; Shibutani, T.; Ohtaki, H.; Numazawa, S.; Shioda, S.; Yoshida, T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J. Neurosci. Res. 2006, 83, 557. [Google Scholar] [CrossRef] [PubMed]
- Woo, N.H.; Teng, H.K.; Siao, C.J.; Chiaruttini, C.; Pang, P.T.; Milner, T.A.; Hempstead, B.L.; Lu, B. Activation of p75ntr by probdnf facilitates hippocampal long-term depression. Nat. Neurosci. 2005, 8, 1069. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Park, K.J.; Hong, E.J.; Kramer, B.M.; Greenberg, M.E.; Kaplan, D.R.; Miller, F.D. Developmental axon pruning mediated by bdnf-p75ntr-dependent axon degeneration. Nat. Neurosci. 2008, 11, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Dora, B.; Adelaide, F. Neuroinflammation and depression: Microglia activation, extracellular microvesicles and microrna dysregulation. Front. Cell. Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef]
- Larrieu, T.; Hilal, M.L.; Fourrier, C.; De Smedt-Peyrusse, V.; Sans, N.; Capuron, L.; Layé, S. Nutritional omega-3 modulates neuronal morphology in the prefrontal cortex along with depression-related behaviour through corticosterone secretion. Transl. Psychiatry 2014, 4, e437. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.G.D.; Souza, L.C.; Goes, A.R.; Fabbro, L.D.; Filho, C.B.; Donato, F.; Prigol, M.; Luchese, C.; Roman, S.S.; Puntel, R.L. Fish oil ameliorates sickness behavior induced by lipopolysaccharide in aged mice through the modulation of kynurenine pathway. J. Nutr. Biochem. 2018, 58, 37. [Google Scholar] [CrossRef] [PubMed]
- De Mello, A.H.; Gassenferth, A.; Schraiber, R.B.; Souza, L.R.; Florentino, D.; Danielski, L.G.; Cittadinsoares, E.C.; Fortunato, J.J.; Petronilho, F.; Quevedo, J. Effects of omega-3 on behavioral and biochemical parameters in rats submitted to chronic mild stress. Metab. Brain Dis. 2014, 29, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Trocha, M.; Szandruk, M.; Słupski, W.; Rymaszewska, J. Effects of supplementation with fish oil and n-3 pufas enriched egg yolk phospholipids on anhedonic-like response and body weight in the rat chronic mild stress model of depression. Die Pharmazie 2013, 68, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Lei, J.; Sun, X.; Liu, G.; Zhao, S. Stress-induced anhedonia correlates with lower hippocampal serotonin transporter protein expression. Brain Res. 2013, 1513, 127. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. N-3 pufa supplementation benefits microglial responses to myelin pathology. Sci. Rep. 2014, 4, 7458. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Ibrahim, A.; Mbodji, K.; Coëffier, M.; Ziegler, F.; Bounoure, F.; Chardigny, J.M.; Skiba, M.; Savoye, G.; Déchelotte, P. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating nf-κb in rats with tnbs-induced colitis. J. Nutr. 2010, 140, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Williamsbey, Y.; Boularan, C.; Vural, A.; Huang, N.N.; Hwang, I.Y.; Chong, S.S.; Kehrl, J.H. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting nf-κb activation and enhancing autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef]
- Bousquet, M.; Gue, K.; Emond, V.; Julien, P.; Kang, J.X.; Cicchetti, F. Transgenic conversion of omega-6 into omega-3 fatty acids in a mouse model of Parkinson’s disease. J. Lipid Res. 2011, 52, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, G.D.; Liang, Z.; Vitale, A.; Jiao, X.Y.; Ju, G.; You, S.W. In vitro beneficial activation of microglial cells by mechanically-injured astrocytes enhances the synthesis and secretion of bdnf through p38mapk. Neurochem. Int. 2012, 61, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Savli, H.; Gulkac, M.D.; Esen, N. The effect of stimulated microglia conditioned media on bdnf gene expression of striatal astrocytes: Quantification by real-time PCR. Int. J. Neurosci. 2004, 114, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Min, X.; Kalueff, A.V.; Cai, S. Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates bdnf function in a rodent model of neuroinflammation induced by central interleukin-1β administration. Eur. J. Nutr. 2017, 57, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.F.; Bernardi, J.R.; Bosa, V.L.; Schuch, I.; Goldani, M.Z.; Kapczinski, F.; Salum, G.A.; Dalmaz, C.; Manfro, G.G.; Silveira, P.P. Correlation between n-3 polyunsaturated fatty acids consumption and bdnf peripheral levels in adolescents. Lipids Health Dis. 2014, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Ertley, R.N.; Lee, H.J.; DeMar, J.C., Jr.; Arnold, J.T.; Rapoport, S.I.; Bazinet, R.P. N-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex bdnf via a p38 mapk-dependent mechanism. Mol. Psychiatry 2007, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize bdnf levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.G.; Hu, Y.; Hua, Y.; Hu, M.; Luo, C.X.; Han, X.; Zhu, X.J.; Wang, B.; Xu, J.S.; Zhu, D.Y. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J. Neurochem. 2010, 103, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Joca, S.R.; Guimarães, F.S. Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology 2006, 185, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Matsumiya, M.; Matsuura, T.; Oishi, M.; Kaibori, M.; Okumura, T.; Nishizawa, M.; Takada, H.; Kwon, A. Peroxidation of n-3 polyunsaturated fatty acids inhibits the induction of inos gene expression in proinflammatory cytokine-stimulated hepatocytes. J. Nutr. Metab. 2011, 2011, 374542. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Chen, M.T.; Zhang, R.; Zhang, Y.; Li, W.; Li, Y.G. Docosahexaenoic acid attenuates doxorubicin-induced cytotoxicity and inflammation by suppressing nf-κb/inos/no signaling pathway activation in h9c2 cardiac cells. J. Cardiovasc. Pharmacol. 2016, 67, 1. [Google Scholar] [CrossRef] [PubMed]

| Genes | Primer | Sequence |
|---|---|---|
| CD11b | Forward | 5′-CCCATGACCTTCCAAGAGAA-3′ |
| Reverse | 5′-AGAGGGCACCTGTCTGGTTA-3′ | |
| BDNF | Forward | 5′-AGCTGAGCGTGTGTGACAGT-3′ |
| Reverse | 5′-TCAGTTGGCCTTTGGATACC-3′ | |
| Trk B | Forward | 5′-CACACACAGGGCTCCTTA-3′ |
| Reverse | 5′-GTCAGCTCAAGCCAGACACA-3′ | |
| p75 | Forward | 5′-CCGATGCTCCTATGGCTACT-3′ |
| Reverse | 5′-CTCTGGGCACTCTTCACACA-3′ | |
| GFAP | Forward | 5′-GAAAGGTTGAATCGCTGGAG-3′ |
| Reverse | 5′-GCCACTGCCTCGTATTGAGT-3′ | |
| â-actin | Forward | 5′-GTCGTACCACTGGCATTGTG-3′ |
| Reverse | 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
| Fatty Acids mg/g Tissue | Saline | LPS | Statistical Effects | ||||
|---|---|---|---|---|---|---|---|
| WT N = 11 | Fat-1 N = 11 | WT N = 11 | Fat-1 N = 10 | LPS | Genotype | LPS × Genotype | |
| EPA (20:5 n-3) | 0.17 ± 0.03 | 0.33 ± 0.05 * | 0.18 ± 0.04 | 0.27 ± 0.03 | NS | <0.01 | NS |
| DPA (22:5 n-3) | 0.19 ± 0.04 | 0.27 ± 0.03 | 0.23 ± 0.04 | 0.29 ± 0.05 | NS | NS | NS |
| DHA (22:6 n-3) | 2.65 ± 0.07 | 3.08 ± 0.05 *** | 2.76 ± 0.04 | 3.15 ± 0.10 ## | NS | <0.001 | NS |
| Subtotal n-3 | 3.01 ± 0.11 | 3.59 ± 0.13 ** | 3.18 ± 0.69 | 3.71 ± 0.11 ## | NS | <0.001 | NS |
| LA (18:2 n-6) | 0.38 ± 0.03 | 0.52 ± 0.05 | 0.50 ± 0.06 | 0.48 ± 0.05 | NS | NS | NS |
| DGLA (20:3 n-6) | 0.12 ± 0.01 | 0.16 ± 0.02 * | 0.015 ± 0.01 | 0.16 ± 0.01 | NS | <0.05 | NS |
| AA (20:4 n-6) | 1.56 ± 0.05 | 1.55 ± 0.06 | 1.60 ± 0.04 | 1.58 ± 0.08 | NS | NS | NS |
| Subtotal n-6 | 2.06 ± 0.05 | 2.21 ± 0.07 | 2.26 ± 0.07 | 2.19 ± 0.08 | NS | NS | NS |
| n-3/n-6 | 1.46 ± 0.04 | 1.68 ± 0.06 | 1.42 ± 0.07 | 1.68 ± 0.03 # | NS | <0.001 | NS |
| AA/DHA | 0.59 ± 0.01 | 0.51 ± 0.02 ** | 0.58 ± 0.01 | 0.50 ± 0.02 ## | NS | <0.001 | NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, M.; Li, Y.; Tang, H.; Zhang, C.; Li, W.; Zhang, Y.; Li, Y.; Zhao, Y.; Song, C. Endogenous Omega (n)-3 Fatty Acids in Fat-1 Mice Attenuated Depression-Like Behavior, Imbalance between Microglial M1 and M2 Phenotypes, and Dysfunction of Neurotrophins Induced by Lipopolysaccharide Administration. Nutrients 2018, 10, 1351. https://doi.org/10.3390/nu10101351
Gu M, Li Y, Tang H, Zhang C, Li W, Zhang Y, Li Y, Zhao Y, Song C. Endogenous Omega (n)-3 Fatty Acids in Fat-1 Mice Attenuated Depression-Like Behavior, Imbalance between Microglial M1 and M2 Phenotypes, and Dysfunction of Neurotrophins Induced by Lipopolysaccharide Administration. Nutrients. 2018; 10(10):1351. https://doi.org/10.3390/nu10101351
Chicago/Turabian StyleGu, Minqing, Yuyu Li, Haiting Tang, Cai Zhang, Wende Li, Yongping Zhang, Yajuan Li, Yuntao Zhao, and Cai Song. 2018. "Endogenous Omega (n)-3 Fatty Acids in Fat-1 Mice Attenuated Depression-Like Behavior, Imbalance between Microglial M1 and M2 Phenotypes, and Dysfunction of Neurotrophins Induced by Lipopolysaccharide Administration" Nutrients 10, no. 10: 1351. https://doi.org/10.3390/nu10101351
APA StyleGu, M., Li, Y., Tang, H., Zhang, C., Li, W., Zhang, Y., Li, Y., Zhao, Y., & Song, C. (2018). Endogenous Omega (n)-3 Fatty Acids in Fat-1 Mice Attenuated Depression-Like Behavior, Imbalance between Microglial M1 and M2 Phenotypes, and Dysfunction of Neurotrophins Induced by Lipopolysaccharide Administration. Nutrients, 10(10), 1351. https://doi.org/10.3390/nu10101351





