Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Study Eligibility Criteria
2.4. Study Quality Assessment and Data Extraction
2.5. Statistical Analysis
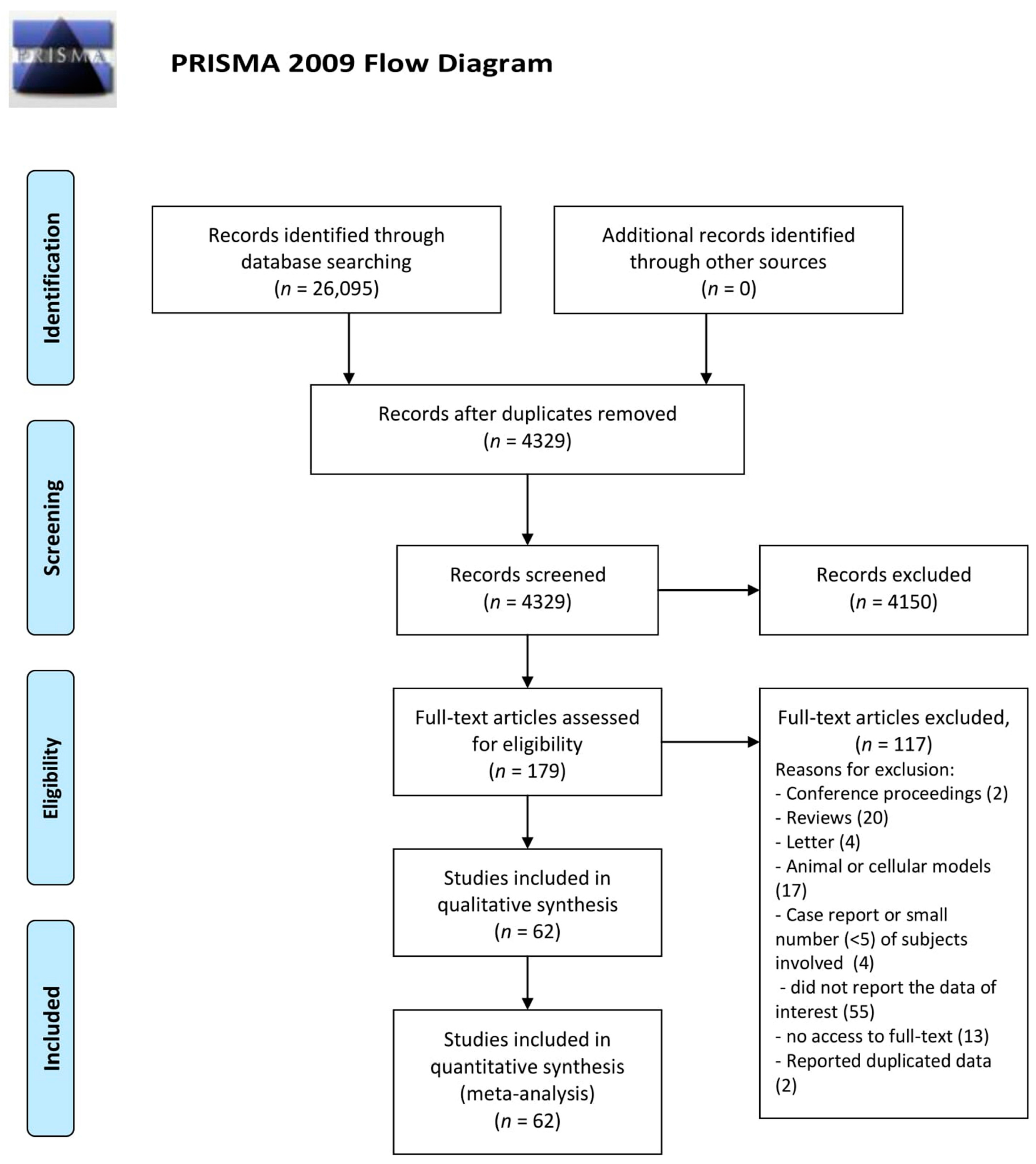
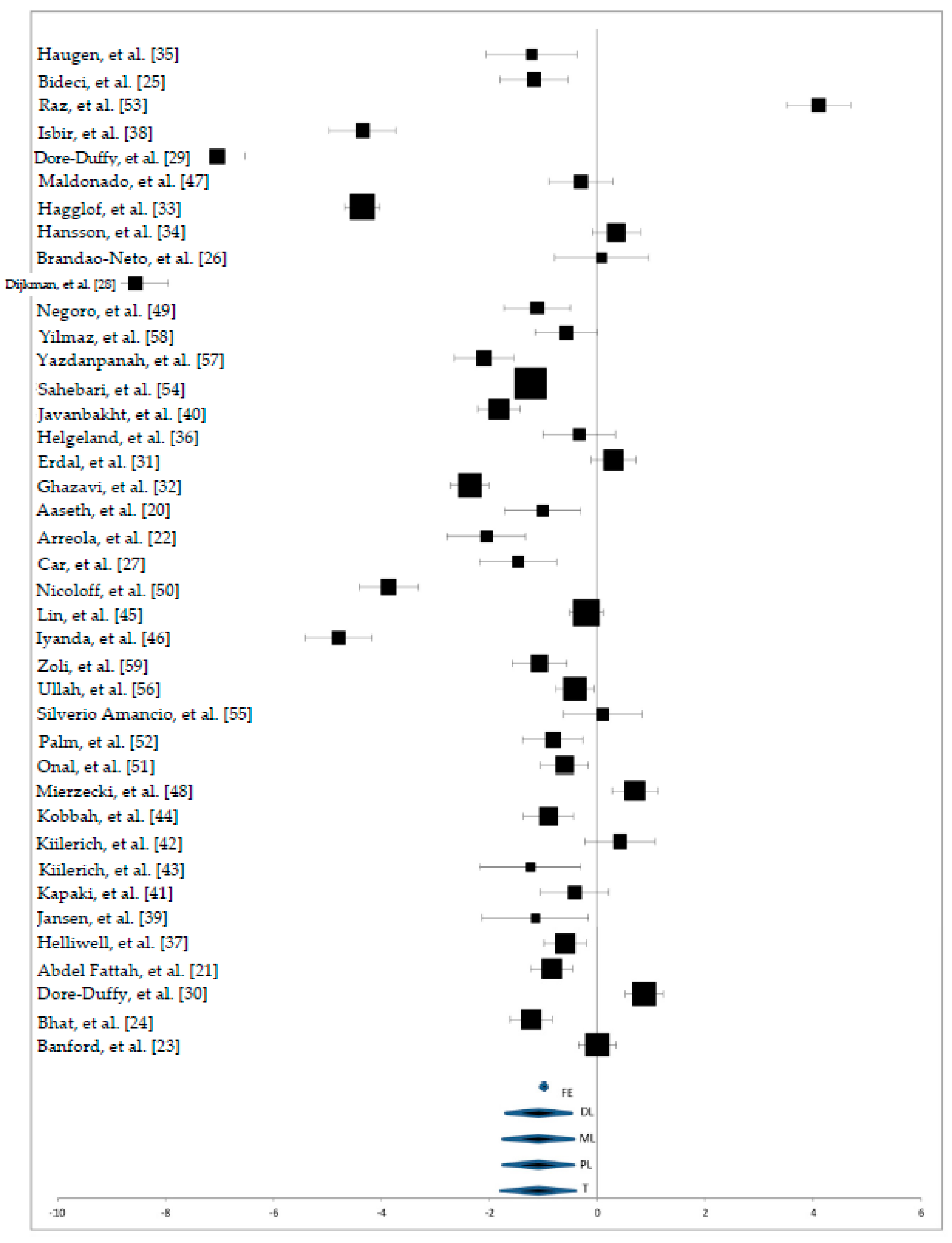
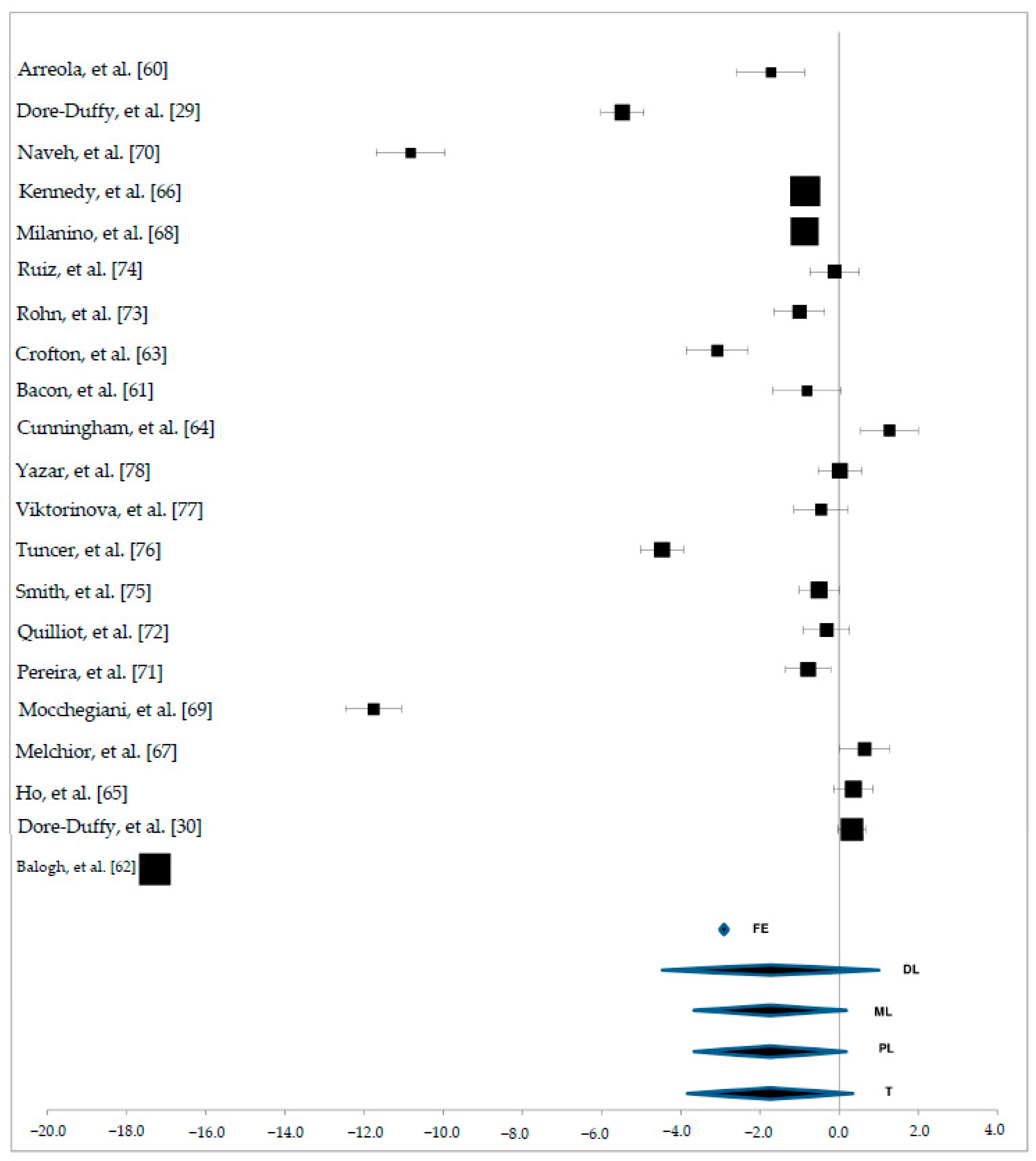
3. Results
3.1. Zn Status and Autoimmune Diseases
3.2. Zn Status in Serum Samples
3.3. Zn Status in Plasma Samples
3.4. Zn Status in Hair, Urine and Cerebrospinal Fluid Samples
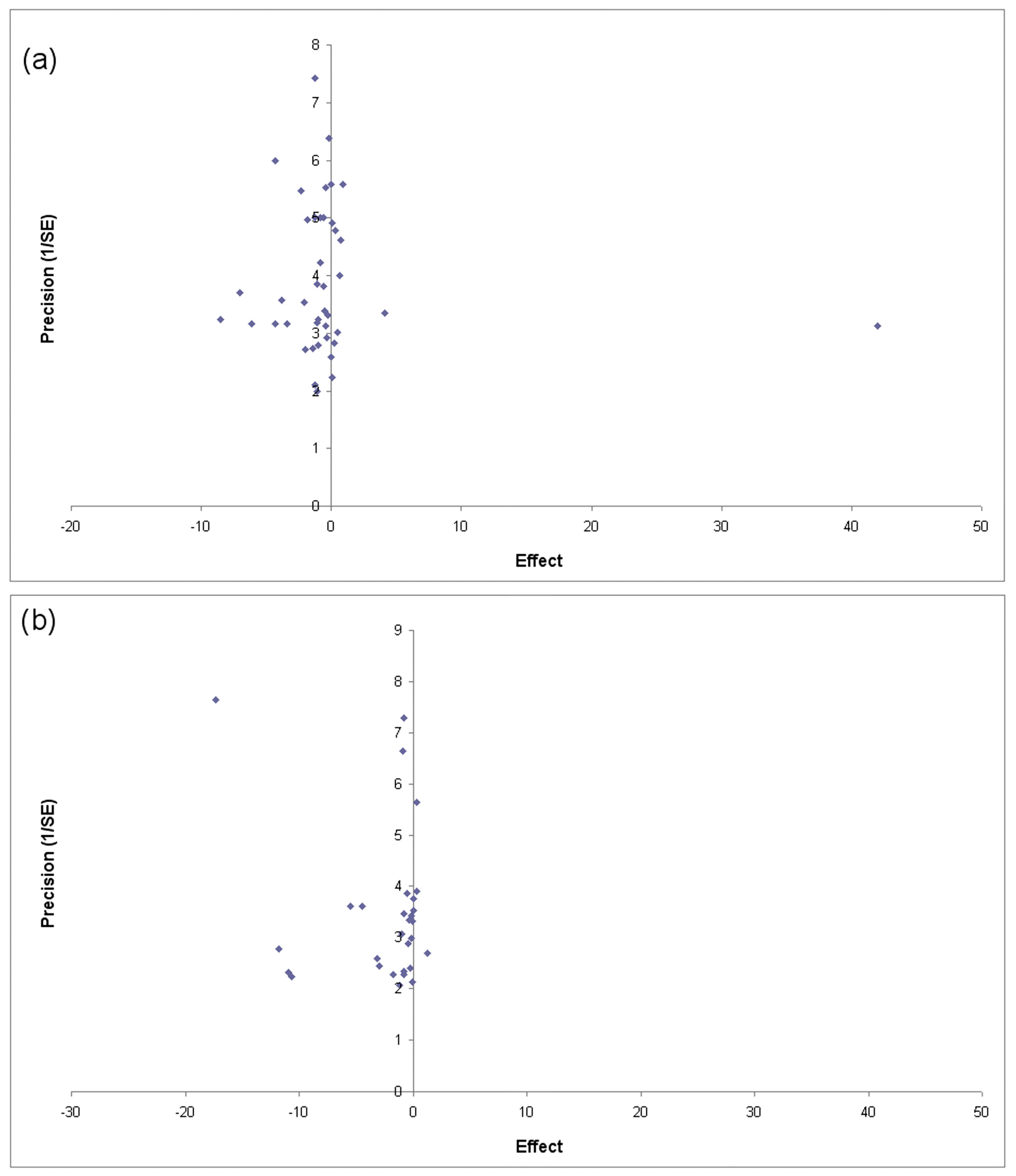
3.5. Publication Bias
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raulin, J. Etude chimique sur la vegetation. Ann. Sci. Nat. Bot. Biol. Veg. 1869, 11, 293–299. [Google Scholar]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D. Cellular transporter for zinc. Nutr. Rev. 2002, 60, 121–124. [Google Scholar] [PubMed]
- Foster, M.; Samman, S. Zinc and Regulation of inflammatory cytokines: Implications for cardiometabolic disease. Nutrients 2012, 4, 676–694. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Wuehler, S.E.; Peerson, J.M.; Brown, K.H. Use of national food balance data to estimate the adequacy of zinc in national food supplies: Methodology and regional estimates. Public Health Nutr. 2005, 8, 812–819. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: http://www.who.int/whr/2002 (accessed on 3 July 2017).
- Chandel, G.; Datta, K.; Datta, S.K. Detection of genomic changes in transgenic Bt rice populations through genetic fingerprinting using amplified fragment length polymorphism (AFLP). GM Crops 2010, 1, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Küry, S.; Dréno, B.; Bézieau, S.; Giraudet, S.; Kharfi, M.; Kamoun, R.; Moisan, J.P. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002, 31, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Wapnir, R.A. Zinc deficiency, malnutrition and the gastrointestinal tract. J. Nutr. 2000, 130, 1388–1392. [Google Scholar]
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J. Nutr. 2007, 137, 135–141. [Google Scholar] [PubMed]
- Prasad, A.S. Clinical manifestations of zinc deficiency. Annu. Rev. Nutr. 1985, 5, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale-Jardieu, P.; Fraker, P.J. Interference in the development of a secondary immune response in mice by zinc deprivation: Persistence of effects. J. Nutr. 1984, 114, 1762–1769. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ 2009, 6, 354–391. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- American Autoimmune Related Diseases Association. Available online: https://www.aarda.org/diseaselist/ (accessed on 3 July 2017).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Munthe, E.; Forre, O.; Steinnes, E. Trace elements in serum and urine of patients with rheumatoid arthritis. Scand. J. Rheumatol. 1978, 7, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, N.S.A.; Atef, M.M.; Al-Qaradaghi, S.M.Q. Evaluation of serum zinc level in patients with newly diagnosed and resistant alopecia areata. Int. J. Dermatol. 2016, 55, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Arreola, F.; Paniagua, R.; Díaz-Bensussen, S.; Urquieta, B.; López-Montaño, E.; Partida-Hernández, G.; Villalpando, S. Bone mineral content, 25-hydroxycalciferol and zinc serum levels in insulin-dependent (type I) diabetic patients. Arch. Investig. Med. 1990, 21, 195–199. [Google Scholar]
- Banford, J.C.; Brown, D.H.; Hazelton, R.A.; McNeil, C.J.; Sturrock, R.D.; Smith, W.E. Serum copper and erythrocyte superoxide dismutase in rheumatoid arthritis. Ann. Rheum. Dis. 1982, 41, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.J.; Manzoor, S.; Khan, A.R.; Qayoom, S. Trace element levels in alopecia areata. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Bideci, A.; Camurdan, M.O.; Cinaz, P.; Dursun, H.; Demirel, F. Serum zinc, insulin-like growth factor-I and insulin-like growth factor binding protein-3 levels in children with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2005, 18, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Brandão-Neto, J.; da Silva, C.A.; Figueiredo, N.B.; Shuhama, T.; da Cunha, N.F.; Dourado, F.B.; Naves, L.A. Lack of acute zinc effects in glucose metabolism in healthy and insulin-dependent diabetes mellitus patients. Biometals 1999, 12, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Car, N.; Car, A.; Granić, M.; Skrabalo, Z.; Momcilović, B. Zinc and copper in the serum of diabetic patients. Biol. Trace Elem. Res. 1992, 32, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Dijkmans, B.A.; van der Voet, G.B.; Cats, A.; de Wolff, F.A. Serum aluminium concentrations in patients with rheumatoid arthritis. Scand. J. Rheumatol. 1987, 16, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Dore-Duffy, P.; Peterson, M.; Catalanotto, F.; Marlow, S.; Ho, S.Y.; Ostrom, M.; Weinstein, A. Zinc profiles in rheumatoid arthritis. Clin. Exp. Rheumatol. 1990, 8, 541–546. [Google Scholar] [PubMed]
- Dore-Duffy, P.; Catalanotto, F.; Donaldson, J.O.; Ostrom, K.M.; Testa, M.A. Zinc in multiple sclerosis. Ann. Neurol. 1983, 14, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Erdal, M.; Sahin, M.; Hasimi, A.; Uckaya, G.; Kutlu, M.; Saglam, K. Trace element levels in hashimoto thyroiditis patients with subclinical hypothyroidism. Biol. Trace Elem. Res. 2008, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Kianbakht, S.; Ghasami, K.; Mosayebi, G. High copper and low zinc serum levels in Iranian patients with multiple sclerosis: A case control study. Clin. Lab. 2012, 58, 161–164. [Google Scholar] [PubMed]
- Hägglöf, B.; Hallmans, G.; Holmgren, G.; Ludvigsson, J.; Falkmer, S. Prospective and retrospective studies of zinc concentrations in serum, blood clots, hair and urine in young patients with insulin-dependent diabetes mellitus. Acta Endocrinol. 1983, 102, 88–95. [Google Scholar] [PubMed]
- Hansson, L.; Huunan-Seppälä, A.; Mattila, A. The content of calcium, magnesium, copper, zinc, lead and chromium in the blood of patients with rheumatoid arthritis. Scand. J. Rheumatol. 1975, 4, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Haugen, M.A.; Høyeraal, H.M.; Larsen, S.; Gilboe, I.M.; Trygg, K. Nutrient intake and nutritional status in children with juvenile chronic arthritis. Scand. J. Rheumatol. 1992, 21, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Helgeland, M.; Svendsen, E.; Førre, O.; Haugen, M. Dietary intake and serum concentrations of antioxidants in children with juvenile arthritis. Clin. Exp. Rheumatol. 2000, 18, 637–641. [Google Scholar] [PubMed]
- Helliwell, M.; Coombes, E.J.; Moody, B.J.; Batstone, G.F.; Robertson, J.C. Nutritional status in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1984, 43, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Isbir, T.; Tamer, L.; Taylor, A.; Isbir, M. Zinc, copper and magnesium status in insulin-dependent diabetes. Diabetes Res. 1994, 26, 41–45. [Google Scholar] [PubMed]
- Jansen, J.; Rosenkranz, E.; Overbeck, S.; Warmuth, S.; Mocchegiani, E.; Giacconi, R.; Weiskirchen, R.; Karges, W.; Rink, L. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J. Nutr. Biochem. 2012, 23, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, M.; Daneshpazhooh, M.; Chams-Davatchi, C.; Eshraghian, M.; Zarei, M.; Chamari, M. Serum selenium, zinc, and copper in early diagnosed patients with pemphigus vulgaris. Iran J. Public Health 2012, 41, 105–109. [Google Scholar] [PubMed]
- Kapaki, E.; Segditsa, J.; Papageorgiou, C. Zinc, copper and magnesium concentration in serum and CSF of patients with neurological disorders. Acta Neurol. Scand. 1989, 79, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kiilerich, S.; Hvid-Jacobsen, K.; Vaag, A.; Sørensen, S.S. 65 Zinc absorption in patients with insulin-dependent diabetes mellitus assessed by whole-body counting technique. Clin. Chim. Acta 1990, 189, 13–18. [Google Scholar] [CrossRef]
- Kiilerich, S.; Christiansen, C. Distribution of serum zinc between albumin and alpha 2-macroglobulin in patients with different zinc metabolic disorders. Clin. Chim. Acta 1986, 154, 1–6. [Google Scholar] [CrossRef]
- Kobbah, A.M.; Hellsing, K.; Tuvemo, T. Early changes of some serum proteins and metals in diabetic children. Acta Paediatr. Scand. 1988, 77, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Tsweng, G.J.; Lee, C.F.; Chen, B.H.; Huang, Y.L. Magnesium, zinc, and chromium levels in children, adolescents, and young adults with type 1 diabetes. Clin. Nutr. 2016, 35, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Iyanda, A.A.; Anetor, J.I.; Oparinde, P. Serum levels of minerals and vitamins in two categories of female alopecia subjects using hair relaxer. DSI 2011, 29, 121–124. [Google Scholar] [CrossRef]
- Maldonado, M.A.; Gil Extremera, B.; Fernández Soto, M.; Ruiz Martínez, M.; González Jiménez, A.; Guijarro Morales, A.; de Dios Luna del Castillo, J. Zinc levels after intravenous administration of zinc sulphate in insulin-dependent diabetes mellitus patients. Klin. Wochenschr. 1991, 69, 640–644. [Google Scholar]
- Mierzecki, A.; Strecker, D.; Radomska, K. A pilot study on zinc levels in patients with rheumatoid arthritis. Biol. Trace Elem. Res. 2011, 143, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Negoro, A.; Umemoto, M.; Fujii, M.; Kakibuchi, M.; Terada, T.; Hashimoto, N.; Sakagami, M. Taste function in Sjögren’s syndrome patients with special reference to clinical tests. Auris Nasus Larynx 2004, 31, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nicoloff, G.; Angelova, M.; Nikolov, A. Serum fibrillin-antifibrillin immune complexes among diabetic children. Vascul. Pharmacol. 2005, 43, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Önal, S.; Nazıroğlu, M.; Çolakm, M.; Bulut, V.; Flores-Arce, M.F. Effects of different medical treatments on serum copper, selenium and zinc levels in patients with rheumatoid arthritis. Biol. Trace Elem. Res. 2011, 142, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Palm, R.; Hallmans, G. Zinc and copper in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1982, 45, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Raz, I.; Havivi, E. Trace elements in blood cells of diabetic subjects. Diabetes Res. 1989, 10, 21–24. [Google Scholar] [PubMed]
- Sahebari, M.; Abrishami-Moghaddam, M.; Moezzi, A.; Ghayour-Mobarhan, M.; Mirfeizi, Z.; Esmaily, H.; Ferns, G. Association between serum trace element concentrations and the disease activity of systemic lupus erythematosus. Lupus 2014, 23, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Silverio Amancio, O.M.; Alves Chaud, D.M.; Yanaguibashi, G.; Esteves Hilário, M.O. Copper and zinc intake and serum levels in patients with juvenile rheumatoid arthritis. Eur. J. Clin. Nutr. 2003, 57, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Ullah, Z.; Ullah, M.I.; Hussain, S.; Kaul, H.; Lone, K.P. Determination of Serum Trace Elements (Zn, Cu, and Fe) in Pakistani Patients with Rheumatoid Arthritis. Biol. Trace Elem. Res. 2017, 175, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, M.P.; Ghayour-Mobarhan, M.; Taji, A.; Javidi, Z.; Pezeshkpoor, F.; Tavallaie, S.; Momenzadeh, A.; Esmaili, H.; Shojaie-Noori, S.; Khoddami, M.; et al. Serum zinc and copper status in Iranian patients with pemphigus vulgaris. Int. J. Dermatol. 2011, 50, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Sari, R.A.; Gundogdu, M.; Kose, N.; Dag, E. Trace elements and some extracellular antioxidant proteins levels in serum of patients with systemic lupus erythematosus. Clin. Rheumatol. 2005, 24, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Zoli, A.; Altomonte, L.; Caricchio, R.; Galossi, A.; Mirone, L.; Ruffini, M.P.; Magaró, M. Serum zinc and copper in active rheumatoid arthritis: Correlation with interleukin 1 beta and tumour necrosis factor alpha. Clin. Rheumatol. 1998, 17, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Arreola, F.; Paniagua, R.; Herrera, J.; Díaz-Bensussen, S.; Mondragón, L.; Bermúdez, J.A.; Pérez Pastén, E.; Villalpando, S. Low plasma zinc and androgen in insulin-dependent diabetes mellitus. Arch. Androl. 1986, 16, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Bacon, M.C.; White, P.H.; Raiten, D.J.; Craft, N.; Margolis, S.; Levanderh, O.A.; Taylor, M.L.; Lipnick, R.N.; Sami, S. Nutritional status and growth in juvenile heumatoid arthritis. Semin. Arthritis Rheum. 1990, 20, 97–106. [Google Scholar] [CrossRef]
- Balogh, Z.; El-Ghobarey, A.F.; Fell, G.S.; Brown, D.H.; Dunlop, J.; Dick, W.C. Plasma zinc and its relationship to clinical symptoms and drug treatment in rheumatoid arthritis. Ann. Rheum. Dis. 1980, 39, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Crofton, R.W.; Glover, S.C.; Ewen, S.W.; Aggett, P.J.; Mowat, N.A.; Mills, C.F. Zinc absorption in celiac disease and dermatitis herpetiformis: A test of small intestinal function. Am. J. Clin. Nutr. 1983, 38, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.J.; Fu, A.; Mearkle, P.L.; Brown, G. Hyperzincuria in individuals with insulin-dependent diabetes mellitus: Concurrent zinc status and the effect of high-dose zinc supplementation. Metabolism 1994, 43, 1558–1562. [Google Scholar] [CrossRef]
- Ho, S.Y.; Catalanotto, F.A.; Lisak, R.P.; Dore-Duffy, P. Zinc in multiple sclerosis. II: Correlation with disease activity and elevated plasma membrane-bound zinc in erythrocytes from patients with multiple sclerosis. Ann. Neurol. 1986, 20, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.C.; Fell, G.S.; Rooney, P.J.; Stevens, W.H.; Dick, W.C.; Buchanan, W.W. Zinc: Its relationship to osteoporosis in rheumatoid arthritis. Scand. J. Rheumatol. 1975, 4, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Melchior, T.; Wiese Simonsen, A.; Johannessen, C.; Binder, C. Plasma zinc concentrations during the first 2 years after diagnosis of insulin-dependent diabetes mellitus: A prospective study. J. Intern. Med. 1989, 226, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Milanino, R.; Frigo, A.; Bambara, L.M.; Marrella, M.; Moretti, U.; Pasqualicchio, M.; Biasi, D.; Gasperini, R.; Mainenti, L.; Velo, G.P. Copper and zinc status in rheumatoid arthritis: Studies of plasma, erythrocytes, and urine, and their relationship to disease activity markers and pharmacological treatment. Clin. Exp. Rheumatol. 1993, 11, 271–281. [Google Scholar] [PubMed]
- Mocchegiani, E.; Boemi, M.; Fumelli, P.; Fabris, N. Zinc-dependent low thymic hormone level in type I diabetes. Diabetes 1989, 38, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Naveh, Y.; Schapira, D.; Ravel, Y.; Geller, E.; Scharf, Y. Zinc metabolism in rheumatoid arthritis: Plasma and urinary zinc and relationship to disease activity. J. Rheumatol. 1997, 24, 643–646. [Google Scholar] [PubMed]
- Pereira, T.C.; Saron, M.L.; Carvalho, W.A.; Vilela, M.M.; Hoehr, N.F.; Hessel, G. Research on zinc blood levels and nutritional status in adolescents with autoimmune hepatitis. Arq. Gastroenterol. 2011, 48, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Quilliot, D.; Dousset, B.; Guerci, B.; Dubois, F.; Drouin, P.; Ziegler, O. Evidence that diabetes mellitus favors impaired metabolism of zinc, copper, and selenium in chronic pancreatitis. Pancreas 2001, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Rohn, R.D.; Pleban, P.; Jenkins, L.L. Magnesium, zinc and copper in plasma and blood cellular components in children with IDDM. Clin. Chim. Acta 1993, 215, 21–28. [Google Scholar] [CrossRef]
- Ruíz, C.; Alegría, A.; Barberá, R.; Farré, R.; Lagarda, M.J. Selenium, Zinc and Copper in Plasma of patients with Type 1 Diabetes Mellitus in Different Metabolic Control States. J. Trace Elem. Med. Biol. 1998, 12, 91–95. [Google Scholar] [CrossRef]
- Smith, D.K.; Feldman, E.B.; Feldman, D.S. Trace element status in multiple sclerosis. Am. J. Clin. Nutr. 1989, 50, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, S.; Kamanli, A.; Akçil, E.; Kavas, G.O.; Seçkin, B.; Atay, M.B. Trace element and magnesium levels and superoxide dismutase activity in rheumatoid arthritis. Biol. Trace Elem. Res. 1999, 68, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Viktorínová, A.; Toserová, E.; Krizko, M.; Duracková, Z. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metabolism 2009, 58, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Yazar, M.; Sarban, S.; Kocyigit, A.; Isikan, E. Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol. Trace Elem. Res. 2005, 106, 123–132. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Brabazon, D.; Naher, S. Interaction between zinc, cadmium, and lead in scalp hair samples of Pakistani and Irish smokers rheumatoid arthritis subjects in relation to controls. Biol. Trace Elem. Res. 2012, 148, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.I.; Talpur, F.N.; Kazi, T.G.; Brabazon, D. Estimation of toxic elements in the samples of different cigarettes and their effect on the essential elemental status in the biological samples of Irish smoker rheumatoid arthritis consumers. Environ. Monit. Assess. 2015, 187, 157. [Google Scholar] [CrossRef] [PubMed]
- Melø, T.M.; Larsen, C.; White, L.R.; Aasly, J.; Sjøbakk, T.E.; Flaten, T.P.; Sonnewald, U.; Syversen, T. Manganese, copper, and zinc in cerebrospinal fluid from patients with multiple sclerosis. Biol. Trace Elem. Res. 2003, 93, 1–8. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 2008, 61, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Marrosu, M.G.; Murru, R.; Murru, M.R.; Costa, G.; Zavattari, P.; Whalen, M.; Cocco, E.; Mancosu, C.; Schirru, L.; Solla, E.; et al. Dissection of the HLA association with multiple sclerosis in the founder isolated population of Sardinia. Hum. Mol. Genet. 2001, 10, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Zavattari, P.; Lampis, R.; Mulargia, A.; Loddo, M.; Angius, E.; Todd, J.A.; Cucca, F. Confirmation of the DRB1-DQB1 loci as the major component of IDDM1 in the isolated founder population of Sardinia. Hum. Mol. Genet. 2000, 9, 2967–2972. [Google Scholar] [CrossRef] [PubMed]
- Pitzalis, M.; Zavattari, P.; Murru, R.; Deidda, E.; Zoledziewska, M.; Murru, D.; Moi, L.; Motzo, C.; Orrù, V.; Costa, G.; et al. Genetic loci linked to Type 1 Diabetes and Multiple Sclerosis families in Sardinia. BMC Med. Genet. 2008, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Orrù, V.; Steri, M.; Sole, G.; Sidore, C.; Virdis, F.; Dei, M.; Lai, S.; Zoledziewska, M.; Busonero, F.; Mulas, A.; et al. Genetic variants regulating immune cell levels in health and disease. Cell 2013, 155, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Steri, M.; Orru, V.; Idda, M.L.; Pitzalis, M.; Pala, M.; Zara, I.; Sidore, C.; Faa, V.; Floris, M.; Deiana, M.; et al. Overexpression of the cytokine BAFF and autoimmunity risk. N. Engl. J. Med. 2017, 376, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Contu, D.; Morelli, L.; Zavattari, P.; Lampis, R.; Angius, E.; Frongia, P.; Murru, D.; Maioli, M.; Francalacci, P.; Todd, J.A.; et al. Sex-related bias and exclusion mapping of the nonrecombinant portion of chromosome Y in human type 1 diabetes in the isolated founder population of Sardinia. Diabetes 2002, 51, 3573–3576. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Guido, D.; Montomoli, C.; Sardu, C.; Sanna, A.; Pretti, S.; Lorefice, L.; Marrosu, M.G.; Valera, P.; Cocco, E. Is Geo-Environmental Exposure a Risk Factor for Multiple Sclerosis? A Population-Based Cross-Sectional Studyin South-Western Sardinia. PLoS ONE 2016, 11, e0163313. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Jojic, V.; Gao, T.; Bhattacharya, S.; Angel, C.J.L.; Furman, D.; Shen-Orr, S.; Dekker, C.L.; Swan, G.E.; Butte, A.J.; et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 2015, 160, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of pathogenic TH 17 cells by inducible salt-sensing kinase SGK1. Nature 2013, 496, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Rink, L. Multiple impacts of zinc on immune function. Metallomics 2014, 6, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000, 182, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, B.; Choi, Y.H.; Hwang, Y.; Kim, D.H.; Cho, S.; Hong, S.J.; Lee, W. Inhibition of interleukin-1β-mediated interleukin-1 receptor-associated kinase 4 phosphorylation by zinc leads to repression of memory T helper type 17 response in humans. Immunology 2015, 146, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Miyai, T.; Fujishiro, H.; Kawamura, M.; Yasuda, T.; Hijikata, A.; Bin, B.; Irié, T.; Tanaka, J.; Atsumi, T.; et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc. Natl. Acad. Sci. USA 2014, 111, 11786–11791. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, E.; Metz, C.H.; Maywald, M.; Hilgers, R.D.; Wessels, I.; Senff, T.; Haase, H.; Jager, M.; Ott, M.; Aspinall, R.; et al. Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol. Nutr. Food Res. 2016, 60, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, E.; Maywald, M.; Hilgers, R.D.; Brieger, A.; Clarner, T.; Kipp, M.; Plumakers, B.; Meyer, S.; Schwerdtle, T.; Rink, L. Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J. Nutr. Biochem. 2016, 29, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, C.; Fukada, T.; Kanamoto, M.; Ohashi, W.; Hojyo, S.; Atsumi, T.; Ueda, N.; Azuma, I.; Hirota, H.; Murakami, M.; et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int. Immunol. 2010, 22, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, M.; Frederiksen, J.L. Zinc in multiple sclerosis: A systematic review and meta-analysis. ASN Neuro 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Socha, K.; Karpinska, E.; Kochanowicz, J.; Soroczynska, J.; Jakoniuk, M.; Wilkiel, M.; Mariak, Z.D.; Borawska, M.H. Dietary habits; concentration of copper, zinc, and Cu-to-Zn ratio in serum and ability status of patients with relapsing-remitting multiple sclerosis. Nutrition 2017, 39, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Frischer, J.M.; Webb, S.M.; Tham, M.; Adiele, R.C.; Robinson, C.A.; Fitz-Gibbon, P.D.; Weigand, S.D.; Metz, I.; Nehzati, S.; et al. Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions. Acta Neuropathol. 2017, 134, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Campo, C.A.; Wellinghausen, N.; Faber, C.; Fischer, A.; Rink, L. Zinc inhibits the mixed lymphocyte culture. Biol. Trace Elem. Res. 2001, 79, 15–22. [Google Scholar] [PubMed]
- Faber, C.; Gabriel, P.; Ibs, K.H.; Rink, L. Zinc in pharmacological doses suppresses allogeneic reaction without affecting the antigenic response. Bone Marrow Transpl. 2004, 33, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Kown, M.H.; van der Steenhoven, T.J.; Jahncke, C.L.; Mari, C.; Lijkwan, M.A.; Koransky, M.L.; Blankenberg, F.G.; Strauss, H.W.; Robbins, R.C. Zinc chloride-mediated reduction of apoptosis as an adjunct immunosuppressive modality in cardiac transplantation. J. Heart Lung Transplant. 2002, 21, 360–365. [Google Scholar] [CrossRef]
- Schubert, C.; Guttek, K.; Grungreiff, K.; Thielitz, A.; Buhling, F.; Reinhold, A.; Brocke, S.; Reinhold, D. Oral zinc aspartate treats experimental autoimmune encephalomyelitis. Biometals 2014, 27, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Rungby, J. Zinc, zinc transporters and diabetes. Diabetologia 2010, 53, 1549–1551. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Karges, W.; Rink, L. Zinc and diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem. 2009, 20, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, T.J.; Bellomo, E.A.; Wijesekara, N.; Loder, M.K.; Baldwin, J.M.; Gyulkhandanyan, A.V.; Koshkin, V.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes–associated variants. Diabetes 2009, 58, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, K.; Ravier, M.A.; Schraenen, A.; Creemers, J.W.; Van de Plas, R.; Granvik, M.; Van Lommel, L.; Waelkens, E.; Chimienti, F.; Rutter, G. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA 2009, 106, 14872–14877. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, N.; Dai, F.; Hardy, A.; Giglou, P.; Bhattacharjee, A.; Koshkin, V.; Chimienti, F.; Gaisano, H.; Rutter, G.; Wheeler, M. Beta cell-specific ZnT8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 2010, 53, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Aburto-Luna, V.; Treviño, S.; Santos-López, G.; Moroni-González, D.; Calva-Cruz, O.; Aguilar-Alonso, P.; León-Chávez, B.A.; Brambila, E. Hepatic mobilization of zinc after an experimental surgery, and its relationship with inflammatory cytokines release, and expression of metallothionein and Zip14 transporter. Inflamm. Res. 2017, 66, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Lamboux, A.; Albarède, F.; Miossec, P. A Feedback Loop between Inflammation and Zn Uptake. PLoS ONE 2016, 11, e0147146. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D.; Uthus, E.O. DNA methylation, cancer susceptibility, and nutrient interactions. Exp. Biol. Med. 2004, 229, 988–995. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Zinc and reproduction: Effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res. B Dev. Reprod. Toxicol. 2010, 89, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Beach, R.S.; Gershwin, M.E.; Hurley, L.S. Gestational zinc deprivation in mice: Persistence of immunodeficiency for three generations. Science 1982, 218, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Tomat, A.L.; Inserra, F.; Veiras, L.; Vallone, M.C.; Balaszczuk, A.M.; Costa, M.A.; Arranz, C. Moderate zinc restriction during fetal and postnatal growth of rats: Effects on adult arterial blood pressure and kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Valera, P.; Zavattari, P.; Albanese, S.; Cicchella, D.; Dinelli, E.; Lima, A.; De Vivo, B. A correlation study between multiple sclerosis and type 1 diabetes incidences and geochemical data in Europe. Environ. Geochem. Health 2014, 36, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Valera, P.; Zavattari, P.; Sanna, A.; Pretti, S.; Marcello, A.; Mannu, C.; Targhetta, C.; Bruno, G.; Songini, M. Zinc and Other Metals Deficiencies and Risk of Type 1 Diabetes: An Ecological Study in the High Risk Sardinia Island. PLoS ONE 2015, 10, e0141262. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, E.; Lima, A.; De Vivo, B.; Albanese, S.; Cicchella, D.; Valera, P. Hydrogeochemical analysis on Italian bottled mineral waters: Effects of geology. J. Geochem. Explor. 2010, 107, 317–335. [Google Scholar] [CrossRef]
- Cicchella, D.; Albanese, S.; De Vivo, B.; Dinelli, E.; Giaccio, L.; Lima, A.; Valera, P. Trace elements and ions in Italian bottled mineral waters: Identification of anomalous values and human health related effects. J. Geochem. Explor. 2010, 107, 336–349. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Comparative study between bottled mineral and tap water in Italy. J. Geochem. Explor. 2012, 112, 368–389. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Major and trace elements in tap water from Italy. J. Geochem. Explor. 2012, 112, 54–75. [Google Scholar] [CrossRef]
- El Amari, K.; Valera, P.; Hibti, M.; Pretti, S.; Marcello, A.; Essarraj, S. Impact of mine tailings on surrounding soils and ground water: Case of Kettara old mine, Morocco. J. Afr. Earth Sci. 2014, 100, 437–449. [Google Scholar] [CrossRef]
- Pompili, M.; Vichi, M.; Dinelli, E.; Pycha, R.; Valera, P.; Albanese, S.; Lima, A.; De Vivo, B.; Cicchella, D.; Fiorillo, A.; et al. Relationships of local lithium concentrations in drinking water to regional suicide rates in Italy. World J. Biol. Psychiatry 2015, 16, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Vichi, M.; Dinelli, E.; Erbuto, D.; Pycha, R.; Serafini, G.; Giordano, G.; Valera, P.; Albanese, S.; Lima, A.; et al. Arsenic: Association of regional concentrations in drinking water with suicide and natural causes of death in Italy. Psychiatry Res. 2017, 249, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Scheib, A.J.; Flight, D.M.A.; Birke, M.; Tarvainen, T.; Locutura, J.; Albanese, S.; Andersson, M.; Arnoldussen, A.; Baritz, R.; Batista, M.J.; et al. The geochemistry of niobium and its distribution and relative mobility in agricultural soils of Europe. Geochem. Explor. Environ. A 2012, 12, 293–302. [Google Scholar] [CrossRef]
- Mann, A.; Reimann, C.; De Caritat, P.; Turner, N.; Birke, M.; Albanese, S.; Andersson, M.; Baritz, R.; Batista, M.J.; Bel-Lan, A.; et al. Mobile metal ion® analysis of european agricultural soils: Bioavailability, weathering, Geogenic patterns and anthropogenic anomalies. Geochem. Explor. Environ. A 2015, 15, 99–112. [Google Scholar] [CrossRef]
- Négrel, P.; Sadeghi, M.; Ladenberger, A.; Reimann, C.; Birke, M.; Albanese, S.; Andersson, M.; Baritz, R.; Batista, M.J.; Bel-Lan, A.; et al. Geochemical fingerprinting and source discrimination of agricultural soils at continental scale. Chem. Geol. 2015, 396, 1–15. [Google Scholar] [CrossRef]
- Ladenberger, A.; Demetriades, A.; Reimann, C.; Birke, M.; Sadeghi, M.; Uhlbäck, J.; Andersson, M.; Jonsson, E.; Albanese, S.; Baritz, R.; et al. GEMAS: Indium in agricultural and grazing land soil of Europe—Its source and geochemical distribution patterns. J. Geochem. Explor. 2015, 154, 61–80. [Google Scholar] [CrossRef]
- Birke, M.; Reimann, C.; Oorts, K.; Rauch, U.; Demetriades, A.; Dinelli, E.; Ladenberger, A.; Halamić, J.; Gosar, M.; Jähne-Klingberg, F. The GEMAS Project Team. Use of GEMAS data for risk assessment of cadmium in European agricultural and grazing land soil under the REACH Regulation. Appl. Geochem. 2016, 74, 109–121. [Google Scholar] [CrossRef]
- Birke, M.; Reimann, C.; Rauch, U.; Ladenberger, A.; Demetriades, A.; Jähne-Klingberg, F.; Oorts, K.; Gosar, M.; Dinelli, E.; Halamić, J. GEMAS: Cadmium distribution and its sources in agricultural and grazing land soil of Europe—Original data versus clr-transformed data. J. Geochem. Explor. 2017, 173, 13–30. [Google Scholar] [CrossRef]
- Scheib, A.J.; Birke, M.; Dinelli, E.; Albanese, S.; Andersson, M.; Baritz, R.; Batista, M.J.; Bel-Lan, A.; Cicchella, D.; Demetriades, A.; et al. Geochemical evidence of aeolian deposits in European soils. Boreas 2014, 43, 175–192. [Google Scholar] [CrossRef]
| Authors | Year | Disease | No. | Zn Status (μg/mL) | Direction | ||
|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | ||||
| Aaseth et al. [20] | 1978 | RA | 22 | 12 | 0.654 | 0.850 | low zinc in patients |
| Abdel Fattah et al. [21] | 2016 | AA | 50 | 50 | 0.755 | 0.857 | low zinc in patients |
| Arreola et al. [22] | 1990 | T1D | 22 | 11 | 0.734 | 1.114 | low zinc in patients |
| Banford et al. [23] | 1982 | RA | 85 | 49 | 12.100 | 12.100 | no difference |
| Bhat et al. [24] | 2009 | AA | 50 | 50 | 78.000 | 88.000 | low zinc in patients |
| Bideci et al. [25] | 2005 | T1D | 28 | 15 | 0.961 | 1.231 | low zinc in patients |
| Brandao-Neto et al. [26] | 1999 | T1D | 10 | 10 | 1.040 | 1.020 | no difference |
| Car et al. [27] | 1992 | T1D | 15 | 15 | 0.562 | 0.772 | low zinc in patients |
| Dijkmans et al. [28] | 1987 | RA | 25 | 18 | 0.667 | 0.942 | low zinc in patients |
| Dore-Duffy et al. [29] | 1983 | MS | 63 | 62 | 831.000 | 817.000 | no difference |
| Dore-Duffyet al. [30] | 1990 | RA | 57 | 18 | 0.850 | 0.997 | low zinc in patients |
| Erdal et al. [31] | 2008 | HT | 43 | 49 | 1.093 | 1.015 | no difference |
| Ghazavi et al. [32] | 2012 | MS | 60 | 60 | 0.402 | 1.278 | low zinc in patients |
| Hagglof et al. [33] | 1983 | T1D | 66 | 79 | 0.915 | 1.000 | low zinc in patients |
| Hansson et al. [34] | 1975 | RA | 37 | 70 | 1.066 | 1.055 | low zinc in patients |
| 42 | 26 | 1.052 | 0.965 | low zinc in patients | |||
| Haugen et al. [35] | 1992 | JIA | 8 | 17 | 0.909 | 0.981 | low zinc in patients |
| Helgeland et al. [36] | 2000 | JIA | 14 | 22 | 0.830 | 0.870 | low zinc in patients |
| Helliwell et al. [37] | 1984 | RA | 50 | 50 | 0.804 | 0.883 | low zinc in patients |
| Isbir et al. [38] | 1994 | T1D | 20 | 20 | 0.565 | 0.696 | low zinc in patients |
| Jansen et al. [39] | 2012 | T1D | 8 | 8 | 0.768 | 0.883 | low zinc in patients |
| Javanbakht et al. [40] | 2012 | PV | 43 | 58 | 0.906 | 0.988 | no difference |
| Kapaki et al. [41] | 1989 | MS | 15 | 28 | 1.030 | 1.100 | no difference |
| Kiilerich et al. [42] | 1986 | T1D | 7 | 12 | 0.798 | 0.948 | low zinc in patients |
| Kiilerich et al. [43] | 1990 | T1D | 10 | 104 | 1.007 | 0.948 | no difference |
| Kobbah et al. [44] | 1988 | T1D | 30 | 44 | 0.785 | 0.909 | low zinc in patients |
| Lin et al. [45] | 2016 | T1D | 88 | 76 | 0.910 | 0.940 | no difference |
| Iyanda et al. [46] | 2011 | AA | 20 | 20 | 0.792 | 0.933 | low zinc in patients |
| 20 | 20 | 0.782 | 0.933 | low zinc in patients | |||
| Maldonado et al. [47] | 1991 | T1D | 22 | 22 | 1.111 | 1.197 | no difference |
| Mierzecki et al. [48] | 2011 | RA | 74 | 30 | 0.801 | 0.720 | low zinc in patients |
| Negoro et al. [49] | 2004 | SS | 31 | 15 | 0.706 | 0.866 | low zinc in patients |
| Nicoloff et al. [50] | 2005 | T1D | 35 | 20 | 0.675 | 1.268 | low zinc in patients |
| Onal et al. [51] | 2011 | RA | 32 | 52 | 0.430 | 0.748 | low zinc in patients |
| Palm et al. [52] | 1982 | MS | 21 | 21 | 0.850 | 0.968 | low zinc in patients |
| 29 | 29 | 0.791 | 0.863 | low zinc in patients | |||
| Raz et al. [53] | 1989 | T1D | 23 | 22 | 0.928 | 0.170 | low zinc in patients |
| Sahebari et al. [54] | 2014 | SLE | 123 | 100 | 0.701 | 0.860 | low zinc in patients |
| Silverio Amancio et al. [55] | 2003 | JIA | 20 | 10 | 0.897 | 0.900 | no difference |
| 21 | 13 | 0.976 | 0.950 | no difference | |||
| Ullah et al. [56] | 2017 | RA | 61 | 61 | 0.856 | 0.959 | low zinc in patients |
| Yazdanpanah et al. [57] | 2011 | PV | 25 | 25 | 0.770 | 1.207 | low zinc in patients |
| Yilmaz et al. [58] | 2005 | SLE | 27 | 20 | 0.875 | 0.990 | low zinc in patients |
| Zoli et al. [59] | 1998 | RA | 57 | 20 | 85.600 | 108.100 | low zinc in patients |
| Authors | Year | Disease | No. | Zn Status (μg/mL) | Direction | ||
|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | ||||
| Arreola et al. [60] | 1986 | T1D | 9 | 12 | 73.490 | 112.460 | low zinc in patients |
| Bacon et al. [61] | 1990 | JIA | 8 | 9 | 0.805 | 0.983 | low zinc in patients |
| JIA | 14 | 9 | 0.859 | 0.983 | low zinc in patients | ||
| JIA | 12 | 9 | 0.875 | 0.983 | low zinc in patients | ||
| Balogh et al. [62] | 1980 | RA | 140 | 100 | 11.740 | 15.100 | low zinc in patients |
| Crofton et al. [63] | 1983 | CD | 12 | 15 | 0.582 | 0.974 | low zinc in patients |
| CD | 10 | 15 | 0.628 | 0.974 | low zinc in patients | ||
| Cunningham et al. [64] | 1994 | T1D | 14 | 15 | 0.950 | 0.910 | no difference |
| Dore-Duffy et al. [29] | 1983 | MS | 68 | 60 | 845.000 | 788.000 | low zinc in patients |
| Dore-Duffy et al. [30] | 1990 | RA | 57 | 17 | 0.795 | 0.890 | low zinc in patients |
| Ho et al. [65] | 1986 | MS | 45 | 23 | 0.890 | 0.880 | high zinc in patients |
| Kennedy et al. [66] | 1975 | RA | 113 | 100 | 0.857 | 0.990 | low zinc in patients |
| Melchior et al. [67] | 1989 | T1D | 14 | 36 | 0.947 | 0.943 | no difference |
| T1D | 12 | 36 | 0.879 | 0.817 | no difference | ||
| Milanino et al. [68] | 1993 | RA | 120 | 70 | 0.895 | 1.019 | low zinc in patients |
| RA | 10 | 0.526 | 0.106 | low zinc in patients | |||
| Mocchegiani et al. [69] | 1989 | T1D | 15 | 16 | 0.793 | 1.064 | low zinc in patients |
| Naveh et al. [70] | 1997 | RA | 13 | 8 | 0.590 | 1.110 | low zinc in patients |
| RA | 16 | 8 | 0.600 | 1.110 | low zinc in patients | ||
| Pereira et al. [71] | 2011 | AH | 23 | 25 | 0.719 | 0.807 | low zinc in patients |
| Quilliot et al. [72] | 2001 | T1D | 25 | 20 | 0.940 | 0.970 | low zinc in patients |
| Rohn et al. [73] | 1993 | T1D | 45 | 12 | 0.942 | 0.981 | no difference |
| Ruiz et al. [74] | 1998 | T1D | 16 | 9 | 1.020 | 1.079 | no difference |
| T1D | 13 | 7 | 1.046 | 1.059 | no difference | ||
| T1D | 31 | 19 | 1.020 | 1.040 | no difference | ||
| T1D | 34 | 24 | 1.046 | 1.040 | no difference | ||
| T1D | 31 | 17 | 1.033 | 1.046 | no difference | ||
| T1D | 25 | 14 | 1.013 | 1.059 | no difference | ||
| Smith et al. [75] | 1989 | MS | 27 | 33 | 0.987 | 1.000 | no difference |
| Tuncer et al. [76] | 1999 | RA | 38 | 20 | 1.087 | 1.253 | low zinc in patients |
| Viktorinova et al. [77] | 2009 | T1D | 11 | 34 | 0.885 | 0.942 | no difference |
| Yazar et al. [78] | 2005 | RA | 25 | 25 | 0.663 | 0.658 | no difference |
| Authors | Year | Disease | No. | Biological Sample | Zn Status (μg/g) | Direction | ||
|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | |||||
| Afridi et al. [79] | 2015 | RA | 15 | 14 | Hair | 122.00 | 178.00 | low zinc in patients |
| 15 | 12 | 117.00 | 167.00 | low zinc in patients | ||||
| 12 | 13 | 135.00 | 203.00 | low zinc in patients | ||||
| 11 | 13 | 126.00 | 203.00 | low zinc in patients | ||||
| Afridi et al. [80] | 2012 | RA | 39 | 47 | Hair | 112.00 | 225.00 | low zinc in patients |
| 34 | 52 | 138.00 | 250.00 | low zinc in patients | ||||
| 23 | 22 | 122.00 | 178.00 | low zinc in patients | ||||
| 20 | 19 | 135.00 | 203.00 | low zinc in patients | ||||
| Hagglof et al. [33] | 1983 | T1D | 74 | 30 | Hair | 160.90 | 190.80 | low zinc in patients |
| Mierzecki et al. [48] | 2011 | RA | 71 | 75 | Hair | 150.37 | 150.37 | no difference |
| Kiilerich et al. [43] | 1990 | T1D | 10 | 28 | Urine | 1006.85 | 509.96 | high zinc in patients |
| Milanino et al. [68] | 1993 | RA | 75 | 50 | Urine | 437.9 | 457.50 | no difference |
| Maldonado et al. [47] | 1991 | T1D | 13 | 8 | Urine | 353 | 984.00 | low zinc in patients |
| Naveh et al. [70] | 1997 | RA | 16 | 8 | Urine | 538 | 984.00 | low zinc in patients |
| 22 | 22 | Urine | 1396 | 611.00 | high zinc in patients | |||
| Kapaki et al. [41] | 1989 | MS | 15 | 28 | CSF | 34.73 | 34.70 | no difference |
| Melo et al. [81] | 2003 | MS | 18 | 19 | CSF | 19.00 | 23.50 | no difference |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. https://doi.org/10.3390/nu10010068
Sanna A, Firinu D, Zavattari P, Valera P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients. 2018; 10(1):68. https://doi.org/10.3390/nu10010068
Chicago/Turabian StyleSanna, Alessandro, Davide Firinu, Patrizia Zavattari, and Paolo Valera. 2018. "Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis" Nutrients 10, no. 1: 68. https://doi.org/10.3390/nu10010068
APA StyleSanna, A., Firinu, D., Zavattari, P., & Valera, P. (2018). Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients, 10(1), 68. https://doi.org/10.3390/nu10010068








