Diatom Phenology in the Southern Ocean: Mean Patterns, Trends and the Role of Climate Oscillations
Abstract
:1. Introduction
2. Data and Methods
2.1. Satellite Data
2.2. Fronts Position
2.3. Maximum Sea Ice Extent
2.4. Climate Indices
2.5. Phenological Indices
2.6. Statistical Analysis
3. Results and Discussion
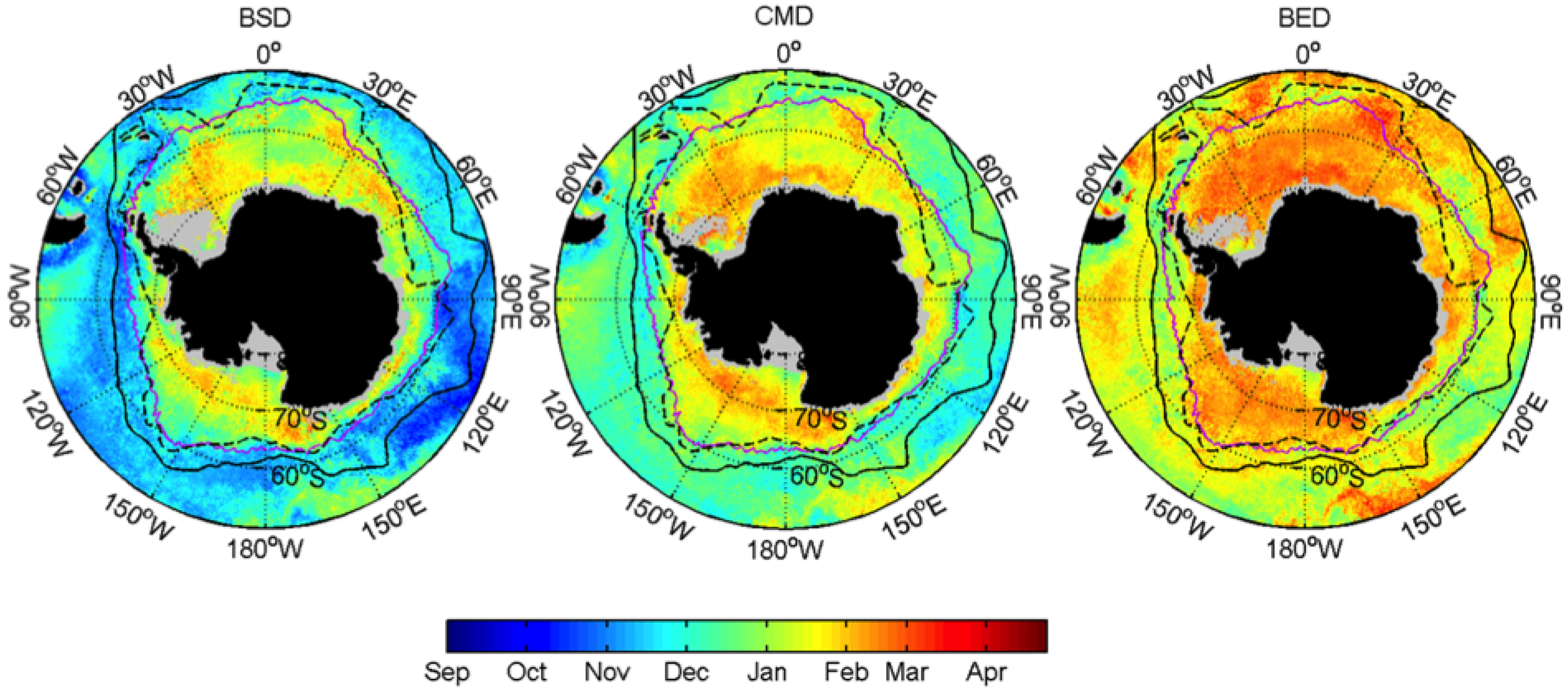
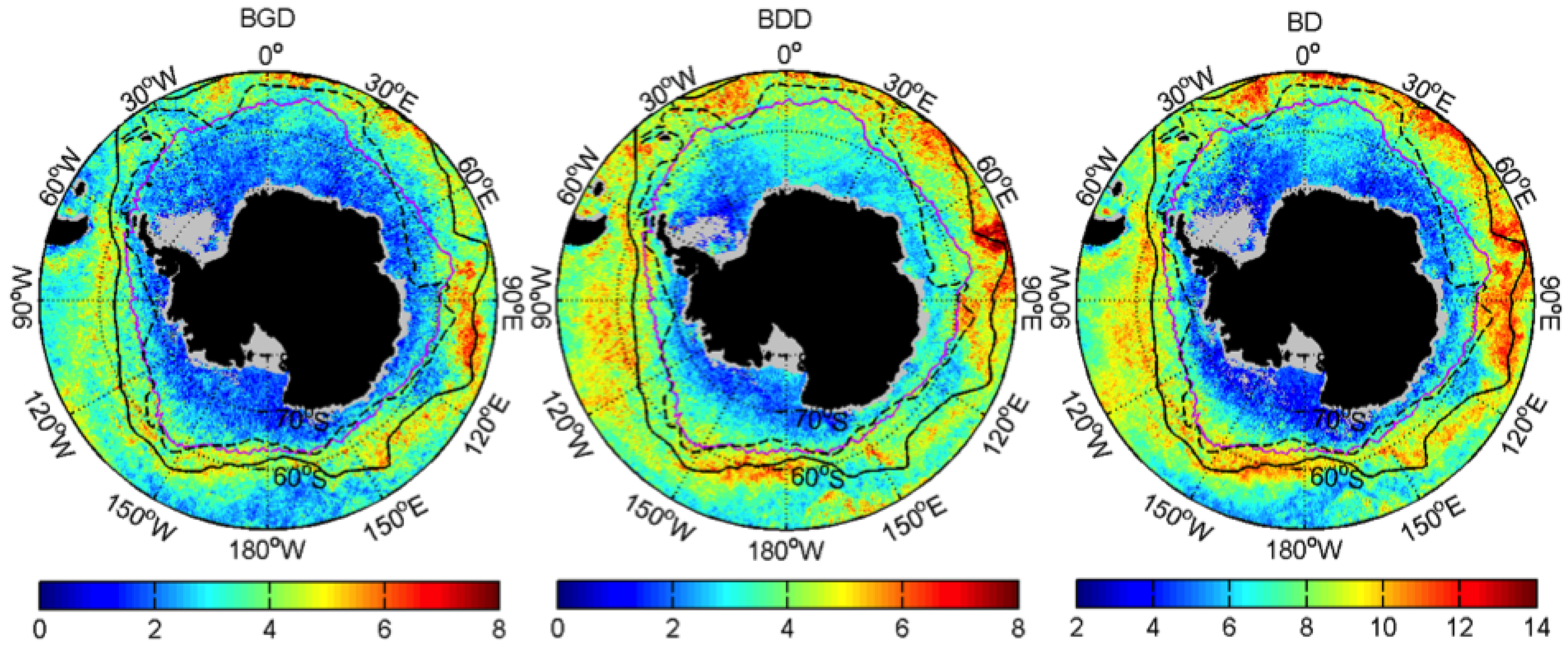
3.1. Mean Patterns
3.2. Interannual Variability
3.2.1. Trends
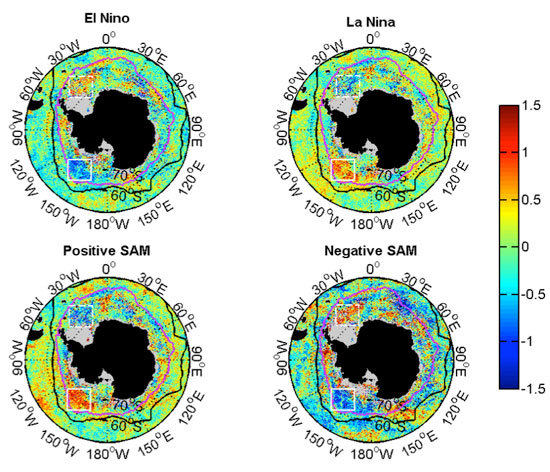
3.2.2. Relationships with ENSO and SAM
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kahru, M.; Brotas, V.; Manzano-sarabia, M.; Mitchell, B. Are phytoplankton blooms occurring earlier in the Arctic? Glob. Change Biol. 2011, 17, 1733–1739. [Google Scholar] [CrossRef]
- Ardyna, M.; Babin, M.; Gosselin, M.; Devred, E.; Rainville, L.; Tremblay, J.É. Recent Arctic Ocean sea ice loss triggers novel fall phytoplankton blooms. Geophys. Res. Lett. 2014, 41, 6207–6212. [Google Scholar] [CrossRef]
- Carranza, M.M.; Gille, S.T. Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a through the summer. J. Geophys. Res.: Oceans 2015, 120, 304–323. [Google Scholar] [CrossRef]
- Cole, H.S.; Henson, S.; Martin, A.P.; Yool, A. Basin-wide mechanisms for spring bloom initiation: How typical is the North Atlantic? ICES J. Mar. Sci.: J. Conseil 2015, 72, 2029–2040. [Google Scholar] [CrossRef]
- Racault, M.F.; Le Quéré, C.; Buitenhuis, E.; Sathyendranath, S.; Platt, T. Phytoplankton phenology in the global ocean. Ecol. Indic. 2012, 14, 152–163. [Google Scholar] [CrossRef]
- Edwards, M.; Richardson, A.J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 2004, 430, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Platt, T.; Fuentes-Yaco, C.; Frank, K.T. Marine ecology: Spring algal bloom and larval fish survival. Nature 2003, 423, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Koeller, P.; Fuentes-Yaco, C.; Platt, T.; Sathyendranath, S.; Richards, A.; Ouellet, P.; Orr, D.; Skúladóttir, U.; Wieland, K.; Savard, L.; et al. Basin-scale coherence in phenology of shrimps and phytoplankton in the North Atlantic Ocean. Science 2009, 324, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, S.; Fauchereau, N.; Swart, S.; Monteiro, P. Regional scale characteristics of the seasonal cycle of chlorophyll in the Southern Ocean. Biogeosciences 2011, 8, 2849–2866. [Google Scholar] [CrossRef]
- Sallée, J.B.; Llort, J.; Tagliabue, A.; Lévy, M. Characterization of distinct bloom phenology regimes in the Southern Ocean. ICES J. Mar. Sci.: J. Conseil 2015, 72, 1985–1998. [Google Scholar] [CrossRef]
- Borrione, I.; Schlitzer, R. Distribution and recurrence of phytoplankton blooms around South Georgia, Southern Ocean. Biogeosciences 2013, 10, 217–231. [Google Scholar] [CrossRef]
- Taylor, M.H.; Losch, M.; Bracher, A. On the drivers of phytoplankton blooms in the Antarctic marginal ice zone: A modeling approach. J. Geophys. Res.: Oceans 2013, 118, 63–75. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Annual cycles of sea ice and phytoplankton in Cape Bathurst polynya, southeastern Beaufort Sea, Canadian Arctic. Geophys. Res. Lett. 2004. [Google Scholar] [CrossRef]
- Montes-Hugo, M.; Vernet, M.; Martinson, D.; Smith, R.; Iannuzzi, R. Variability on phytoplankton size structure in the western Antarctic Peninsula (1997–2006). Deep Sea Res. Part II: Top. Stud. Oceanogr. 2008, 55, 2106–2117. [Google Scholar] [CrossRef]
- Smith, R.C.; Martinson, D.G.; Stammerjohn, S.E.; Iannuzzi, R.A.; Ireson, K. Bellingshausen and western Antarctic Peninsula region: Pigment biomass and sea-ice spatial/temporal distributions and interannual variabilty. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2008, 55, 1949–1963. [Google Scholar] [CrossRef]
- Alvain, S.; Le Quéré, C.; Bopp, L.; Racault, M.F.; Beaugrand, G.; Dessailly, D.; Buitenhuis, E.T. Rapid climatic driven shifts of diatoms at high latitudes. Remote Sens. Environ. 2013, 132, 195–201. [Google Scholar] [CrossRef]
- McPhaden, M.J.; Zebiak, S.E.; Glantz, M.H. ENSO as an integrating concept in earth science. Science 2006, 314, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.W.; Wallace, J.M. Annular modes in the extratropical circulation. Part I: Month-to-month variability*. J. Clim. 2000, 13, 1000–1016. [Google Scholar] [CrossRef]
- Penland, C.; Sun, D.Z.; Capotondi, A.; Vimont, D.J. A brief introduction to El Nino and La Nina. In Climate Dynamics: Why Does Climate Vary? Elsevier: Amsterdam, The Netherlands, 2010; pp. 53–64. [Google Scholar]
- Lovenduski, N.S. Impact of the Southern Annular Mode on Southern Ocean Circulation and Biogeochemistry; University of California: Los Angeles, CA, USA, 2007. [Google Scholar]
- Pohl, B.; Fauchereau, N.; Reason, C.; Rouault, M. Relationships between the Antarctic Oscillation, the Madden-Julian Oscillation, and ENSO, and consequences for rainfall analysis. J. Clim. 2010, 23, 238–254. [Google Scholar] [CrossRef]
- Lovenduski, N.S.; Gruber, N. Impact of the Southern Annular Mode on Southern Ocean circulation and biology. Geophys. Res. Lett. 2005. [Google Scholar] [CrossRef]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, W.; Gersonde, R.; Medlin, L.K.; Mann, D.G. The origin and evolution of the diatoms: Their adaptation to a planktonic existence. In Evolution of Primary Producers in the Sea; Elsevier: Amsterdam, The Netherlands, 2007; pp. 207–249. [Google Scholar]
- Smetacek, V. Diatoms and the ocean carbon cycle. Protist 1999, 150, 25–32. [Google Scholar] [CrossRef]
- Smetacek, V. Role of sinking in diatom life-history cycles: Ecological, evolutionary and geological significance. Mar. Biol. 1985, 84, 239–251. [Google Scholar] [CrossRef]
- Raven, J.; Waite, A. The evolution of silicification in diatoms: Inescapable sinking and sinking as escape? New Phytol. 2004, 162, 45–61. [Google Scholar] [CrossRef]
- Smetacek, V.; Assmy, P.; Henjes, J. The role of grazing in structuring Southern Ocean pelagic ecosystems and biogeochemical cycles. Antarct. Sci. 2004, 16, 541–558. [Google Scholar] [CrossRef]
- Assmy, P.; Smetacek, V.; Montresor, M.; Klaas, C.; Henjes, J.; Strass, V.H.; Arrieta, J.M.; Bathmann, U.; Berg, G.M.; Breitbarth, E.; et al. Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic Circumpolar Current. Proc. Natl. Acad. Sci. USA 2013, 110, 20633–20638. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.S.; Gregg, W.W. Interannual variation in phytoplankton primary production at a global scale. Remote Sens. 2013, 6, 1–19. [Google Scholar] [CrossRef]
- Tréguer, P.J.; De La Rocha, C.L. The world ocean silica cycle. Annu. Rev. Mar. Sci. 2013, 5, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Tréguer, P.J. The southern ocean silica cycle. Comptes Rendus Geosci. 2014, 346, 279–286. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Krasemann, H. Climate Assessment Report: Ocean Colour Climate Change Initiative (OC-CCI) – Phase One. Technical Report, ESA OC-CCI. 2014. Available online: http://www.esa-oceancolour-cci.org/?q=documents (accessed on 9 May 2016).
- Steinmetz, F.; Deschamps, P.Y.; Ramon, D. Atmospheric correction in presence of sun glint: Application to MERIS. Opt. Express 2011, 19, 9783–9800. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gordon, H.R. A simple, moderately accurate, atmospheric correction algorithm for SeaWiFS. Remote Sens. Environ. 1994, 50, 231–239. [Google Scholar] [CrossRef]
- Krasemann, H.; Belo-Couto, A.; Brando, V.; Brewin, R.J.; Brockmann, C.; Brotas, V.; Doerffer, R.; Feng, H.; Frouin, R.; Gould, R.; et al. Product Validation and Inter–Comparison Report; Technical Report 1, Ocean Colour Climate Change Initiative; Helmholtz-Zentrum Geesthacht: Geesthacht, Germany, 2014. [Google Scholar]
- Soppa, M.A.; Hirata, T.; Silva, B.; Dinter, T.; Peeken, I.; Wiegmann, S.; Bracher, A. Global Retrieval of Diatom Abundance Based on Phytoplankton Pigments and Satellite Data. Remote Sens. 2014, 6, 10089–10106. [Google Scholar] [CrossRef]
- Hirata, T.; Hardman-Mountford, N.; Brewin, R.; Aiken, J.; Barlow, R.; Suzuki, K.; Isada, T.; Howell, E.; Hashioka, T.; Noguchi-Aita, M.; et al. Synoptic relationships between surface Chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Kahru, M.; Hewes, C.D. Deep chlorophyll a maxima (DCMs) in pelagic Antarctic waters. II. Relation to bathymetric features and dissolved iron concentrations. Mar. Ecol. Progr. Ser. 2005, 297, 71–81. [Google Scholar] [CrossRef]
- Fennel, K.; Boss, E. Subsurface maxima of phytoplankton and chlorophyll: Steady-state solutions from a simple model. Limnol. Oceanogr. 2003, 48, 1521–1534. [Google Scholar] [CrossRef]
- Parslow, J.S.; Boyd, P.W.; Rintoul, S.R.; Griffiths, F.B. A persistent subsurface chlorophyll maximum in the Interpolar Frontal Zone south of Australia: Seasonal progression and implications for phytoplankton-light-nutrient interactions. J. Geophys. Res.: Oceans 2001, 106, 31543–31557. [Google Scholar] [CrossRef]
- Racault, M.F.; Sathyendranath, S.; Platt, T. Impact of missing data on the estimation of ecological indicators from satellite ocean-colour time-series. Remote Sens. Environ. 2014, 152, 15–28. [Google Scholar] [CrossRef]
- Sallée, J.; Speer, K.; Morrow, R. Southern Ocean fronts and their variability to climate modes. J. Clim. 2008, 21, 3020–3039. [Google Scholar] [CrossRef]
- Orsi, A.H.; Whitworth, T.; Nowlin, W.D. On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Res. Part I: Oceanogr. Res. Pap. 1995, 42, 641–673. [Google Scholar] [CrossRef]
- Fetterer, F.; Knowles, K.; Meier, W.; Savoie, M. Sea Ice Index. Boulder, CO: National Snow and Ice Data Center. Digit. Media 2002, 6. Available online: ftp://sidads.colorado.edu/DATASETS/NOAA/G02135/shapefiles/ (accessed on 13 May 2016). [Google Scholar]
- Wolter, K.; Timlin, M.S. Monitoring ENSO in COADS with a seasonally adjusted principal component index. In Proceedings of the 17th Climate Diagnostics Workshop, Norman, OK, USA, 18–23 October1993; pp. 52–57.
- Mo, K.C. Relationships between low-frequency variability in the Southern Hemisphere and sea surface temperature anomalies. J. Clim. 2000, 13, 3599–3610. [Google Scholar] [CrossRef]
- Siegel, D.; Doney, S.; Yoder, J. The North Atlantic spring phytoplankton bloom and Sverdrup’s critical depth hypothesis. Science 2002, 296, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.A.; Thomas, A.C. Interannual variability in timing of bloom initiation in the California Current System. J. Geophys. Res.: Oceans 2007. [Google Scholar] [CrossRef]
- Henson, S.A.; Raitsos, D.; Dunne, J.P.; McQuatters-Gollop, A. Decadal variability in biogeochemical models: Comparison with a 50-year ocean colour dataset. Geophys. Res. Lett. 2009. [Google Scholar] [CrossRef]
- Brody, S.R.; Lozier, M.S.; Dunne, J.P. A comparison of methods to determine phytoplankton bloom initiation. J. Geophys. Res.: Oceans 2013, 118, 2345–2357. [Google Scholar] [CrossRef]
- Kwok, R.; Comiso, J. Southern Ocean climate and sea ice anomalies associated with the Southern Oscillation. J. Clim. 2002, 15, 487–501. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Lowry, K.E.; van Dijken, G.L. Annual changes in sea ice and phytoplankton in polynyas of the Amundsen Sea, Antarctica. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2012, 71, 5–15. [Google Scholar] [CrossRef]
- Sallée, J.; Speer, K.; Rintoul, S. Zonally asymmetric response of the Southern Ocean mixed-layer depth to the Southern Annular Mode. Nat. Geosci. 2010, 3, 273–279. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Doney, S.C.; Lima, I.; Boss, E.S.; Siegel, D.A. Annual cycles of ecological disturbance and recovery underlying the subarctic Atlantic spring plankton bloom. Glob. Biogeochem. Cycles 2013, 27, 526–540. [Google Scholar] [CrossRef]
- Tagliabue, A.; Sallée, J.B.; Bowie, A.R.; Lévy, M.; Swart, S.; Boyd, P.W. Surface-water iron supplies in the Southern Ocean sustained by deep winter mixing. Nat. Geosci. 2014, 7, 314–320. [Google Scholar] [CrossRef]
- Sokolov, S.; Rintoul, S.R. On the relationship between fronts of the Antarctic Circumpolar Current and surface chlorophyll concentrations in the Southern Ocean. J. Geophys. Res.: Oceans 2007. [Google Scholar] [CrossRef]
- Blain, S.; Quéguiner, B.; Armand, L.; Belviso, S.; Bombled, B.; Bopp, L.; Bowie, A.; Brunet, C.; Brussaard, C.; Carlotti, F.; et al. Effect of natural iron fertilization on carbon sequestration in the Southern Ocean. Nature 2007, 446, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Planquette, H.; Statham, P.J.; Fones, G.R.; Charette, M.A.; Moore, C.M.; Salter, I.; Nedelec, F.H.; Taylor, S.L.; French, M.; Baker, A.R.; et al. Dissolved iron in the vicinity of the Crozet Islands, Southern Ocean. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2007, 54, 1999–2019. [Google Scholar] [CrossRef]
- Borrione, I.; Aumont, O.; Nielsdóttir, M.; Schlitzer, R. Sedimentary and atmospheric sources of iron around South Georgia, Southern Ocean: A modelling perspective. Biogeosciences 2014, 11, 1981–2001. [Google Scholar] [CrossRef]
- IOCCG. Ocean Colour Remote Sensing in Polar Seas; IOCCG Report Series, No. 16; International Ocean-Colour Coordinating Group: Dartmouth, NS, Canada, 2015. [Google Scholar]
- Bélanger, S.; Ehn, J.K.; Babin, M. Impact of sea ice on the retrieval of water-leaving reflectance, chlorophyll a concentration and inherent optical properties from satellite ocean color data. Remote Sens. Environ. 2007, 111, 51–68. [Google Scholar] [CrossRef]
- Wang, M.; Son, S.; Shi, W. Evaluation of MODIS SWIR and NIR-SWIR atmospheric correction algorithms using SeaBASS data. Remote Sens. Environ. 2009, 113, 635–644. [Google Scholar] [CrossRef]
- Maheshwari, M.; Singh, R.K.; Oza, S.R.; Kumar, R. An investigation of the southern ocean surface temperature variability using long-term optimum interpolation SST data. ISRN Oceanogr. 2013, 2013, 392632. [Google Scholar] [CrossRef]
- Maksym, T.; Stammerjohn, S.E.; Ackley, S.; Massom, R. Antarctic sea ice—A polar opposite? Oceanography 2012, 25, 140–151. [Google Scholar] [CrossRef]
- Henson, S.A.; Sarmiento, J.L.; Dunne, J.P.; Bopp, L.; Lima, I.D.; Doney, S.C.; John, J.; Beaulieu, C. Detection of anthropogenic climate change in satellite records of ocean chlorophyll and productivity. Biogeosciences 2010, 7, 621–640. [Google Scholar] [CrossRef]
- Siegel, D.A.; Buesseler, K.O.; Doney, S.C.; Sailley, S.F.; Behrenfeld, M.J.; Boyd, P.W. Global assessment of ocean carbon export by combining satellite observations and food-web models. Glob. Biogeochem. Cycles 2014, 28, 181–196. [Google Scholar] [CrossRef]
- Rousseaux, C.S.; Gregg, W.W. Recent decadal trends in global phytoplankton composition. Glob. Biogeochem. Cycles 2015, 29, 1674–1688. [Google Scholar] [CrossRef]
- Lefebvre, W.; Goosse, H.; Timmermann, R.; Fichefet, T. Influence of the Southern Annular Mode on the sea ice–ocean system. J. Geophys. Res.: Oceans 2004. [Google Scholar] [CrossRef]
- Hauck, J.; Völker, C.; Wang, T.; Hoppema, M.; Losch, M.; Wolf-Gladrow, D.A. Seasonally different carbon flux changes in the Southern Ocean in response to the southern annular mode. Glob. Biogeochem. Cycles 2013, 27, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, M.L.; Thompson, D.W. Observed relationships between the El Niño-Southern Oscillation and the extratropical zonal-mean circulation. J. Clim. 2006, 19, 276–287. [Google Scholar] [CrossRef]
- Hopkins, J.; Henson, S.A.; Painter, S.C.; Tyrrell, T.; Poulton, A.J. Phenological characteristics of global coccolithophore blooms. Glob. Biogeochem. Cycles 2015, 29, 239–253. [Google Scholar] [CrossRef]
- Smith, W.O., Jr.; Sedwick, P.N.; Arrigo, K.R.; Ainley, D.G.; Orsi, A.H. The Ross Sea in a sea of change. Oceanography 2012, 25, 90–103. [Google Scholar] [CrossRef]
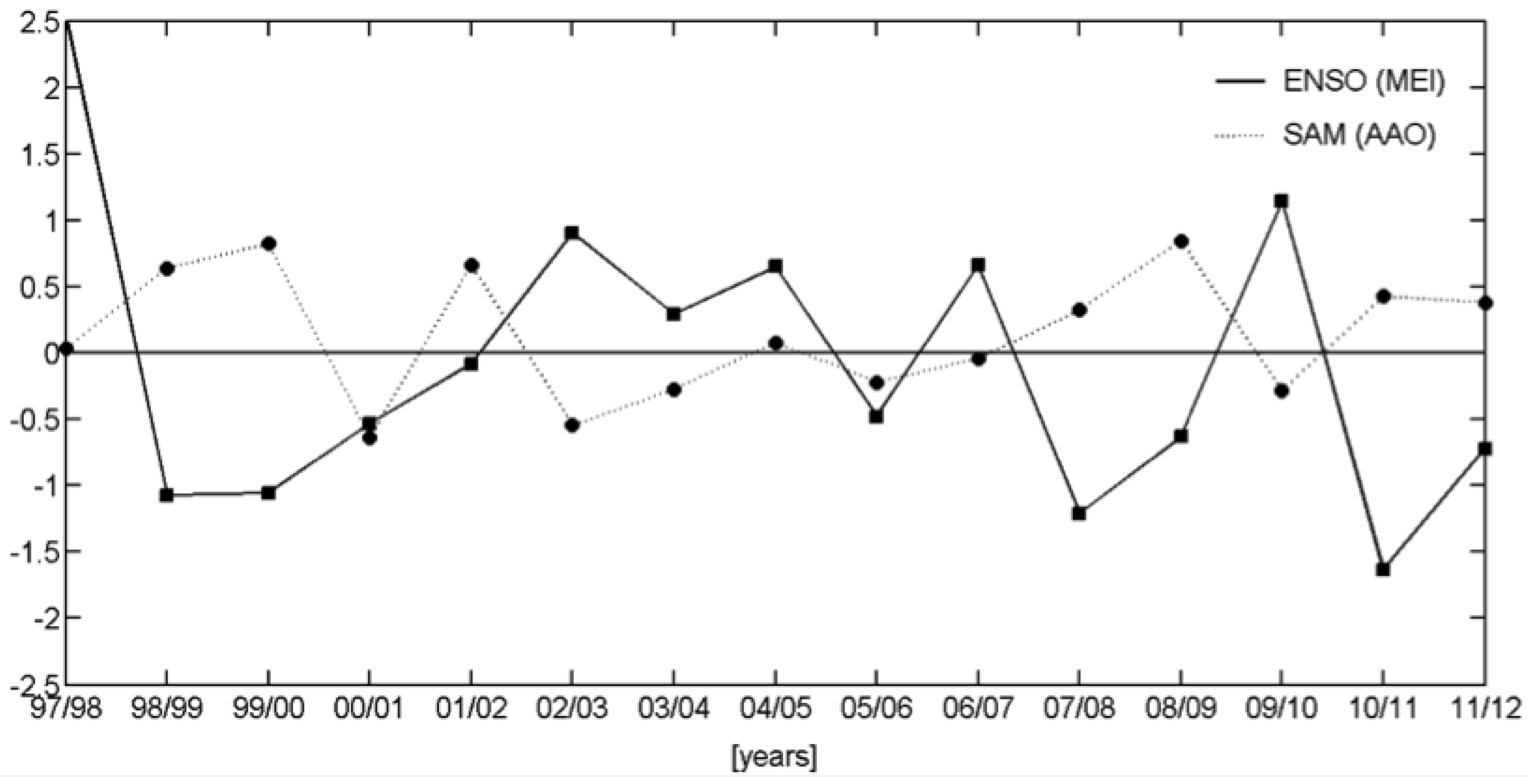


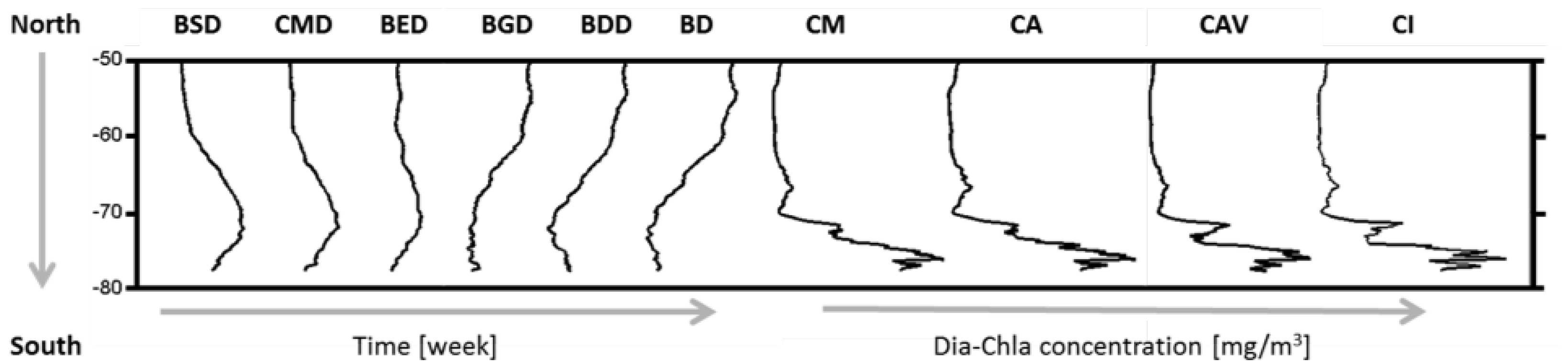


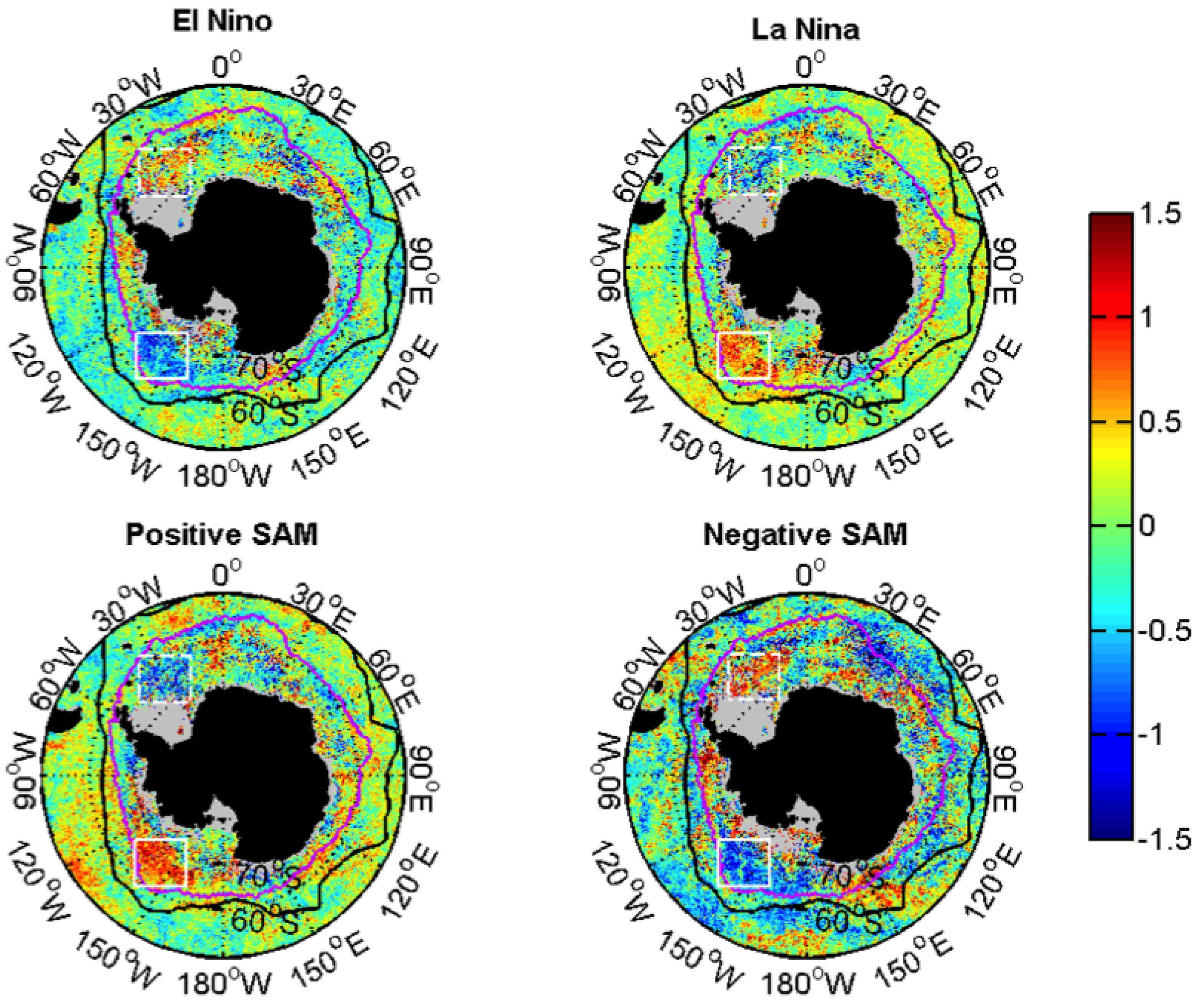
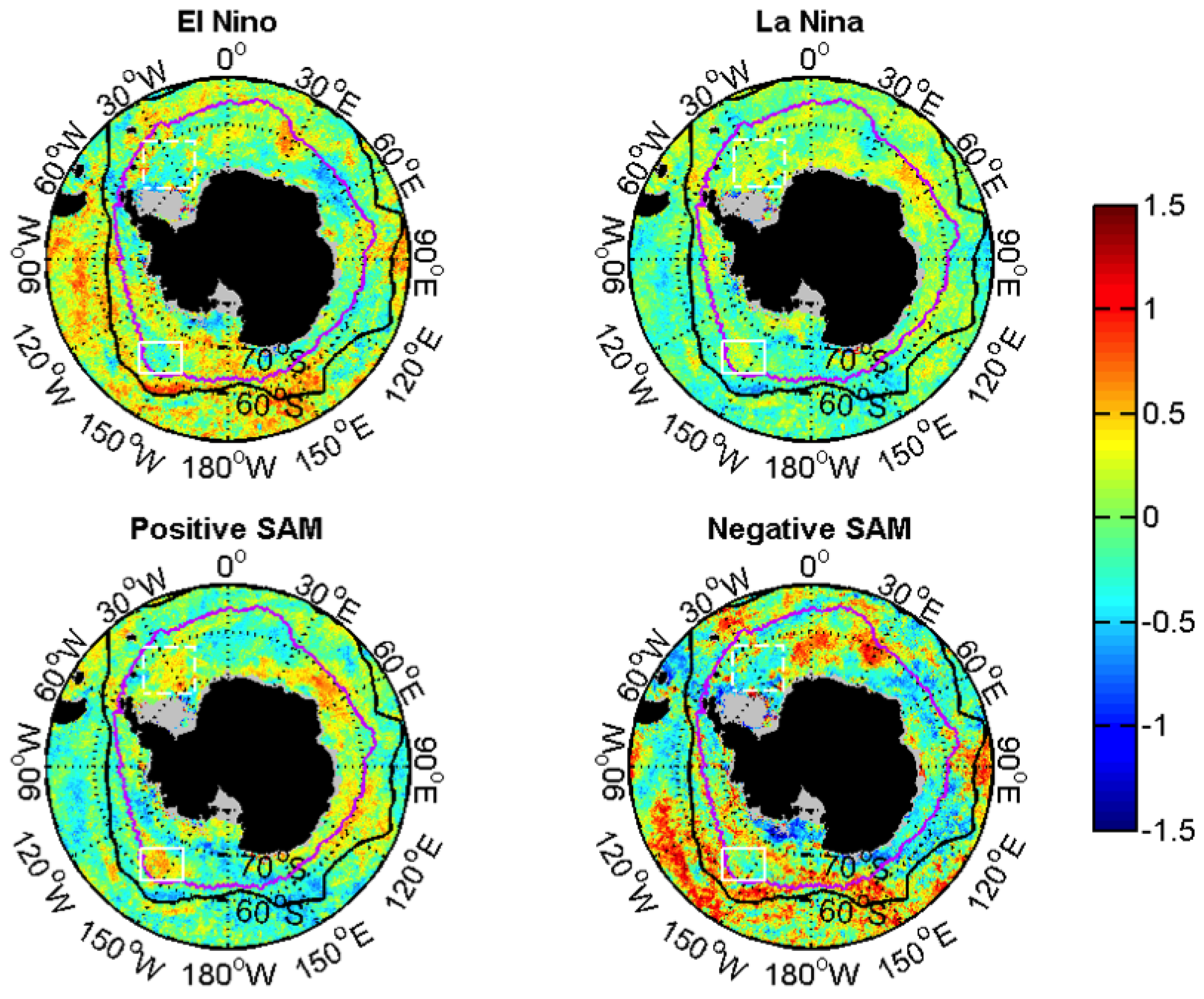
| Events | ENSO | SAM |
|---|---|---|
| 1997/1998 | + | |
| 1998/1999 | − | + |
| 1999/2000 | − | + |
| 2000/2001 | − | − |
| 2001/2002 | + | |
| 2002/2003 | + | |
| 2003/2004 | + | − |
| 2004/2005 | + | |
| 2005/2006 | − | |
| 2006/2007 | + | |
| 2007/2008 | − | + |
| 2008/2009 | − | + |
| 2009/2010 | + | − |
| 2010/2011 | − | + |
| 2011/2012 | − | + |
| Index | Abbreviation | Unit |
|---|---|---|
| Bloom Start Date | BSD | Week |
| Date of Dia-Chla Maximum | CMD | Week |
| Bloom End Date | BED | Week |
| Bloom Duration | BD | Week |
| Bloom Growth Duration | BGD | Week |
| Bloom Decline Duration | BDD | Week |
| Dia-Chla Amplitude | CA | mg·m |
| Dia-Chla Maximum | CM | mg·m |
| Dia-Chla averaged over BGD | CAV | mg·m |
| Dia-Chla integrated over BGD | CI | mg·m |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soppa, M.A.; Völker, C.; Bracher, A. Diatom Phenology in the Southern Ocean: Mean Patterns, Trends and the Role of Climate Oscillations. Remote Sens. 2016, 8, 420. https://doi.org/10.3390/rs8050420
Soppa MA, Völker C, Bracher A. Diatom Phenology in the Southern Ocean: Mean Patterns, Trends and the Role of Climate Oscillations. Remote Sensing. 2016; 8(5):420. https://doi.org/10.3390/rs8050420
Chicago/Turabian StyleSoppa, Mariana A., Christoph Völker, and Astrid Bracher. 2016. "Diatom Phenology in the Southern Ocean: Mean Patterns, Trends and the Role of Climate Oscillations" Remote Sensing 8, no. 5: 420. https://doi.org/10.3390/rs8050420
APA StyleSoppa, M. A., Völker, C., & Bracher, A. (2016). Diatom Phenology in the Southern Ocean: Mean Patterns, Trends and the Role of Climate Oscillations. Remote Sensing, 8(5), 420. https://doi.org/10.3390/rs8050420