Estimating the Total Nitrogen Concentration of Reed Canopy with Hyperspectral Measurements Considering a Non-Uniform Vertical Nitrogen Distribution
Abstract
:1. Introduction
2. Materials and Methods
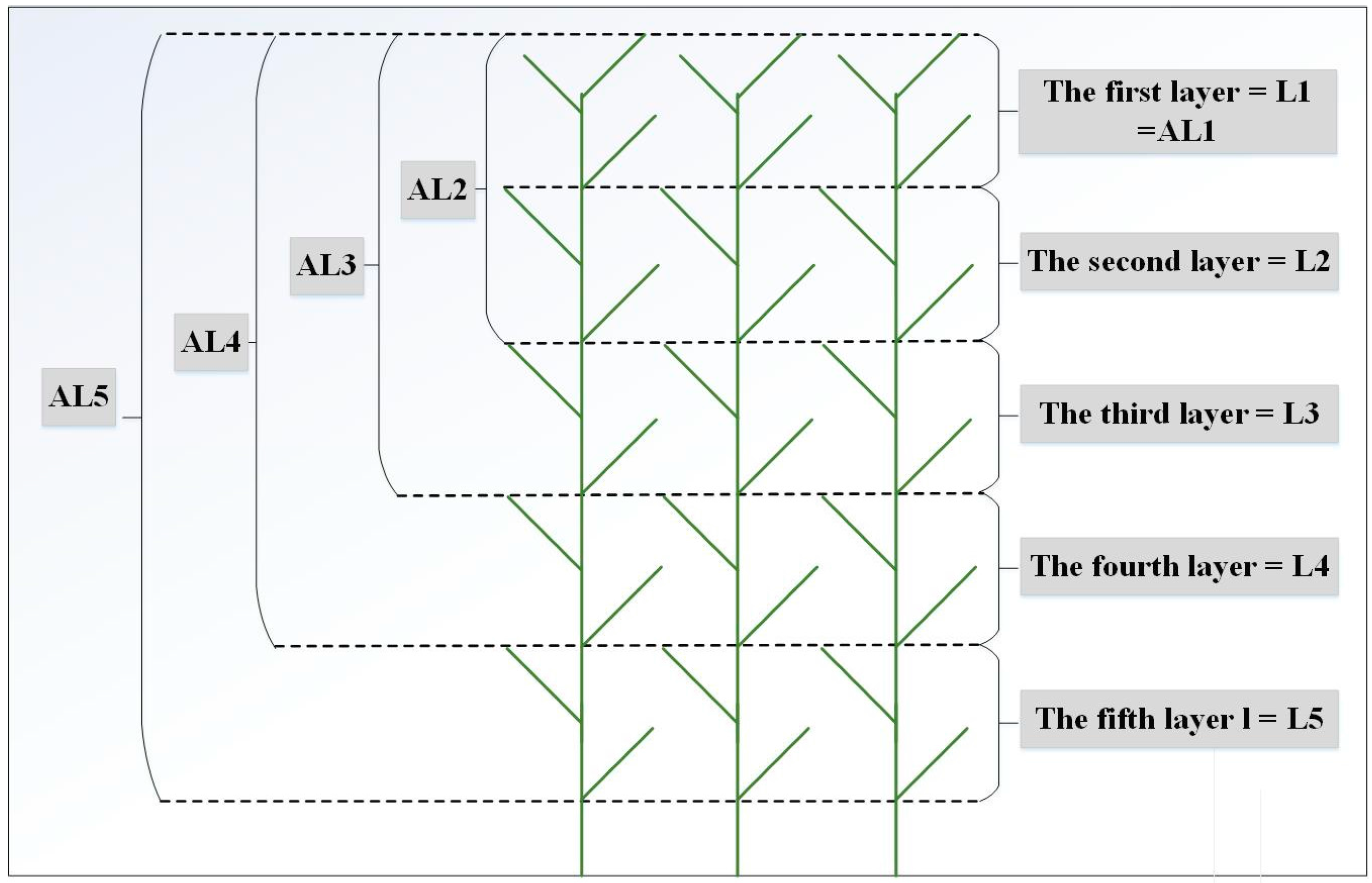
2.1. Field Experiments
2.2. Experimental Design and Spectral Reflectance Measurement
2.3. Spectral Indices
2.4. Nitrogen Concentration Measurements
2.5. Modeling and Validation of Datasets
3. Results
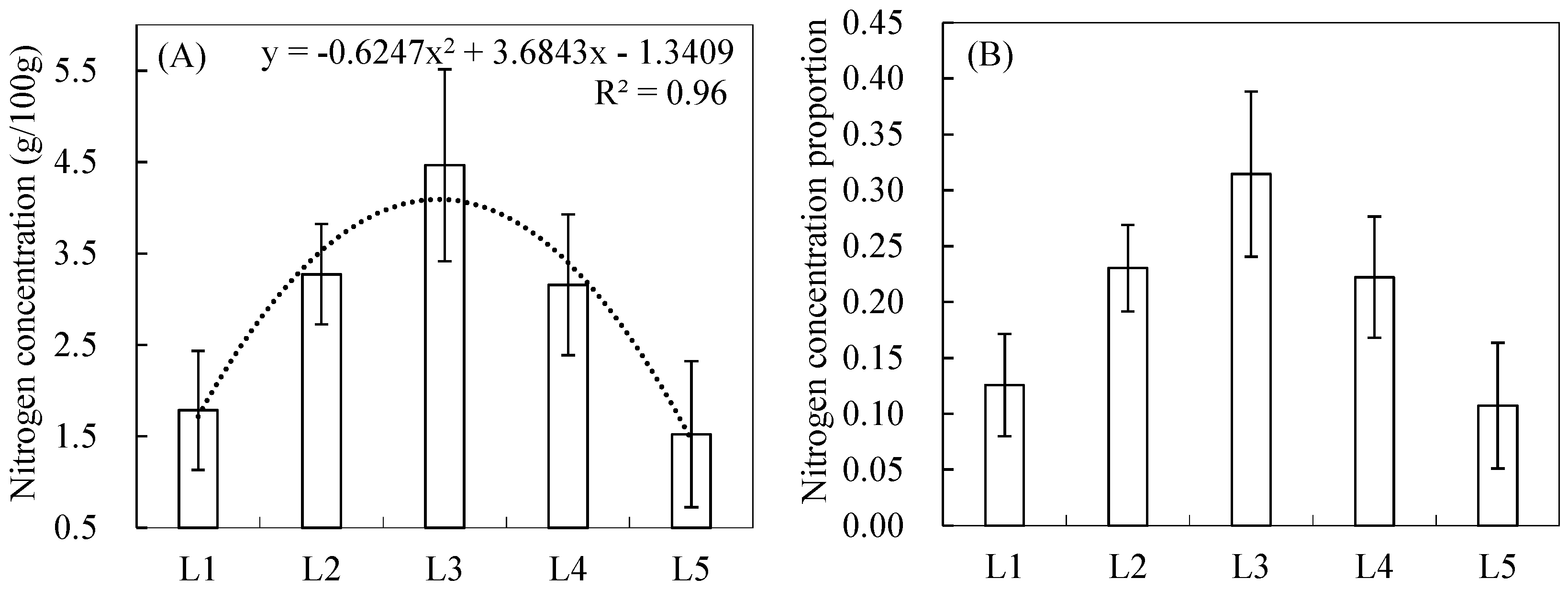
3.1. Vertical Distribution Pattern of Nitrogen Concentrations within the Reed Canopy
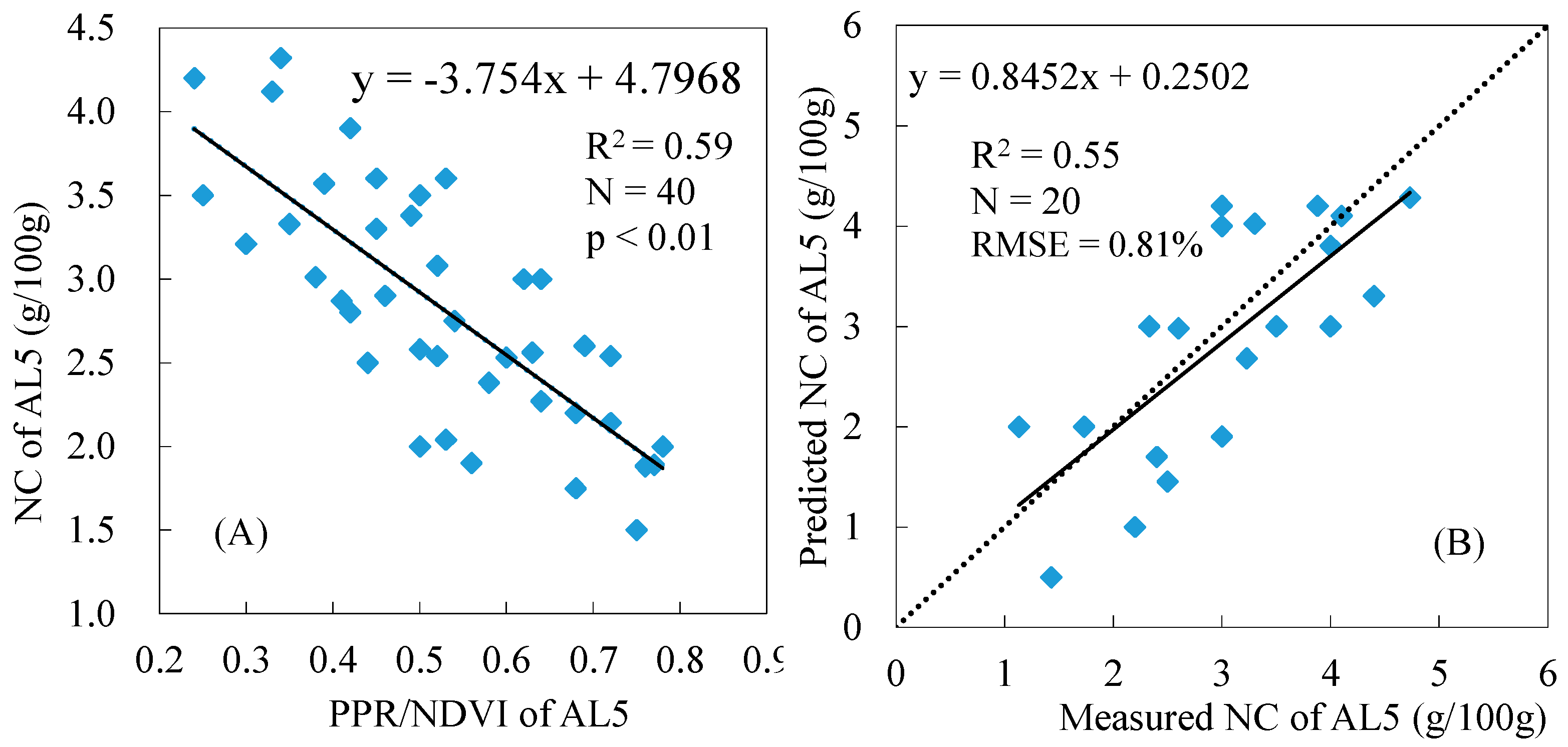
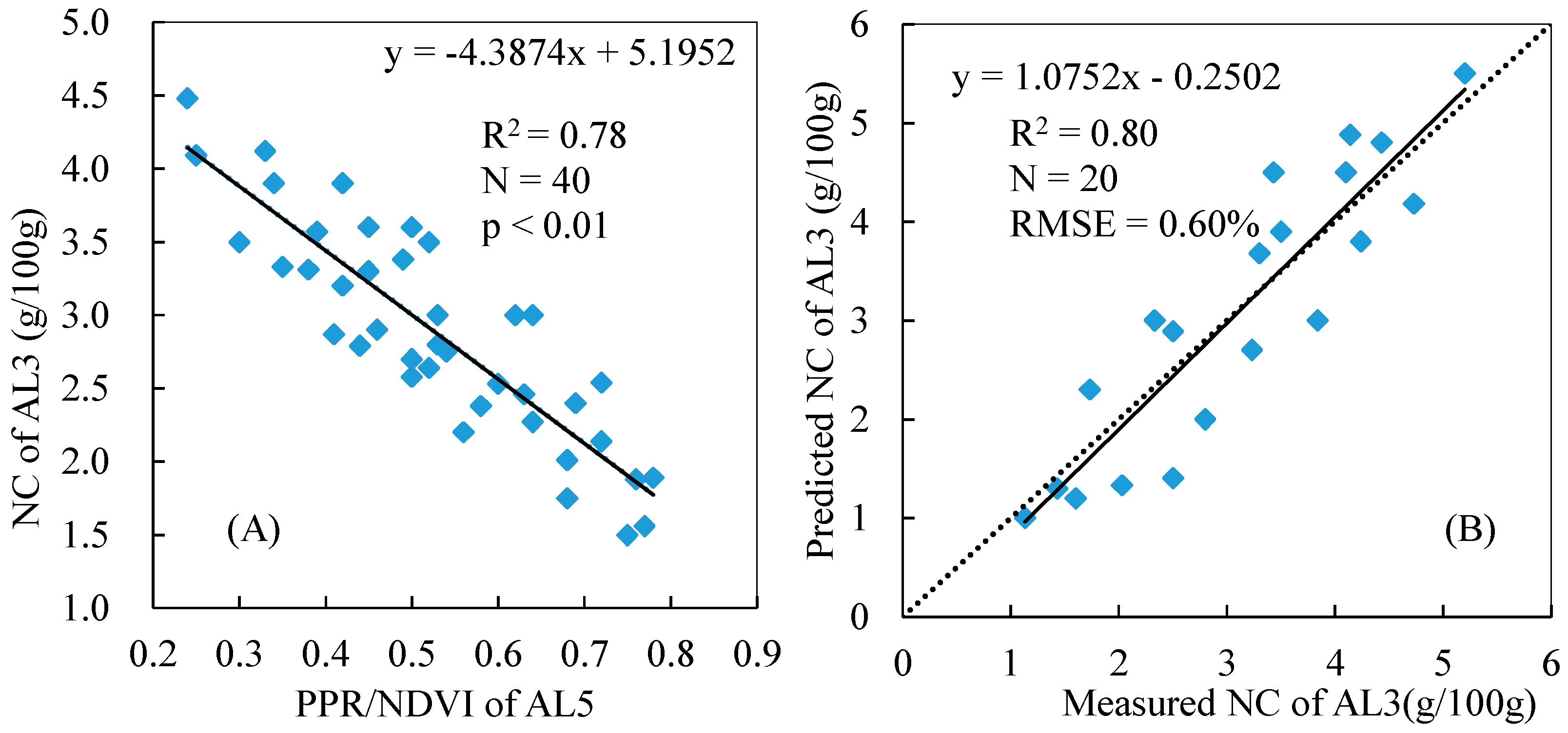
3.2. Estimation of Nitrogen Concentrations Using Spectral Indices
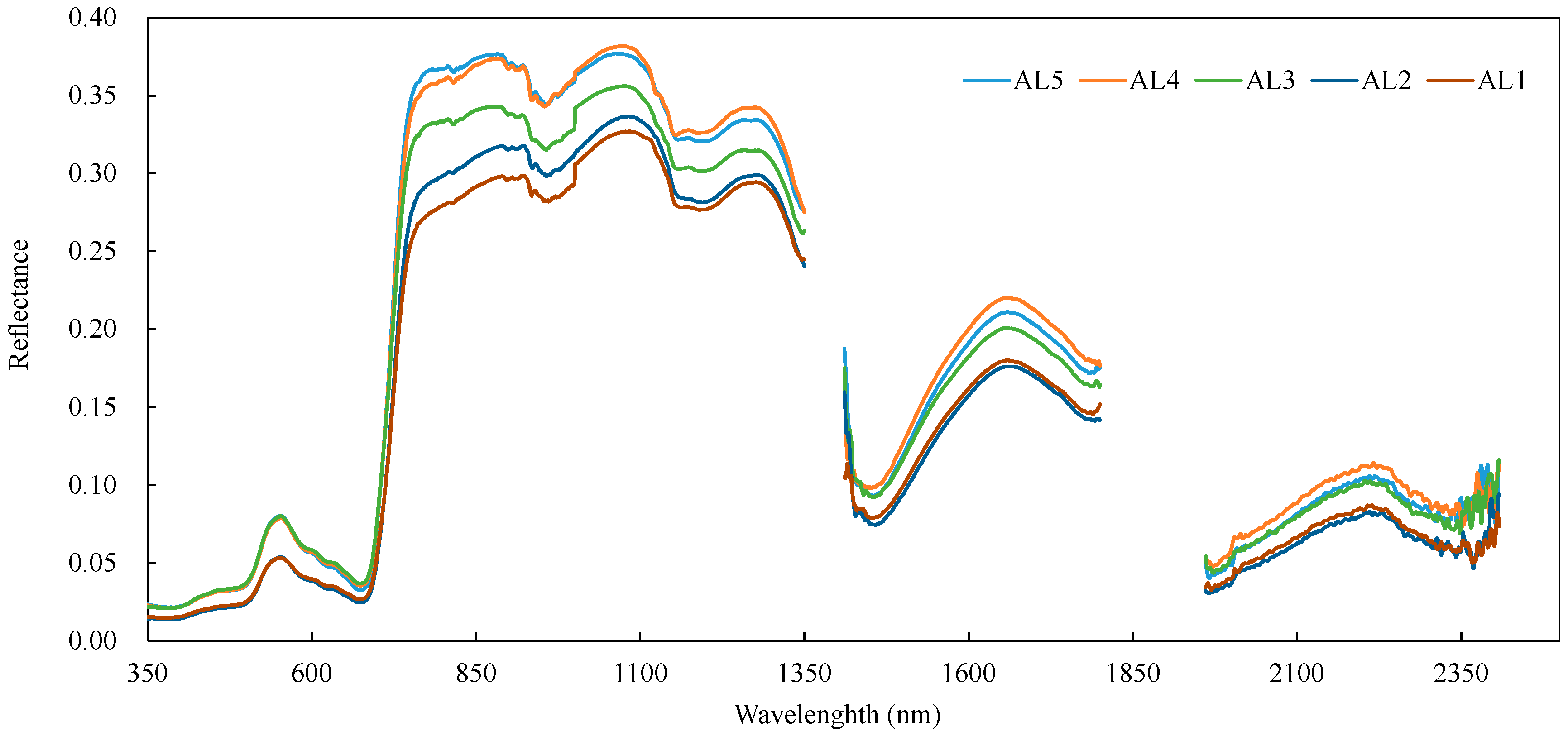
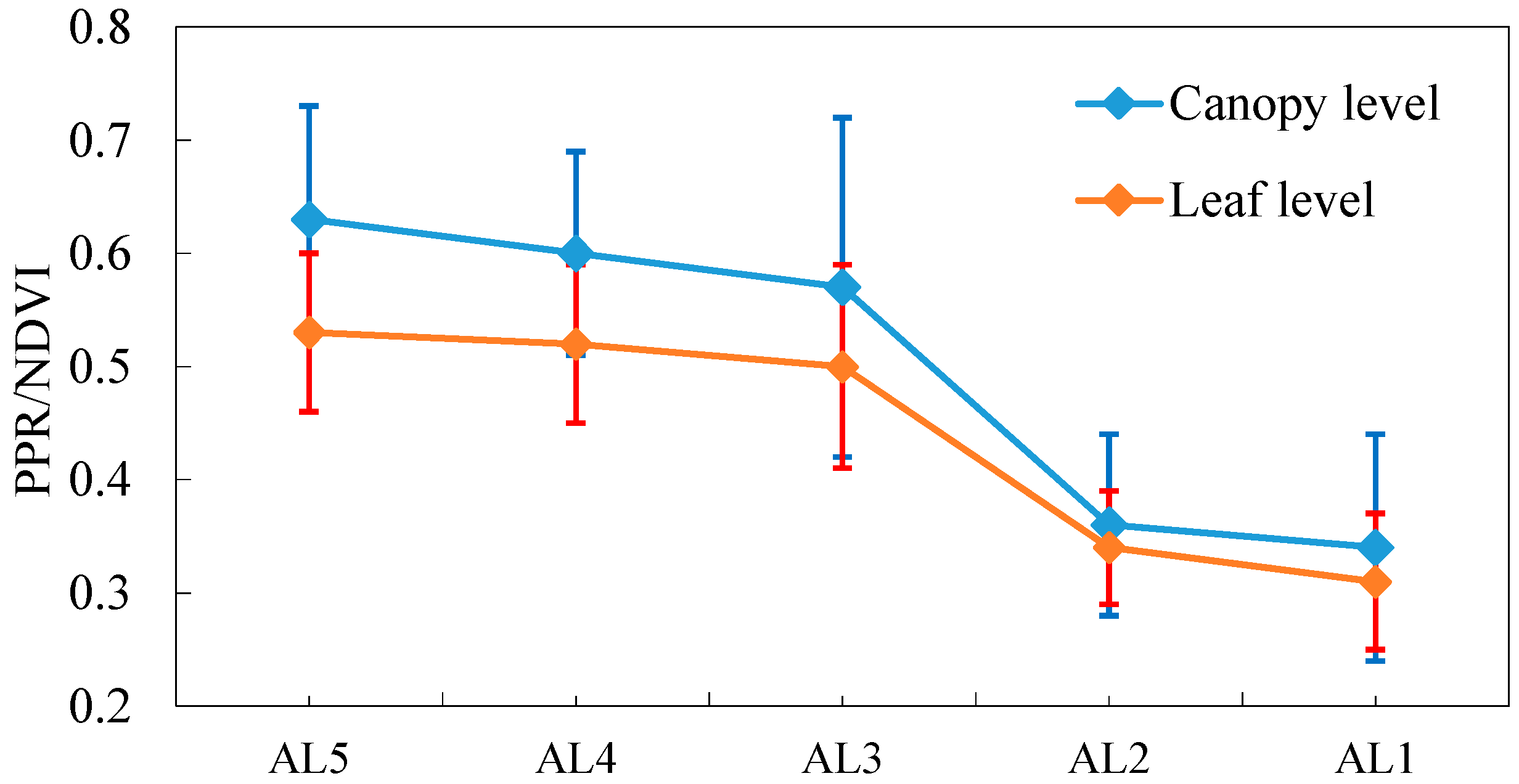
3.3. Variations in the Spectral Index for Different Layers of the Reed Canopies
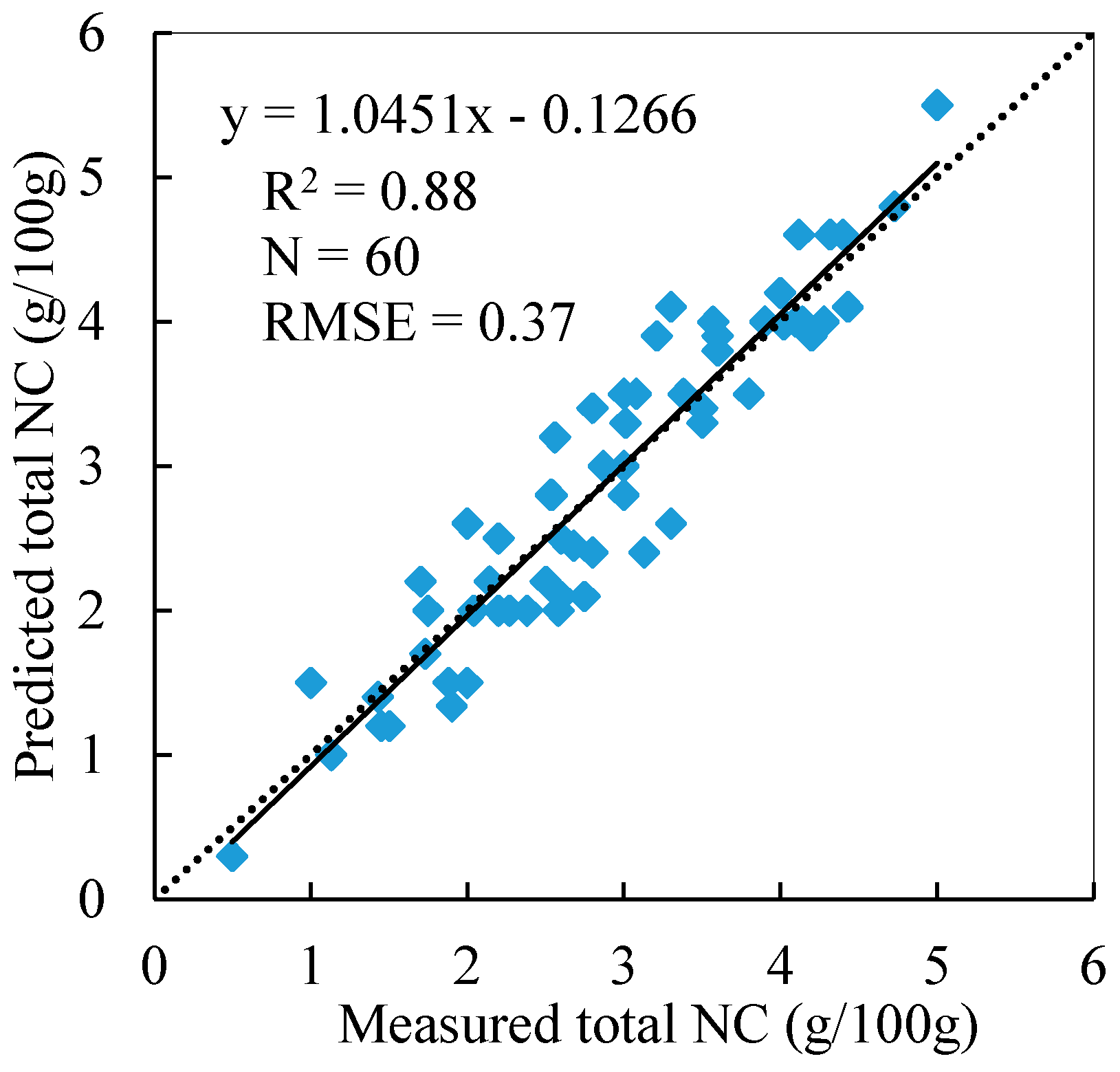
3.4. Total NC Estimation of Reed Canopies
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goldman, M.A.; Needelman, B.A. Chapter one—Wetland restoration and creation for nitrogen removal: Challenges to developing a watershed-scale approach in the chesapeake bay coastal plain. In Advances in Agronomy; Donald, L.S., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 1–38. [Google Scholar]
- Feng, L.; Yang, G.; Zhu, L.; Xu, X.; Gao, F.; Mu, J.; Xu, Y. Enhancement removal of endocrine-disrupting pesticides and nitrogen removal in a biofilm reactor coupling of biodegradable phragmites communis and elastic filler for polluted source water treatment. Bioresour. Technol. 2015, 187, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kang, H.; Ko, D.; Lee, D. Nitrogen removal from a riverine wetland: A field survey and simulation study of phragmites japonica. Ecol. Eng. 2002, 18, 467–475. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhao, X.; Song, X.; Gong, J. The inhibition and adaptability of four wetland plant species to high concentration of ammonia wastewater and nitrogen removal efficiency in constructed wetlands. Bioresour. Technol. 2016, 202, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Errecart, P.M.; Agnusdei, M.G.; Lattanzi, F.A.; Marino, M.A. Leaf nitrogen concentration and chlorophyll meter readings as predictors of tall fescue nitrogen nutrition status. Field Crop. Res. 2012, 129, 46–58. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Meza, C.M.; Rivera, J.P.; Alonso, L.; Moreno, J. A red-edge spectral index for remote sensing estimation of green lai over agroecosystems. Eur. J. Agron. 2013, 46, 42–52. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.L.; Peng, Y.; Gitelson, A.A.; Arkebauer, T.J.; Pimstein, A.; Herrmann, I.; Karnieli, A.; Rundquist, D.C.; Bonfil, D.J. Estimating green lai in four crops: Potential of determining optimal spectral bands for a universal algorithm. Agric. For. Meteorol. 2014, 192–193, 140–148. [Google Scholar] [CrossRef]
- Rana, P.; Gautam, B.; Tokola, T. Optimizing the number of training areas for modeling above-ground biomass with als and multispectral remote sensing in subtropical nepal. Int. J. Appl. Earth Obs. 2016, 49, 52–62. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L.; Schaepman, M.E. Estimating canopy water content using hyperspectral remote sensing data. Int. J. Appl. Earth Obs. 2010, 12, 119–125. [Google Scholar] [CrossRef]
- Neumann, M.; Saatchi, S.S.; Ulander, L.M.; Fransson, J.E. Assessing performance of l-and p-band polarimetric interferometric sar data in estimating boreal forest above-ground biomass. IEEE Trans. Geosci. Remote Sens. 2012, 50, 714–726. [Google Scholar] [CrossRef]
- Jin, X.; Li, Z.; Feng, H.; Xu, X.; Yang, G. Newly combined spectral indices to improve estimation of total leaf chlorophyll content in cotton. IEEE 2014, 7, 4589–4600. [Google Scholar] [CrossRef]
- Tian, X.; Su, Z.; Chen, E.; Li, Z.; van der Tol, C.; Guo, J.; He, Q. Reprint of: Estimation of forest above-ground biomass using multi-parameter remote sensing data over a cold and arid area. Int. J. Appl. Earth Obs. 2012, 17, 102–110. [Google Scholar] [CrossRef]
- Viana, H.; Aranha, J.; Lopes, D.; Cohen, W.B. Estimation of crown biomass of pinus pinaster stands and shrubland above-ground biomass using forest inventory data, remotely sensed imagery and spatial prediction models. Ecol. Model. 2012, 226, 22–35. [Google Scholar] [CrossRef]
- He, L.; Zhang, H.-Y.; Zhang, Y.-S.; Song, X.; Feng, W.; Kang, G.-Z.; Wang, C.-Y.; Guo, T.-C. Estimating canopy leaf nitrogen concentration in winter wheat based on multi-angular hyperspectral remote sensing. Eur. J. Agron. 2016, 73, 170–185. [Google Scholar] [CrossRef]
- Li, F.; Gnyp, M.L.; Jia, L.; Miao, Y.; Yu, Z.; Koppe, W.; Bareth, G.; Chen, X.; Zhang, F. Estimating n status of winter wheat using a handheld spectrometer in the north China plain. Field Crop. Res. 2008, 106, 77–85. [Google Scholar] [CrossRef]
- Ryu, C.; Suguri, M.; Umeda, M. Multivariate analysis of nitrogen content for rice at the heading stage using reflectance of airborne hyperspectral remote sensing. Field Crop. Res. 2011, 122, 214–224. [Google Scholar] [CrossRef]
- Wang, W.; Yao, X.; Yao, X.; Tian, Y.; Liu, X.; Ni, J.; Cao, W.; Zhu, Y. Estimating leaf nitrogen concentration with three-band vegetation indices in rice and wheat. Field Crop. Res. 2012, 129, 90–98. [Google Scholar] [CrossRef]
- Hirose, T.; Werger, M. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 1987, 72, 520–526. [Google Scholar] [CrossRef]
- Chen, J.-L.; Reynolds, J.F.; Harley, P.C.; Tenhunen, J.D. Coordination theory of leaf nitrogen distribution in a canopy. Oecologia 1993, 93, 63–69. [Google Scholar] [CrossRef]
- Werger, M.; Hirose, T. Leaf nitrogen distribution and whole canopy photosynthetic carbon gain in herbaceous stands. Vegetatio 1991, 97, 11–20. [Google Scholar]
- Field, C. Allocating leaf nitrogen for the maximization of carbon gain: Leaf age as a control on the allocation program. Oecologia 1983, 56, 341–347. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Huang, W.; Yang, G. Non-uniform vertical nitrogen distribution within plant canopy and its estimation by remote sensing: A review. Field Crop. Res. 2013, 142, 75–84. [Google Scholar] [CrossRef]
- Lötscher, M.; Stroh, K.; Schnyder, H. Vertical leaf nitrogen distribution in relation to nitrogen status in grassland plants. Ann. Bot. 2003, 92, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Gausman, H. Leaf reflectance vs. Leaf chlorophyll and carotenoid concentrations for eight crops. Agron. J. 1977, 69, 799–802. [Google Scholar] [CrossRef]
- Gusewell, S. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Li, J.; Zhou, X.; Li, B. Dynamics of litter decomposition of dieback phragmites in spartina-invaded salt marshes. Ecol. Eng. 2016, 90, 459–465. [Google Scholar] [CrossRef]
- Saltonstall, K.; Lambert, A.; Meyerson, L. Genetics and reproduction of common (phragmites australis) and giant reed (arundo donax). Invasive Plant Sci. Manag. 2010, 3, 495–505. [Google Scholar] [CrossRef]
- Meng, H.; Wang, X.; Tong, S.; Lu, X.; Hao, M.; An, Y.; Zhang, Z. Seed germination environments of typha latifolia and phragmites australis in wetland restoration. Ecol. Eng. 2016. [Google Scholar] [CrossRef]
- Tanaka, T.S.T.; Irbis, C.; Kumagai, H.; Inamura, T. Timing of harvest of phragmites australis (cav.) trin. Ex steudel affects subsequent canopy structure and nutritive value of roughage in subtropical highland. J. Environ. Manag. 2016, 166, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, J.; Lipping, T. Spectral characteristics of common reed beds: Studies on spatial and temporal variability. Remote Sens. 2016, 8. [Google Scholar] [CrossRef]
- Colwell, J.E. Vegetation canopy reflectance. Remote Sens. Environ. 1974, 3, 175–183. [Google Scholar] [CrossRef]
- Daughtry, C.; Walthall, C.; Kim, M.; De Colstoun, E.B.; McMurtrey, J. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Vina, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Eitel, J.; Long, D.; Gessler, P.; Smith, A. Using in-situ measurements to evaluate the new rapideye™ satellite series for prediction of wheat nitrogen status. Int. J. Remote Sens. 2007, 28, 4183–4190. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P. Evaluation of the meris terrestrial chlorophyll index (MTCI). Adv. Space Res. 2007, 39, 100–104. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Metternicht, G. Vegetation indices derived from high-resolution airborne videography for precision crop management. Int. J. Remote Sens. 2003, 24, 2855–2877. [Google Scholar] [CrossRef]
- Rouse, J., Jr. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA: Hampton, VA, USA, 1974.
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Zhao, C.; Zhao, M.; Huang, W.; Wang, C. Vertical distribution of nitrogen in different layers of leaf and stem and their relationship with grain quality of winter wheat. J. Plant Nutr. 2005, 28, 73–91. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Yang, G.; Feng, H. Variations in crop variables within wheat canopies and responses of canopy spectral characteristics and derived vegetation indices to different vertical leaf layers and spikes. Remote Sens. Environ. 2015, 169, 358–374. [Google Scholar] [CrossRef]
- Monsi, M.; Saeki, T. On the factor light in plant communities and its importance for matter production. Ann. Bot. 2005, 95, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Werger, M.; Pons, T.; Van Rheenen, J. Canopy structure and leaf nitrogen distribution in a stand of lysimachia vulgaris l. As influenced by stand density. Oecologia 1988, 77, 145–150. [Google Scholar] [CrossRef]
- Bindraban, P. Impact of canopy nitrogen profile in wheat on growth. Field Crop. Res. 1999, 63, 63–77. [Google Scholar] [CrossRef]
- Bertheloot, J.; Martre, P.; Andrieu, B. Dynamics of light and nitrogen distribution during grain filling within wheat canopy. Plant Physiol. 2008, 148, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Pozo, A.D.; Dennett, M. Analysis of the distribution of light, leaf nitrogen, and photosynthesis within the canopy of vicia faba l. At two contrasting plant densities. Aust. J. Agric. Res. 1999, 50, 183–189. [Google Scholar] [CrossRef]
- Anten, N.P.; Werger, M.J.; Medina, E. Nitrogen distribution and leaf area indices in relation to photosynthetic nitrogen use efficiency in savanna grasses. Plant Ecol. 1998, 138, 63–75. [Google Scholar] [CrossRef]
- Lemaire, G.; Onillon, B.; Gosse, G.; Chartier, M.; Allirand, J. Nitrogen distribution within a lucerne canopy during regrowth: Relation with light distribution. Ann. Bot. 1991, 68, 483–488. [Google Scholar]
- Knyazikhin, Y.; Schull, M.A.; Stenberg, P.; Mõttus, M.; Rautiainen, M.; Yang, Y.; Marshak, A.; Carmona, P.L.; Kaufmann, R.K.; Lewis, P. Hyperspectral remote sensing of foliar nitrogen content. Proc. Natl. Acad. Sci. USA 2013, 110, E185–E192. [Google Scholar] [CrossRef] [PubMed]
- Yoder, B.J.; Pettigrew-Crosby, R.E. Predicting nitrogen and chlorophyll content and concentrations from reflectance spectra (400–2500 nm) at leaf and canopy scales. Remote Sens. Environ. 1995, 53, 199–211. [Google Scholar] [CrossRef]
- Jin, X.; Diao, W.; Xiao, C.; Wang, F.; Chen, B.; Wang, K.; Li, S.-K. Estimation of wheat nitrogen status under drip irrigation with canopy spectral indices. J. Agric. Sci. 2015, 153, 1281–1291. [Google Scholar] [CrossRef]
| Index | Formula | Reference |
|---|---|---|
| Red edge model (CIred edge) | (R750/R720) − 1 | [34] |
| Modified triangular vegetation index 2 (MTVI2) | 1.5(1.2(R800 − R550) − 2.5(R670 − R550))/sqrt((2R800 + 1)2 − (6R800 − 5sqrt(R670)) − 0.5) | [35] |
| Modified chlorophyll absorption ratio index (MCARI) | (R700 − R670 − 0.2(R700 − R550))(R700/R670) | [33] |
| Combined Index I (MCARI/MTVI2) | MCARI/MTVI2 | [36] |
| Transformed chlorophyll absorption in reflectance index (TCARI) | 3((R700 − R670) − 0.2(R700 − R550)(R700/R670)) | [37] |
| Optimized soil-adjusted vegetation index (OSAVI) | 1.16(R800 − R670)/(R800 + R670 + 0.16) | [38] |
| Combined Index II (TCARI/OSAVI) | TCARI/OSAVI | [37] |
| MERIS terrestrial chlorophyll index (MTCI) | (R750 − R710)/(R710 − R680) | [39] |
| Structure insensitive pigment index (SIPI) | (R800 − R445)/(R800 − R680) | [40] |
| Plant pigment ratio (PPR) | (R550 − R450)/(R550 + R450) | [41] |
| Normalized difference vegetation index (NDVI) | (R800 − R670)/(R800 + R670) | [42] |
| Modified MERIS terrestrial chlorophyll index (MMTCI) | [(R750 − R680 + 0.03) (R750 − R710)]/(R710 − R680) | [39] |
| Double-peak canopy nitrogen index (DCNI) | ((R720 − R700)/(R700 − R670))/(R720 − R670 + 0.03) | [43] |
| Combined Index III (PPR/NDVI) | PPR/NDVI | [12] |
| Spectral Index | LAI | NC | ||
|---|---|---|---|---|
| R2 | RMSE (%) | R2 | RMSE (%) | |
| CIred edge | 0.26 | 1.1 | 0.56 | 0.34 |
| MTVI2 | 0.78 | 0.63 | 0.14 | 1.28 |
| MCARI | 0.58 | 0.80 | 0.51 | 0.51 |
| MCARI/MTVI2 | 0.18 | 1.60 | 0.57 | 0.42 |
| TCARI | 0.21 | 1.11 | 0.57 | 0.38 |
| OSAVI | 0.62 | 0.91 | 0.48 | 1.25 |
| TCARI/OSAVI | 0.16 | 1.90 | 0.58 | 0.41 |
| MTCI | 0.57 | 1.50 | 0.52 | 0.32 |
| SIPI | 0.40 | 1.78 | 0.46 | 0.60 |
| PPR | 0.42 | 1.26 | 0.56 | 0.35 |
| NDVI | 0.70 | 0.34 | 0.55 | 0.51 |
| MMTCI | 0.19 | 1.50 | 0.50 | 0.44 |
| DCNI | 0.16 | 1.60 | 0.59 | 0.51 |
| PPR/NDVI | 0.19 | 1.68 | 0.62 | 0.41 |
| Index | Rv (%) | |||
|---|---|---|---|---|
| (AL5-AL4)/AL5 | (AL4-AL3)/AL4 | (AL3-AL2)/AL3 | (AL2-AL1)/AL2 | |
| MCARI/MTVI2 | 3.5 ± 0.51 | 4.1 ± 0.65 | 28.8 ± 6.11 | 4.46 ± 1.12 |
| TCARI/OSAVI | 3.2 ± 0.42 | 3.9 ± 0.78 | 26.7 ± 4.24 | 4.23 ± 0.98 |
| MMTCI | −4.12 ± 0.87 | −4.01 ± 0.88 | −32.32 ± 4.13 | −4.13 ± 0.97 |
| DCNI | −4.34 ± 0.92 | −4.26 ± 0.56 | −34.56 ± 6.01 | −4.07 ± 1.28 |
| PPR/NDVI | 4.76 ± 0.65 | 5 ± 0.71 | 36.84 ± 5.50 | 5.56 ± 1.21 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Ma, R.; Feng, H.; Li, X. Estimating the Total Nitrogen Concentration of Reed Canopy with Hyperspectral Measurements Considering a Non-Uniform Vertical Nitrogen Distribution. Remote Sens. 2016, 8, 789. https://doi.org/10.3390/rs8100789
Luo J, Ma R, Feng H, Li X. Estimating the Total Nitrogen Concentration of Reed Canopy with Hyperspectral Measurements Considering a Non-Uniform Vertical Nitrogen Distribution. Remote Sensing. 2016; 8(10):789. https://doi.org/10.3390/rs8100789
Chicago/Turabian StyleLuo, Juhua, Ronghua Ma, Huihui Feng, and Xinchuan Li. 2016. "Estimating the Total Nitrogen Concentration of Reed Canopy with Hyperspectral Measurements Considering a Non-Uniform Vertical Nitrogen Distribution" Remote Sensing 8, no. 10: 789. https://doi.org/10.3390/rs8100789
APA StyleLuo, J., Ma, R., Feng, H., & Li, X. (2016). Estimating the Total Nitrogen Concentration of Reed Canopy with Hyperspectral Measurements Considering a Non-Uniform Vertical Nitrogen Distribution. Remote Sensing, 8(10), 789. https://doi.org/10.3390/rs8100789








