Assessment of the Applicability of Hue from In Situ Spectral Measurements to Remote Sensing of Plant Phenology
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. In Situ Data
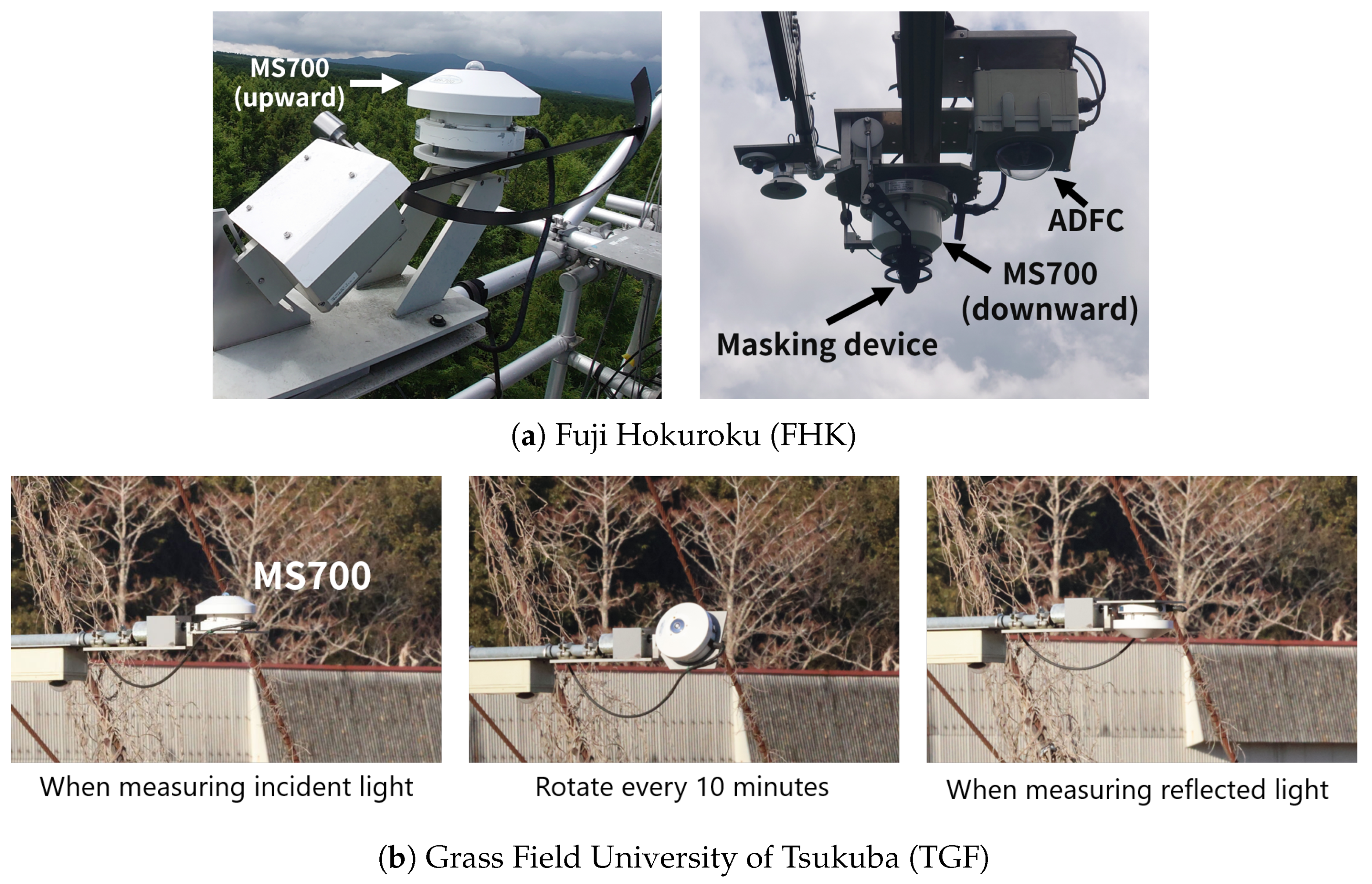
2.2.1. Hemispherical Spectral Radiometer: HSSR
2.2.2. Automatic Digital Fisheye Camera: ADFC
2.3. Analysis of HSSR Data
2.3.1. Simulation of Satellite Channels
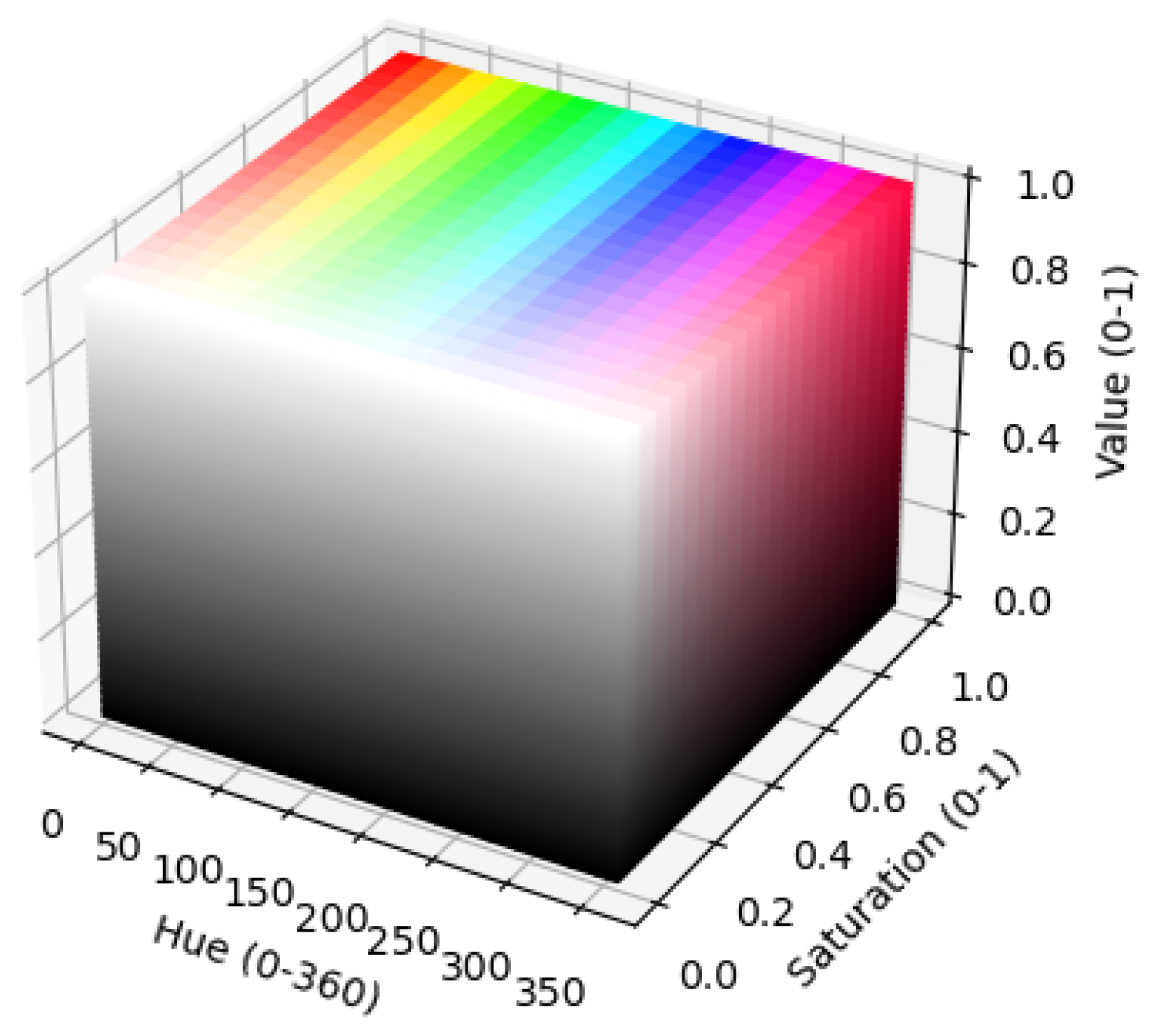
2.3.2. Calculation of VIs and Hue
3. Results
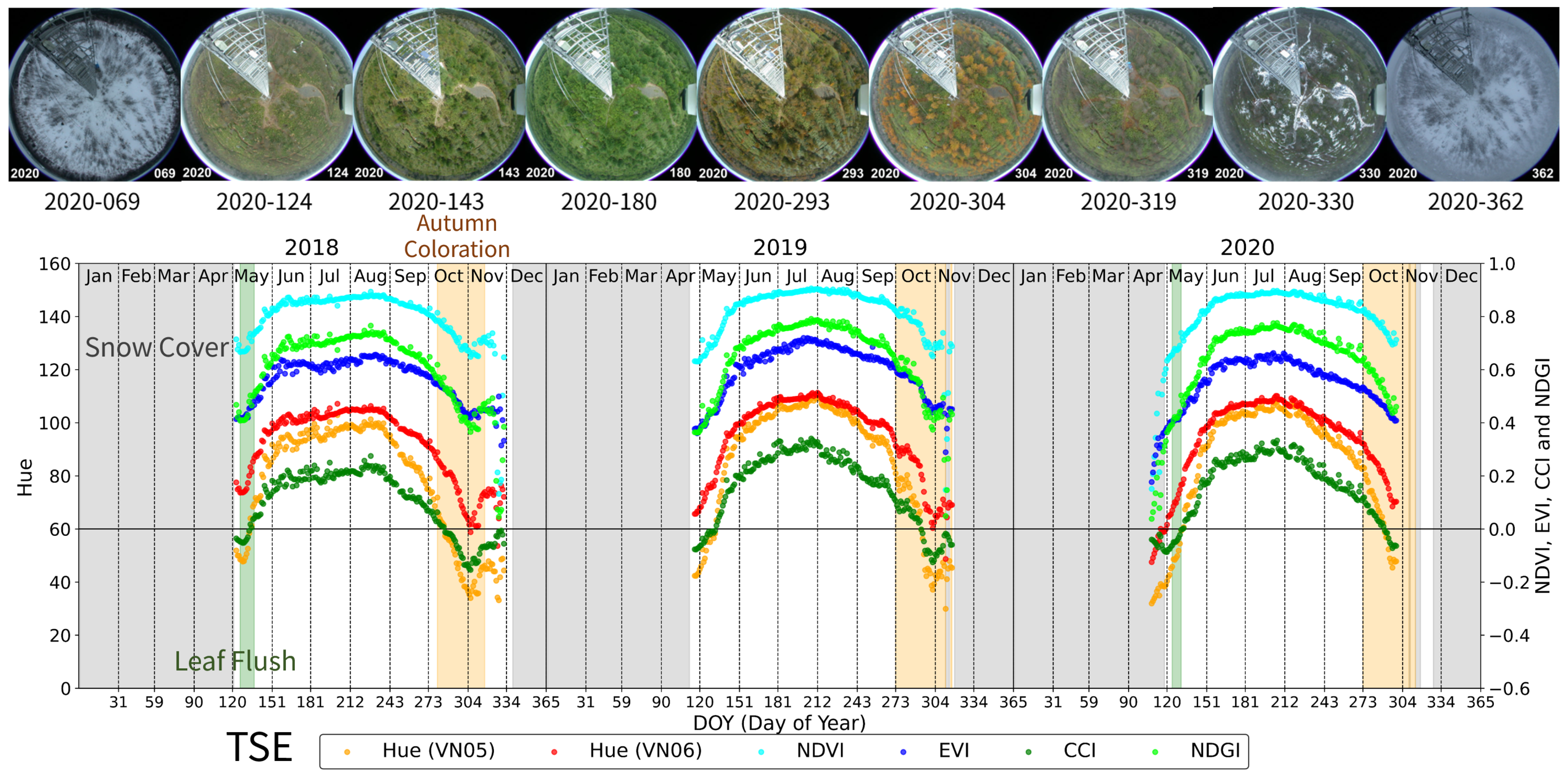
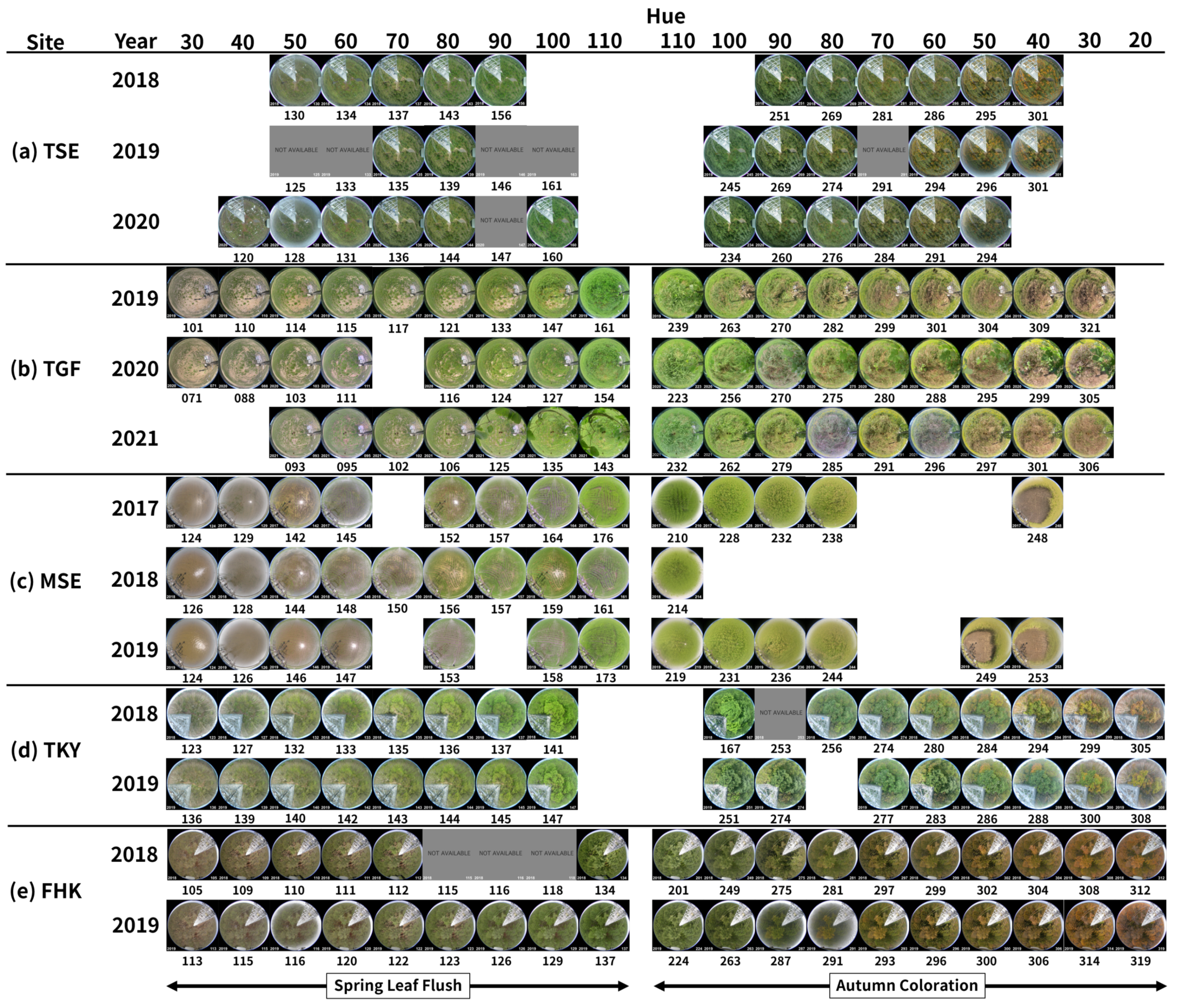
3.1. Teshio Site (TSE): Deciduous Needle-Leaved Forest (DNF)
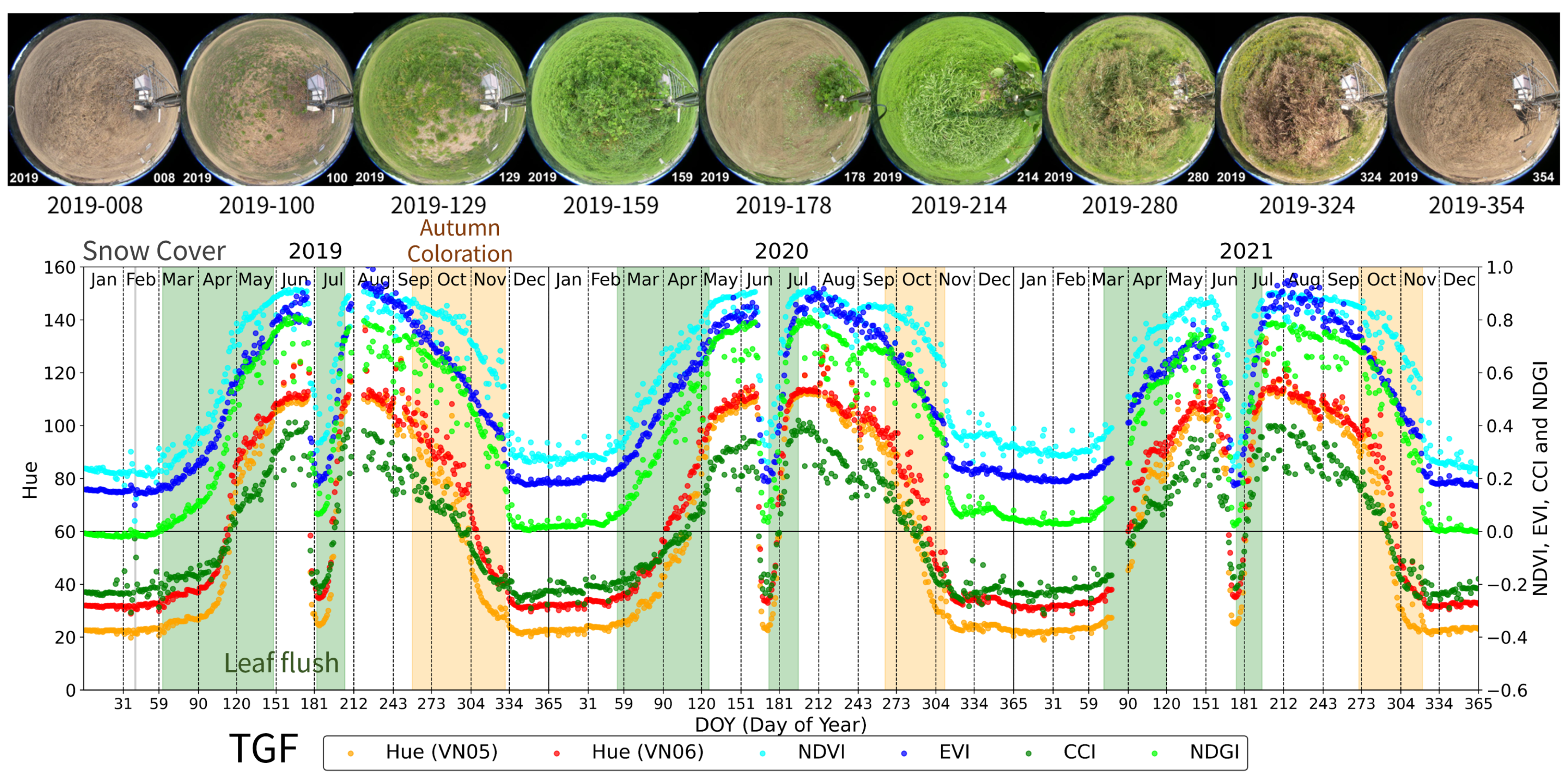
3.2. Grass Field of CRiED in the University of Tsukuba (TGF): Grassland
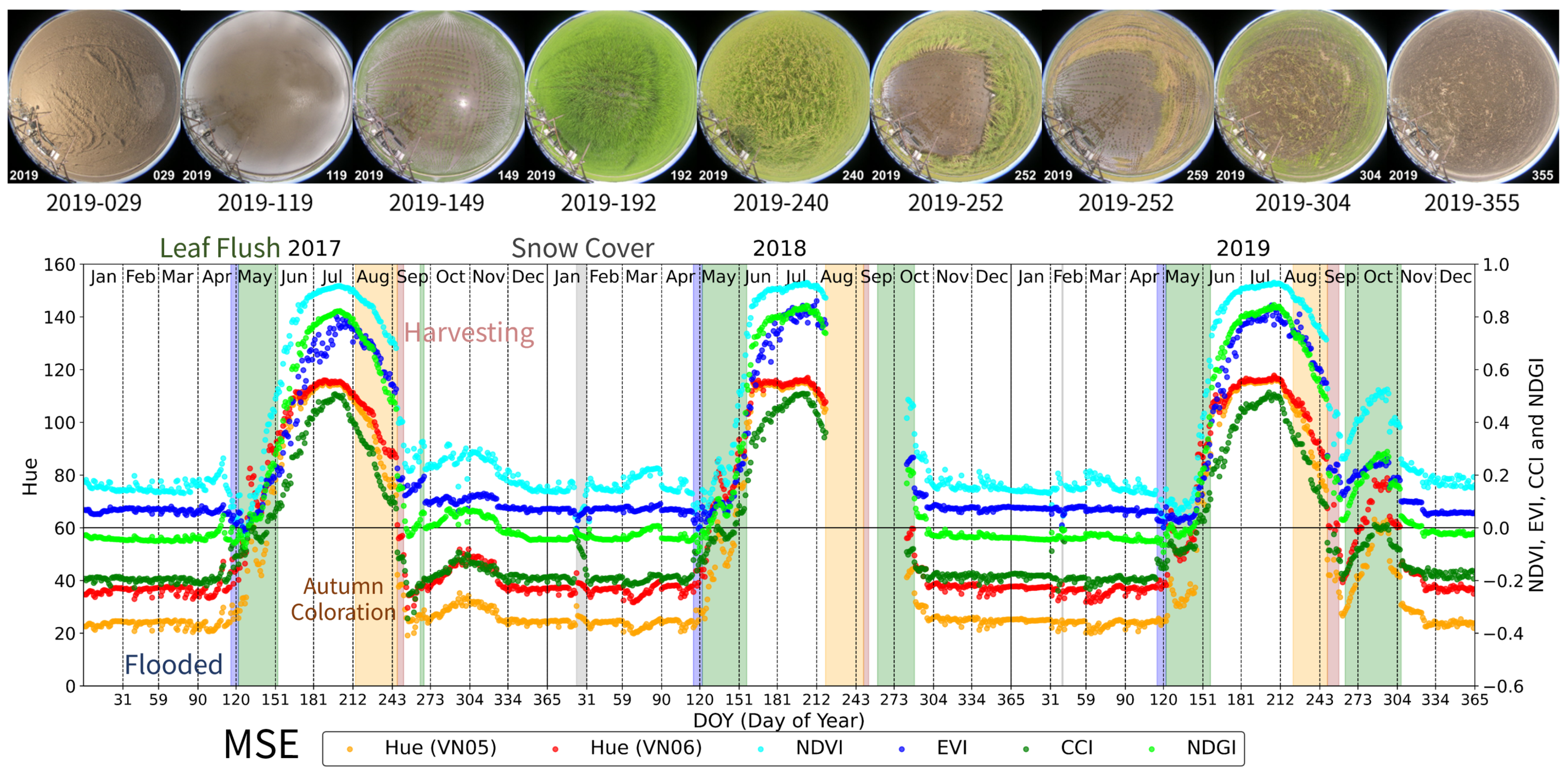
3.3. Mase Site (MSE): Rice Paddy
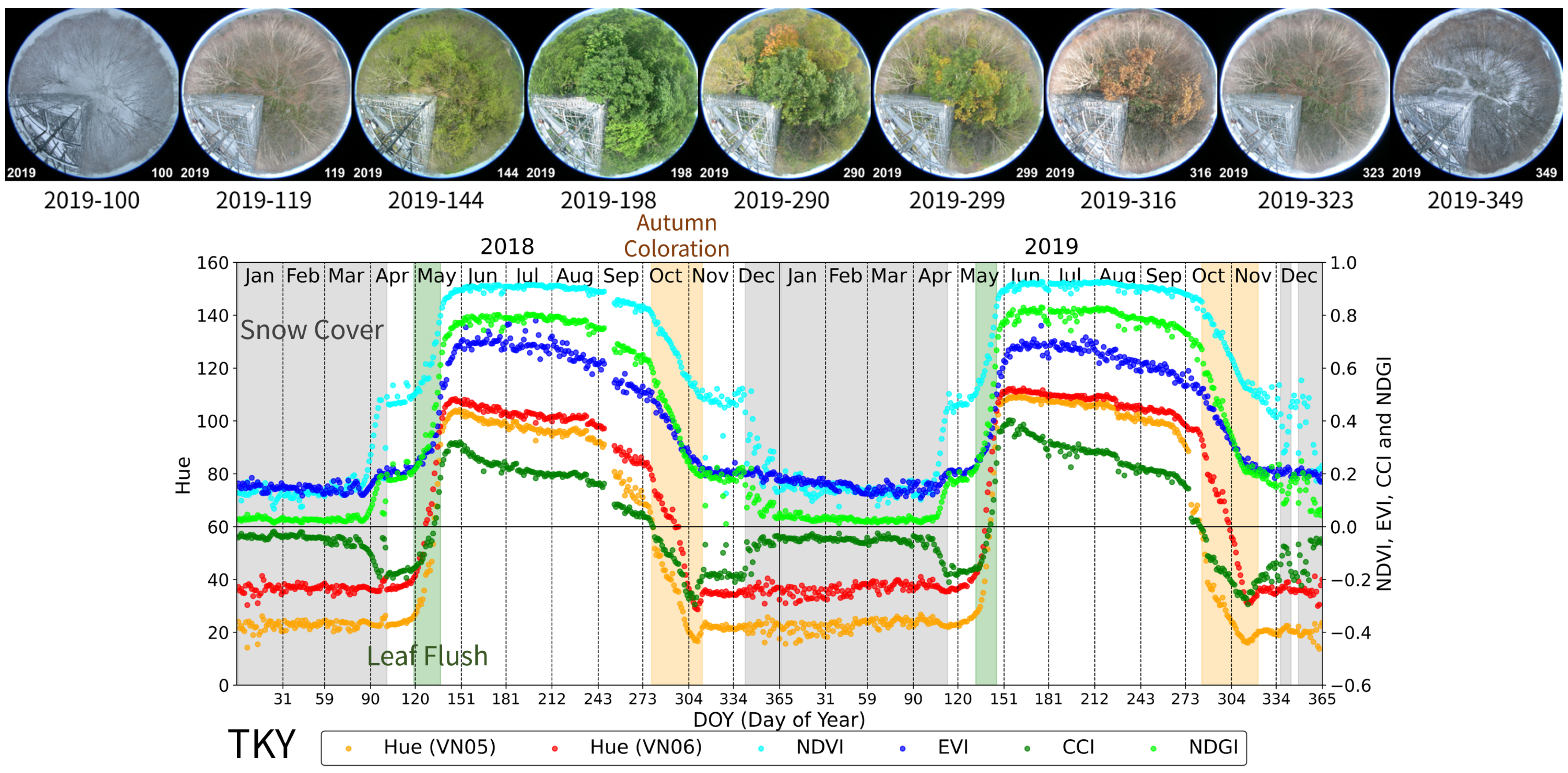
3.4. Takayama Site (TKY): Deciduous Broad-Leaved Forest (DBF)
3.5. Fuji Hokuroku Site (FHK): Deciduous Needle-Leaved Forest (DNF)
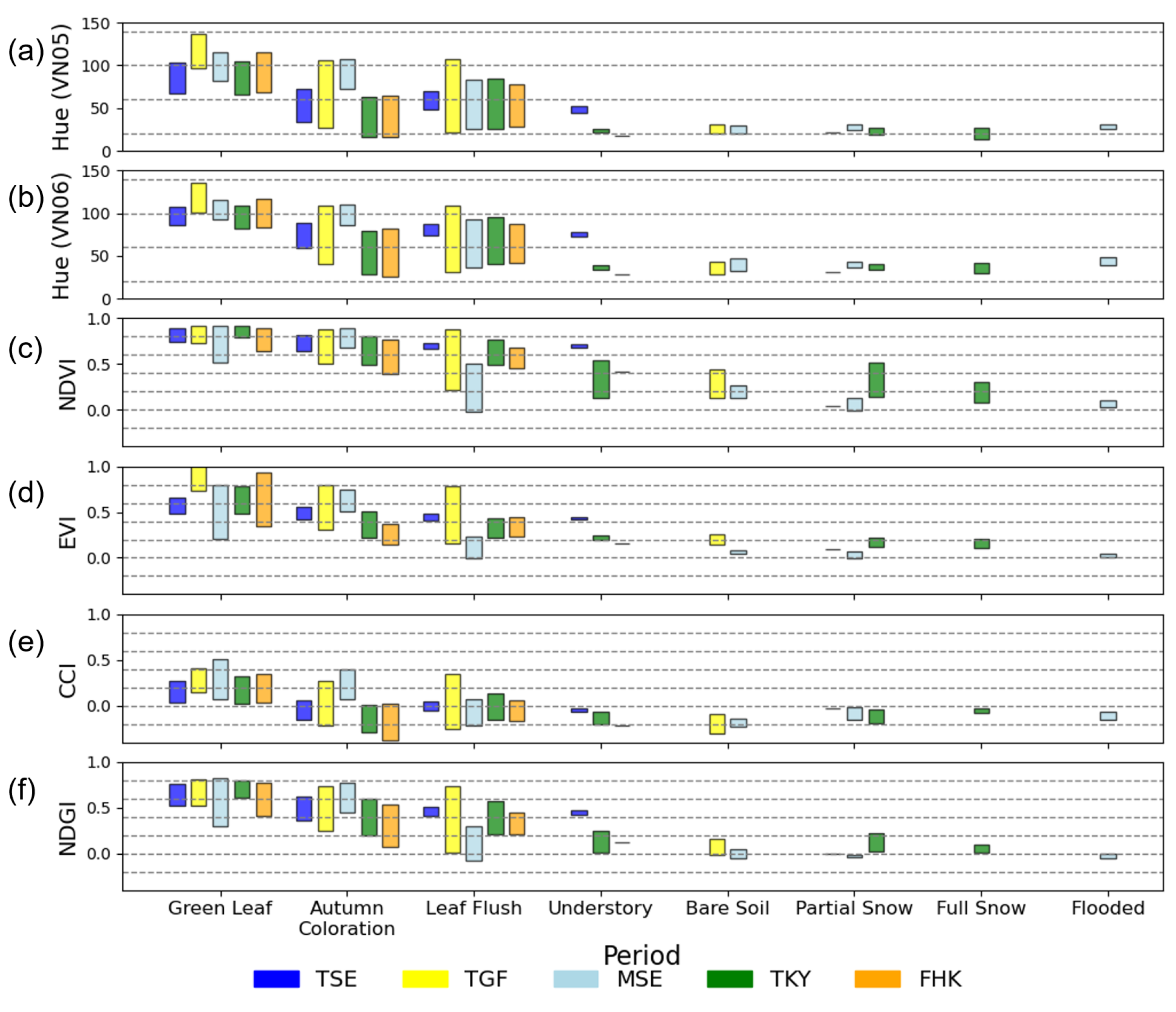
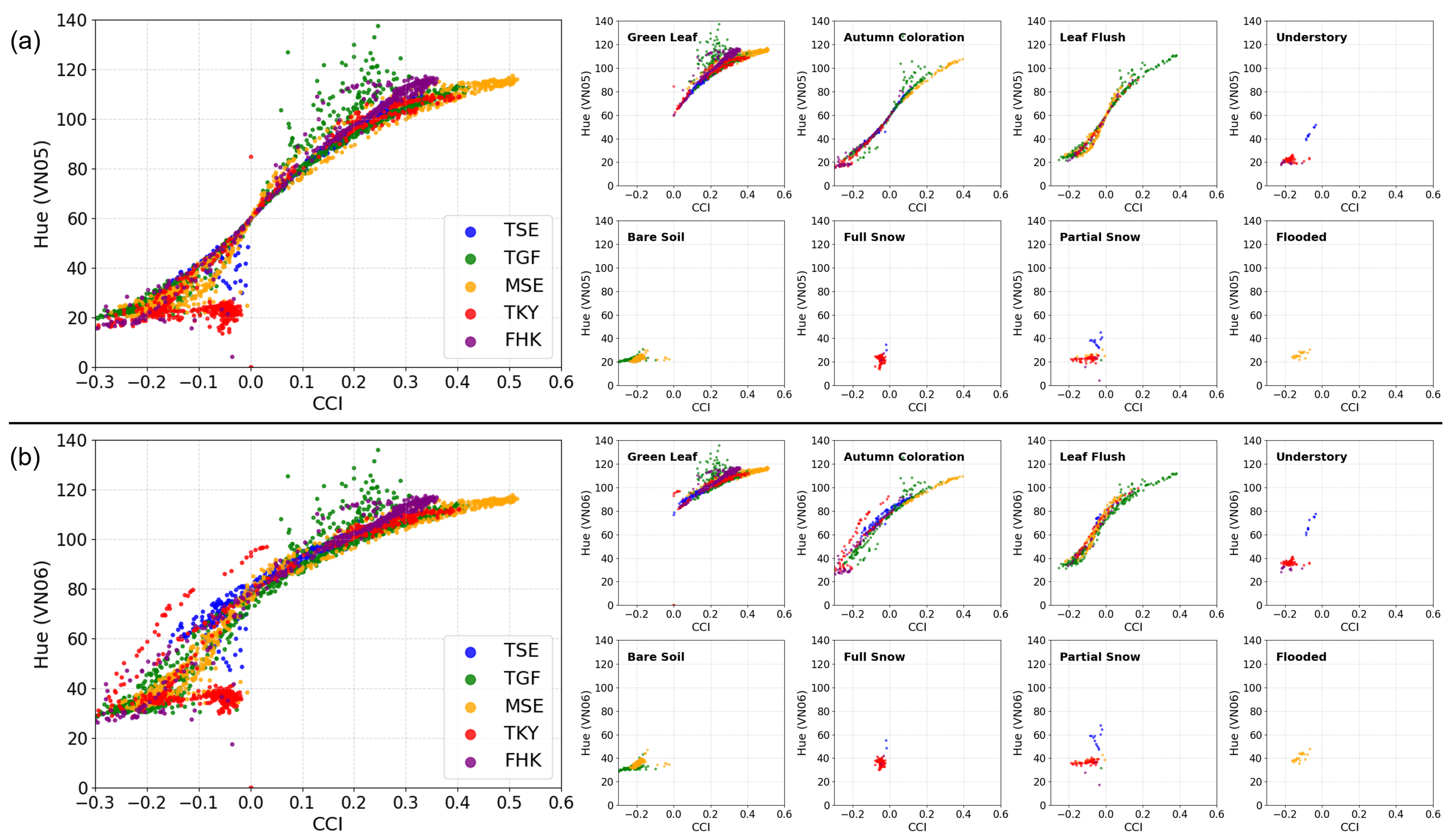
3.6. Relationship Between Spectral Indices and Site Condition
- Leaf Flush period: At TSE, TGF, TKY, and FHK, this period was defined as the time from the onset of budburst to the subsequent development and expansion of leaves. At MSE, it was defined as the period from rice planting to the full coverage of the soil by green leaves.
- Green Leaf period: This is a period of green canopy covered with green leaves after the leaf flush. At TSE, TGF, TKY, and FHK, it ended by the start of the autumn coloration. At MSE, it ended with the earing of rice.
- Autumn Coloration period: This is a period during which the canopy or dominant species changed leaf color from green to yellow or red, ending with leaf fall. At MSE, it was defined as the period from earing of rice to harvesting.
- Understory Vegetation period (only TSE, TKY, and FHK): This period refers to times when understory vegetation was visible before the canopy leaf flush in spring or after leaf fall in autumn.
- Bare Soil period (only TGF and MSE): Defined as the period when the bare soil surface was exposed, observed at TGF and MSE.
- Snow Cover period: Refers to the period during which snow was present, including the snowmelt phase.
- –
- Full Snow Cover: Ground surface mostly covered by snow (more than 90%).
- –
- Partial Snow Cover: Only part of the ground surface covered by snow (10%–90%).
- Flooded period (only MSE): At MSE, it is defined as the period of surface water flooding prior to rice planting.
| Site ID | Year | Green Leaf | Autumn Coloration | Leaf Flush | Understory Vegetation | Bare Soil | Full Snow | Partial Snow | Flooded |
|---|---|---|---|---|---|---|---|---|---|
| TSE | 2018 | 138–279 | 280–317 | 126–137 | 122–125, 324 | 1–110, 339–365 | 111–121 | ||
| 2019 | 134–272 | 273–311, 316–317 | 1–105, 312 332–365 | 106–111, 313–314, 319–331 | |||||
| 2020 | 132–272 | 273–308, 310–313 | 124–131 | 119–123, 318–320 | 1–100, 114, 309, 315, 336–365 | 101–113, 115–118 316–317, 328–335 | |||
| TGF | 2019 | 149–177, 205–257 | 258–330 | 62–148, 183–204 | 1–39, 42–61, 331–365 | 40 | |||
| 2020 | 126–164, 196–263 | 264–310 | 54–125, 173–195 | 1–53, 350–365 | |||||
| 2021 | 120–168, 195–270 | 271–320 | 71–119, 175–194 | 1–70, 321–365 | |||||
| MSE | 2017 | 153–213 | 214–246 | 122–152, 265–268 | 1–114, 326–365 | 116–121 | |||
| 2018 | 157–218 | 219–248 | 122–156, 260–288 | 1–22, 31–113,298–365 | 23–33 | 114–121 | |||
| 2019 | 157–221 | 222–248 | 122–156, 263–306 | 1–39, 41–114, 324–365 | 40 | 115–121 | |||
| TKY | 2018 | 137–278 | 279–312 | 119–136 | 101–118, 313–341 | 1–81, 353–357, 362–365 | 82–100, 342–352, 358–361 | ||
| 2019 | 132–245 | 284–321 | 132–145 | 113–131, 322–336, 344–348, 351–353, 355–356 | 1–103, 357–365 | 104–112, 337–343, 349–350, 354 | |||
| FHK | 2018 | 115–296 | 297–330 | 96–114 | 1–21, 64–66, 68–79, 88–95, 331–365 | 22–59, 67, 80–86 | 60–63, 87 | ||
| 2019 | 124–287 | 288–329 | 107–123 | 1–31, 35–61, 63–69, 71–99, 104–106, 330–332, 335–356 | 100–102, 357–365 | 32–34, 62, 70, 103, 333–334 |
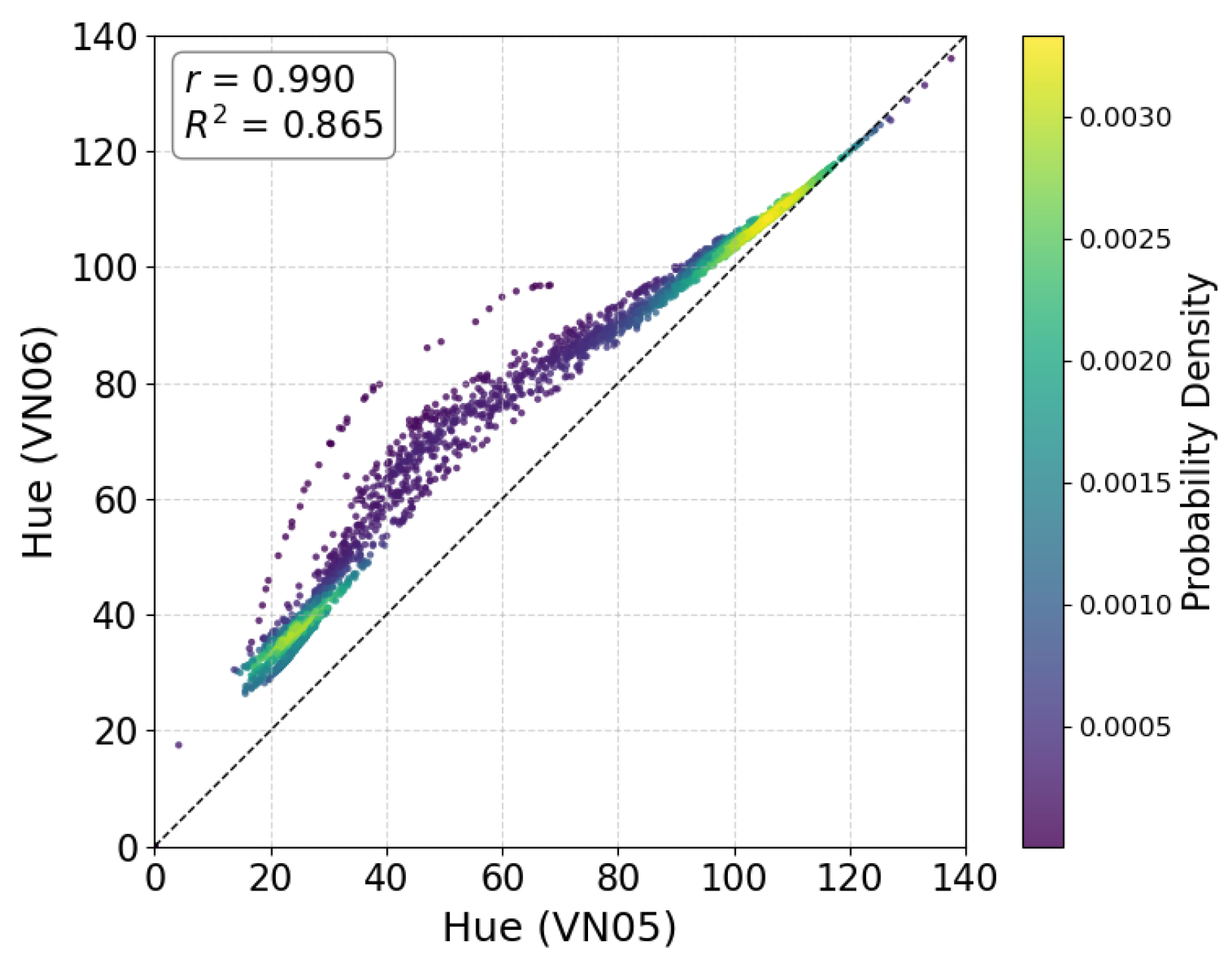
3.7. Comparison Between Hue (VN05) and Hue (VN06)
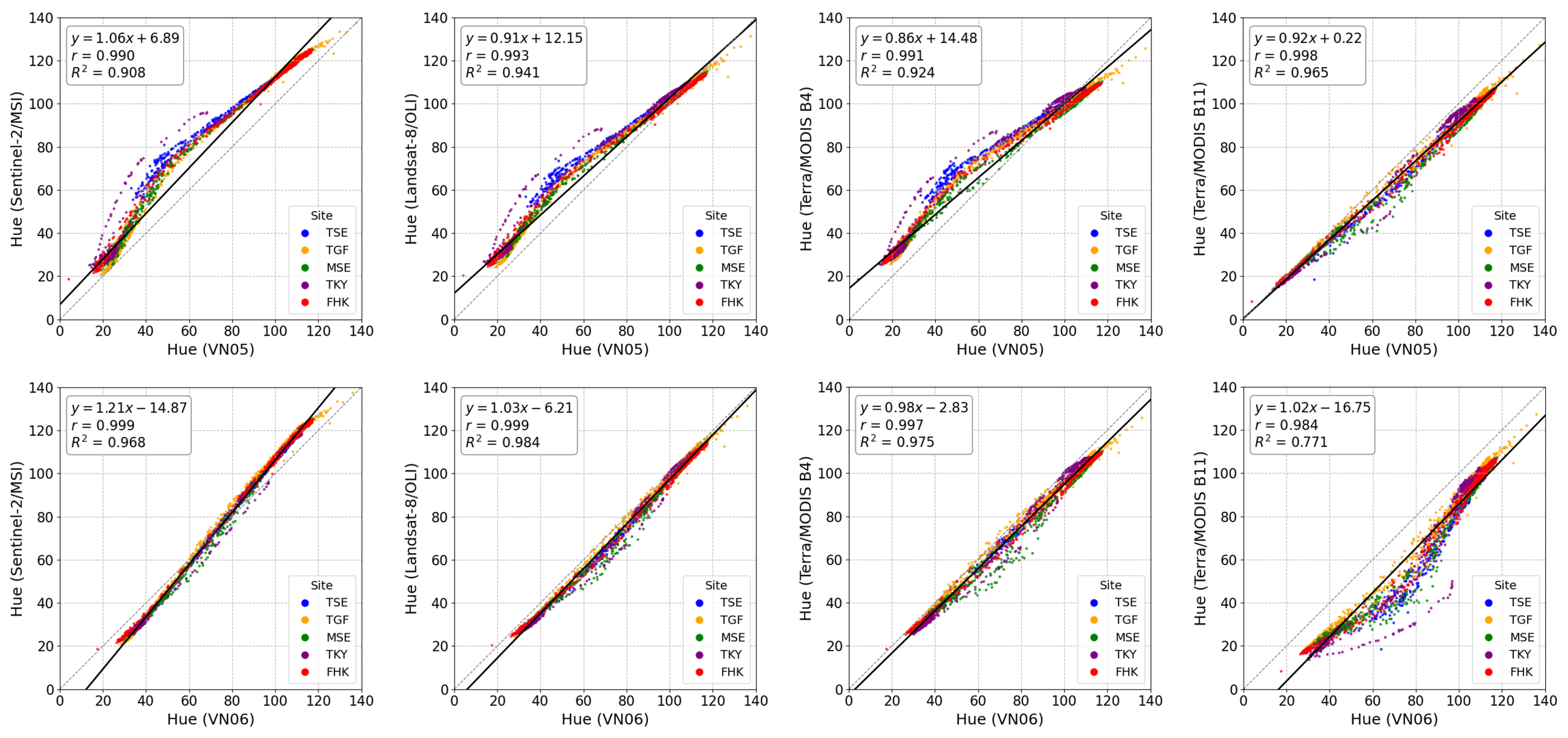
3.8. Comparison of Hue Across Multiple Satellite Sensors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Zhou, L.; Ciais, P.; Zhu, B. Variations in satellite-derived phenology in China’s temperate vegetation. Glob. Change Biol. 2006, 12, 672–685. [Google Scholar] [CrossRef]
- Yang, L.H.; Rudolf, E.V. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol. Lett. 2010, 13, 1–10. [Google Scholar] [CrossRef]
- Jeong, S.J.; Ho, C.H.; GIM, H.J.; Brown, M.E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Change Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Gill, A.L.; Gallinat, A.S.; Sanders-DeMott, R.; Rigden, A.J.; Short Gianotti, D.J.; Mantooth, J.A.; Templer, P.H. Changes in autumn senescence in northern hemisphere deciduous trees: A meta-analysis of autumn phenology studies. Ann. Bot. 2015, 116, 875–888. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouet, P.; Johns, D.G.; Jones, I.D.; Leech, D.I. Phenological sensitivity to climate across taxa and trophic levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef]
- AR6 Synthesis Report: Climate Change 2023. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 7 March 2025).
- G20 Climate Risk Atlas: Japan. Available online: https://files.cmcc.it/g20climaterisks/Japan.pdf (accessed on 7 March 2025).
- Climate Change in Japan 2025—Report on Assessment of Observed/Projected Climate Change Relating to the Atmosphere, Land and Oceans. Available online: https://www.data.jma.go.jp/cpdinfo/ccj/index.html (accessed on 27 March 2025).
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Rutishauser, T.; Filella, I. Phenology feedbacks on climate change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef]
- Chuine, I. Why does phenology drive species distribution? Philos. Trans. R. Soc. Biol. Sci. 2010, 365, 3149–3160. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Sano, J. Effect of the snow-free timing and temperature rise on the flushing phenology of seedlings. Jpn. J. Ecol. 2012, 62, 111–120. [Google Scholar]
- Mizuno, Y.; Tachikawa, H.; Sasagawa, T.; Kobayashi, T.; Nasahara, K.N. Impact of high temperature in 2023 and 2024 on spring leaf flush phenology in Japan derived by GCOM-C satellite. Sci. Rep. 2025, 15, 12920. [Google Scholar] [CrossRef]
- Kudo, G.; Ida, T.Y. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 2013, 94, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.T.; Nakao, K.; Ozeki, M.; Hotta, M.; Hamada, T.; Suka, T.; Ohashi, H.; Hirata, A.; Ishigooka, Y.; Matsui, T. Velocity of climate change (VoCC) analyses toward local adaptation to climate change. Pap. Environ. Inf. Sci. 2019, 33, 49–54. [Google Scholar]
- Matsui, T.; Tanaka, N.; Yagihashi, T. Predicting changes in suitable habitats for beech (Fagus crenata Blume) forests under climate warming in Shirakami mountains world natural heritage area, northern Japan. J. Jpn. For. Soc. 2007, 89, 7–13. [Google Scholar] [CrossRef]
- Expanding the Economic Impact of Tourism. Available online: https://www.mlit.go.jp/common/001294468.pdf (accessed on 12 March 2025).
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Holben, B.N.; Tucker, C.J.; Fan, C.J. Spectral assessment of soybean leaf area and leaf biomass. Photogramm. Eng. Remote Sens. 1980, 46, 651–656. [Google Scholar]
- Qiao, K.; Zhu, W.; Xie, Z.; Li, P. Estimating the seasonal dynamics of the leaf area index using piecewise LAI-VI relationships based on phenophases. Remote Sens. 2019, 11, 689. [Google Scholar] [CrossRef]
- Tan, C.W.; Zhang, P.P.; Zhou, X.X.; Wang, Z.X.; Xu, Z.Q.; Mao, W.; Li, W.X.; Huo, Z.Y.; Guo, W.S.; Yun, F. Quantitative monitoring of leaf area index in wheat of different plant types by integrating NDVI and Beer-Lambert law. Sci. Rep. 2020, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Shabanov, N.V.; Zhou, L.; Knyazikhin, Y.; Myneni, R.B.; Tucker, C.J. Analysis of interannual changes in northern vegetation activity observed in AVHRR data from 1981 to 1994. IEEE Trans. Geosci. Remote Sens. 2002, 40, 115–130. [Google Scholar] [CrossRef]
- Delbart, N.; Kergoat, L.; Le Toan, T.; Lhermitte, J.; Picard, G. Determination of phenological dates in boreal regions using normalized difference water index. Remote Sens. Environ. 2005, 97, 26–38. [Google Scholar] [CrossRef]
- Nagai, S.; Nasahara, K.N.; Muraoka, H.; Akiyama, T.; Tsuchida, S. Field experiments to test the use of the normalized-difference vegetation index for phenology detection. Agric. For. Meteorol. 2010, 150, 152–160. [Google Scholar] [CrossRef]
- Cao, R.; Feng, Y.; Liu, X.; Shen, M.; Zhou, J. Uncertainty of vegetation green-up date estimated from vegetation indices due to snowmelt at northern middle and high latitudes. Remote Sens. 2020, 12, 190. [Google Scholar] [CrossRef]
- Delbart, N.; Le Toan, T.; Kergoat, L.; Fedotova, V. Remote sensing of spring phenology in boreal regions: A free of snow-effect method using NOAA-AVHRR and SPOT-VGT data (1982–2004). Remote Sens. Environ. 2006, 101, 52–62. [Google Scholar] [CrossRef]
- Dunn, A.H.; de Beurs, K.M. Land surface phenology of North American mountain environments using moderate resolution imaging spectroradiometer data. Remote Sens. Environ. 2011, 115, 1220–1233. [Google Scholar] [CrossRef]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of green-red vegetation index for remote sensing of vegetation phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef]
- Gonsamo, A.; Chen, J.M.; Price, D.T.; Kurz, W.A.; Wu, C. Land surface phenology from optical satellite measurement and CO2 eddy covariance technique. J. Geophys. Res. Biogeosci. 2012, 117, G03032. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Wu, J.; Tang, Y.; Shi, P.; Black, T.A.; Zhu, K. A snow-free vegetation index for improved monitoring of vegetation spring green-up date in deciduous ecosystems. Remote Sens. Environ. 2017, 196, 1–12. [Google Scholar] [CrossRef]
- Yang, W.; Kobayashi, H.; Wang, C.; Shen, M.; Chen, J.; Matsushita, B.; Tang, Y.; Kim, Y.; Bret-Harte, M.S.; Zona, D.; et al. A semi-analytical snow-free vegetation index for improving estimation of plant phenology in tundra and grassland ecosystems. Remote Sens. Environ. 2019, 228, 31–44. [Google Scholar] [CrossRef]
- Nagai, S.; Inoue, T.; Suzuki, R. Leaf-coloring information published on web sites and its utility in the ground-truthing of satellite remote-sensing data for mapping autumn leaf phenology. Jpn. J. Biometeorol. 2015, 52, 119–129. [Google Scholar]
- Zhao, B.; Donnelly, A.; Schwartz, M.D. Evaluating autumn phenology derived from field observations, satellite data, and carbon flux measurements in a northern mixed forest, USA. Int. J. Biometeorol. 2020, 64, 713–727. [Google Scholar] [CrossRef]
- Takagi, K.; Takami, K.; Murai, R. Mapping Tender Green and Autumn Color using Earth Observation Satellite GCOM-C1. Bull. Kochi Univ. Technol. 2020, 18, 57–63. [Google Scholar]
- Xing, J.; Zhang, Y.C.; Gao, J.C. Research on Improved Infrared and Visible Image Fusion Algorithm Based on HSV Color Space. Mech. Electron. 2022, 40, 15–19. [Google Scholar]
- Meyer, G.E.; Neto, J.C. Verification of color vegetation indices for automated crop imaging applications. Comput. Electron. Agric. 2008, 63, 282–293. [Google Scholar] [CrossRef]
- Yang, W.; Wang, S.; Zhao, X.; Zhang, J.; Feng, J. Greenness identification based on HSV decision tree. Inf. Process. Agric. 2015, 2, 149–160. [Google Scholar] [CrossRef]
- Hassanein, M.; Lari, Z.; El-Sheimy, N. A new vegetation segmentation approach for cropped fields based on threshold detection from hue histograms. Sensors 2018, 18, 1253. [Google Scholar] [CrossRef] [PubMed]
- Beniaich, A.; Silva, M.L.N.; Avalos, F.A.P.; de Menezes, M.D.; Cândido, B.M. Determination of vegetation cover index under different soil management systems of cover plants by using an unmanned aerial vehicle with an onboard digital photographic camera. Semin. Ciências Agrárias 2019, 40, 49–66. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Y.; Chen, X.; Zhang, H.; Liu, C.; Duan, Q. Estimating the aquatic-plant area on a pond surface using a hue-saturation-component combination and an improved Otsu method. Comput. Electron. Agric. 2021, 188, 106372. [Google Scholar] [CrossRef]
- Guan, P.; Zheng, Y.; Lei, G. Analysis of canopy phenology in man-made forests using near-earth remote sensing. Plant Methods 2021, 17, 104. [Google Scholar] [CrossRef]
- Ribeiro, A.L.A.; Maciel, G.M.; Siquieroli, A.C.S.; Luz, J.M.Q.; Gallis, R.B.D.A.; Assis, P.H.D.S.; Catão, H.C.R.M.; Yada, R.Y. Vegetation indices for predicting the growth and harvest rate of lettuce. Agriculture 2023, 13, 1091. [Google Scholar] [CrossRef]
- Song, Z.; Lu, Y.; Ding, Z.; Sun, D.; Jia, Y.; Sun, W. A new remote sensing desert vegetation detection index. Remote Sens. 2023, 15, 5742. [Google Scholar] [CrossRef]
- Biró, L.; Kozma-Bognár, V.; Berke, J. Comparison of RGB indices used for vegetation studies based on structured similarity index (SSIM). J. Plant Sci. Phytopathol. 2024, 8, 007–012. [Google Scholar] [CrossRef]
- Tsuchida, S.; Nishida, K.; Iwao, K.; Kawato, W.; Oguma, H.; Iwasaki, A. Phenological Eyes Network for Validation of Remote Sensing Data. J. Remote Sens. Soc. Jpn. 2005, 25, 282–288. [Google Scholar]
- Nasahara, K.N.; Nagai, S. Development of an in situ observation network for terrestrial ecological remote sensing: The Phenological Eyes Network (PEN). Ecol. Res. 2015, 30, 211–223. [Google Scholar] [CrossRef]
- Nagai, S.; Akitsu, T.; Saitoh, T.M.; Busey, R.C.; Fukuzawa, K.; Honda, Y.; Ichie, T.; Ide, R.; Ikawa, H.; Iwasaki, A.; et al. 8 million phenological and sky images from 29 ecosystems from the Arctic to the tropics: The Phenological Eyes Network. Ecol. Res. 2018, 33, 1091–1092. [Google Scholar] [CrossRef]
- Phenological Eyes Network (PEN). Available online: http://www.pheno-eye.org/ (accessed on 7 March 2025).
- AsiaFlux. Available online: https://www.asiaflux.net/ (accessed on 7 March 2025).
- Japan Long Term Ecological Research Network (JaLTER). Available online: http://www.jalter.org/ (accessed on 7 March 2025).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Sasagawa, T.; Akitsu, T.K.; Ide, R.; Takagi, K.; Takanashi, S.; Nakaji, T.; Nasahara, K.N. Accuracy assessment of photochemical reflectance index (PRI) and chlorophyll carotenoid index (CCI) derived from GCOM-C/SGLI with in situ Data. Remote Sens. 2021, 14, 5352. [Google Scholar] [CrossRef]
- Ide, R.; Hirose, Y.; Oguma, H.; Saigusa, N. Development of a masking device to exclude contaminated reflection during tower-based measurements of spectral reflectance from a vegetation canopy. Agric. For. Meteorol. 2016, 223, 141–150. [Google Scholar] [CrossRef]
- GCOM-C “SHIKISAI” Data Users Handbook version B. 2023. Available online: https://gportal.jaxa.jp/gpr/assets/mng_upload/GCOM-C/GCOM-C_SHIKISAI_Data_Users_Handbook_jp_B.pdf (accessed on 11 March 2025).
- SGLI Sensor Characterization. Available online: https://suzaku.eorc.jaxa.jp/GCOM_C/data/prelaunch/index.html (accessed on 11 March 2025).
- Li, M.; Yang, W.; Kondoh, A. Improving remote estimation of vegetation phenology using GCOM-C/SGLI land surface reflectance data. Remote Sens. 2022, 14, 4027. [Google Scholar] [CrossRef]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef]
- Yin, G.; Verger, A.; Descals, A.; Filella, I.; Peñuelas, J. A broadband green-red vegetation index for monitoring gross primary production phenology. J. Remote Sens. 2022, 2022, 9764982. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Tucker, C.J.; Pinzon, J.E.; Brown, M.E.; Slayback, D.A.; Pak, E.W.; Mahoney, R.; Vermote, E.F.; El Saleous, N. An extended AVHRR 8-km NDVI dataset compatible with MODIS and SPOT vegetation NDVI data. Int. J. Remote Sens. 2005, 26, 4485–4498. [Google Scholar] [CrossRef]
- Pinzon, J.E.; Tucker, C.J. A non-stationary 1981–2012 AVHRR NDVI3g time series. Remote Sens. 2014, 6, 6929–6960. [Google Scholar] [CrossRef]
- Simpson, J.J.; Stitt, J.R. A procedure for the detection and removal of cloud shadow from AVHRR data over land. IEEE Trans. Geosci. Remote Sens. 1998, 36, 880–897. [Google Scholar] [CrossRef]
- Yang, X.; Zuo, X.; Xie, W.; Li, Y.; Guo, S.; Zhang, H. A correction method of NDVI topographic shadow effect for rugged terrain. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2022, 15, 8456–8472. [Google Scholar] [CrossRef]
- Ma, Y.; He, T.; McVicar, T.R.; Liang, S.; Liu, T.; Peng, W.; Song, D.; Tian, F. Quantifying how topography impacts vegetation indices at various spatial and temporal scales. Remote Sens. Environ. 2024, 312, 114311. [Google Scholar] [CrossRef]
- Lorenzi, L.; Melgani, F.; Mercier, G. A complete processing chain for shadow detection and reconstruction in VHR images. IEEE Trans. Geosci. Remote Sens. 2012, 50, 3440–3452. [Google Scholar] [CrossRef]

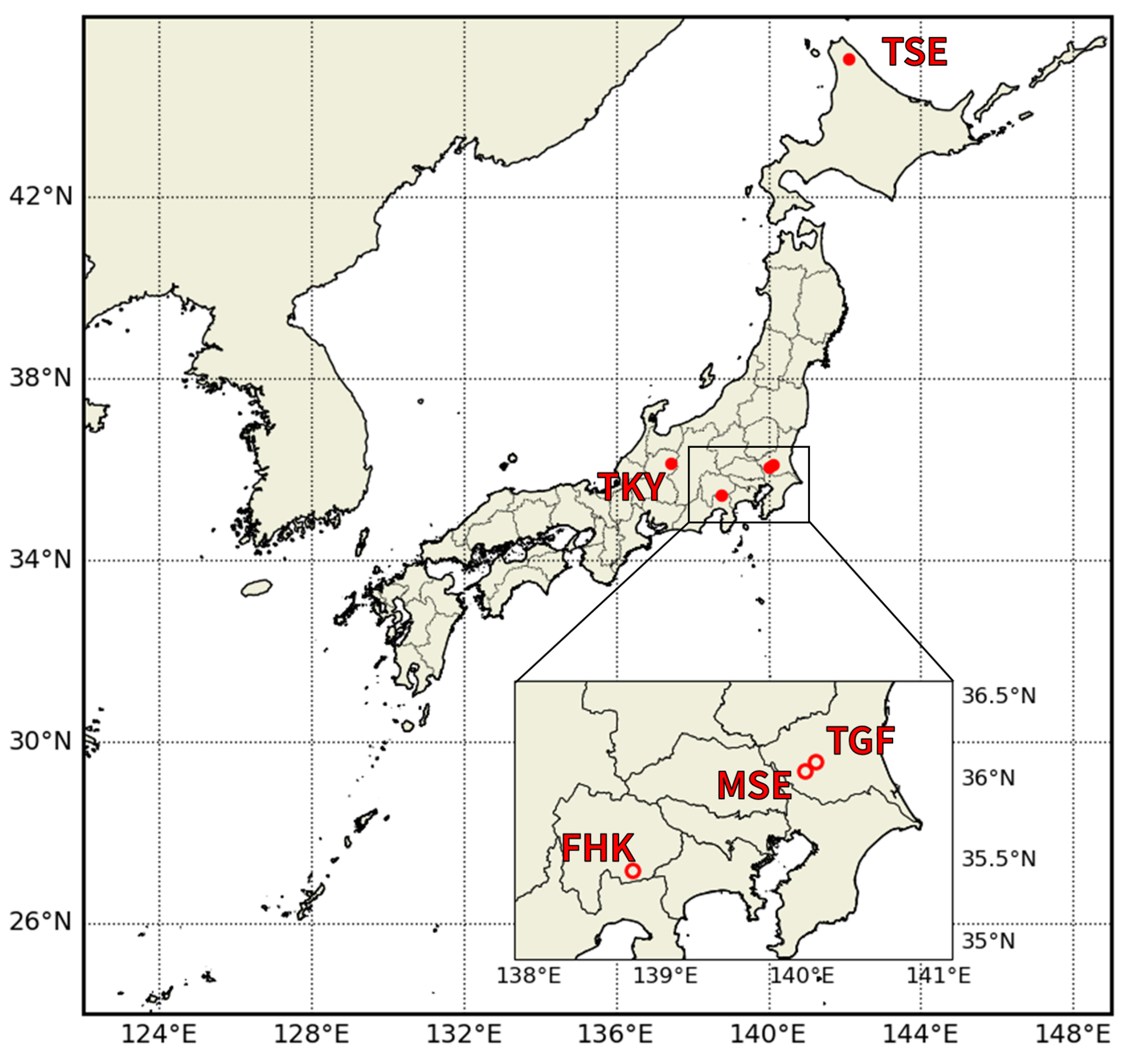
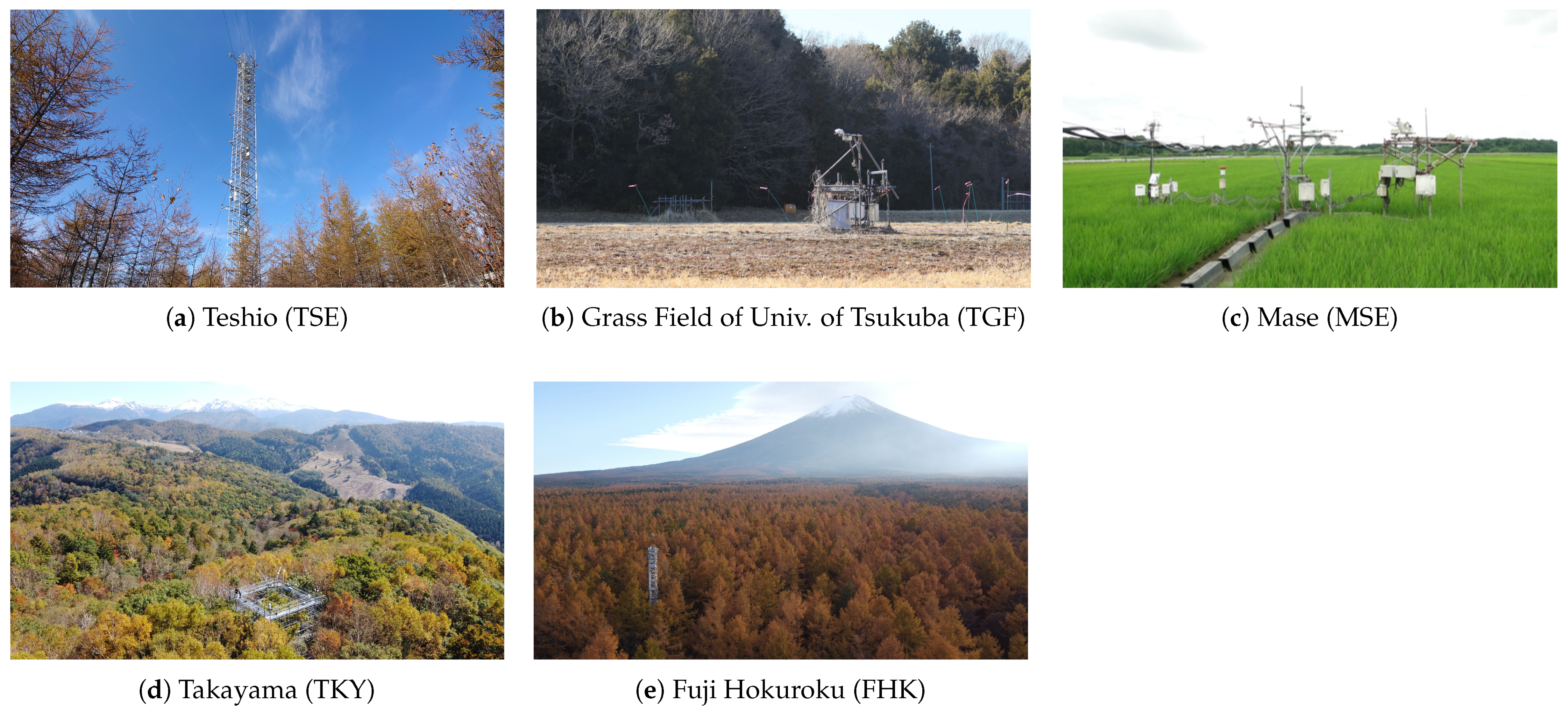



| Site ID | Site Name | Forest Type | Latitude, Longitude (WGS84), Altitude | Köppen- Geiger Climate Classification [53] | Dominant Species |
|---|---|---|---|---|---|
| TSE | Teshio | Mixed, DNF | 45°03′21″N, 142°06′26″E, 70 m | Dfb | Hybrid larch (Larix kaempferi and L. gmelinii), Sasa senanensis, and S. kurilensis |
| TGF | Grass Field of CRiED in the University of Tsukuba | Grassland | 36°06′35″N, 140°06′00″E, 27 m | Cfb | Miscanthus sinensis, Imperata cylindrica, Solidago altissima, and Pueraria lobata subsp. lobata |
| MSE | Mase | Rice Paddy | 36°03′14″N, 140°01′37″E, 13 m | Cfb | Oryza sativa L. (cultivar: Koshihikari) |
| TKY | Takayama | DBF | 36°8′43″N, 137°25′25″E, 1420 m | Dfb | Quercus crispula, Betula ermanii, and Sasa senanensis |
| FHK | Fuji Hokuroku | DNF | 35°26′37″N, 138°45′53″E, 1100 m | Cfb | Larix kaempferi, Pinus densiflora, Cornus controversa, and Quercus crispula |
| Channel | Center Wavelength [nm] | Band Width [nm] | Spatial Resolution [m] |
|---|---|---|---|
| VN01 | 379.9 | 10.6 | 250 |
| VN02 | 412.3 | 10.3 | 250 |
| VN03 | 443.3 | 10.1 | 250 |
| VN04 | 490.0 | 10.3 | 250 |
| VN05 | 529.7 | 19.1 | 250 |
| VN06 | 566.1 | 19.8 | 250 |
| VN07 | 672.3 | 22.0 | 250 |
| VN08 | 672.4 | 21.9 | 250 |
| VN09 | 763.1 | 11.4 | 250 |
| VN10 | 867.1 | 20.9 | 250 |
| VN11 | 867.4 | 20.8 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, Y.; Sasagawa, T.; Takahashi, Y.; Ide, R.; Kobayashi, T.; Muraoka, H.; Takagi, K.; Ono, K.; Nasahara, K.N. Assessment of the Applicability of Hue from In Situ Spectral Measurements to Remote Sensing of Plant Phenology. Remote Sens. 2025, 17, 2767. https://doi.org/10.3390/rs17162767
Mizuno Y, Sasagawa T, Takahashi Y, Ide R, Kobayashi T, Muraoka H, Takagi K, Ono K, Nasahara KN. Assessment of the Applicability of Hue from In Situ Spectral Measurements to Remote Sensing of Plant Phenology. Remote Sensing. 2025; 17(16):2767. https://doi.org/10.3390/rs17162767
Chicago/Turabian StyleMizuno, Yuki, Taiga Sasagawa, Yoshiyuki Takahashi, Reiko Ide, Toshiyuki Kobayashi, Hiroyuki Muraoka, Kentaro Takagi, Keisuke Ono, and Kenlo Nishida Nasahara. 2025. "Assessment of the Applicability of Hue from In Situ Spectral Measurements to Remote Sensing of Plant Phenology" Remote Sensing 17, no. 16: 2767. https://doi.org/10.3390/rs17162767
APA StyleMizuno, Y., Sasagawa, T., Takahashi, Y., Ide, R., Kobayashi, T., Muraoka, H., Takagi, K., Ono, K., & Nasahara, K. N. (2025). Assessment of the Applicability of Hue from In Situ Spectral Measurements to Remote Sensing of Plant Phenology. Remote Sensing, 17(16), 2767. https://doi.org/10.3390/rs17162767









