Abstract
Drought disturbances are becoming more frequent with global warming. Accurately assessing the regulatory effect of drought on vegetation phenology is key to understanding terrestrial ecosystem response mechanisms in the context of climate change. Previous studies on cumulative and lagged effects of drought on vegetation growth have mostly focused on a single vegetation type or the overall vegetation NDVI, overlooking the possible influence of different adaptation strategies of different vegetation types and differences in drought effects on different phenological nodes. This study investigates the cumulative and lagged effects of drought on vegetation phenology across a region of East Asia from 2001 to 2020 using NDVI data and the Standardized Precipitation Evapotranspiration Index (SPEI). We analyzed the start of growing season (SOS) and end of growing season (EOS) responses to drought across four vegetation types: deciduous needleleaf forests (DNFs), deciduous broadleaf forests (DBFs), shrublands, and grasslands. Results reveal contrasting phenological responses: drought delayed SOS in grasslands through a “drought escape” strategy but advanced SOS in forests and shrublands. All vegetation types showed earlier EOS under drought stress. Cumulative drought effects were strongest on DNFs, SOS, and shrubland SOS, while lagged effects dominated DBFs and grassland SOS. Drought impacts varied with moisture conditions: they were stronger in dry regions for SOS but more pronounced in humid areas for EOS. By confirming that drought effects vary by vegetation type and phenology node, these findings enhance our understanding of vegetation adaptation strategies and ecosystem responses to climate stress.
1. Introduction
The impacts of global warming on ecosystems are far-reaching and complex. Changes in vegetation phenology (the timing of cyclical events in the life cycle of plants) are an important indicator of how vegetation responds to a changing environment. Vegetation phenology in turn plays a key role in terrestrial ecosystem dynamics and in guiding agricultural production [1]. Many studies have confirmed that vegetation phenology is influenced by meteorological factors such as temperature, radiation, and precipitation [2,3]. These influences vary among vegetation types, such as forests and grasslands [4,5,6,7,8].
Droughts—periods of extreme water scarcity—are expected to increase as the climate warms [9,10,11]. The cascading effects of drought can alter the structure and function of ecosystems. In recent decades, drought disturbances have been occurring more frequently; the area affected by drought has increased; and the impacts of drought on terrestrial ecosystems, biodiversity, and agricultural production are increasingly becoming a focus of global concern [12,13,14,15]. Drought directly modulates the physiological processes of plants by altering water availability, which in turn reshapes vegetation phenology, but these effects vary among the phenological nodes of different vegetation types [16,17]. Studies have implicated drought conditions in either accelerating [18] or delaying [19] the start of the vegetation growing season (start of season, or SOS) in different settings. Vegetation spring phenology is also constrained by background climatic conditions such as spring temperature and precipitation [20]. Winter moisture and temperature conditions at high latitudes influence the sensitivity of vegetation SOS to spring drought [21]. For shrubs, it was found that drought led to an earlier SOS, thereby extending the growing season [22]. However, in a study on Canadian grasslands, it was found that drought led to an earlier termination of the vegetation growing season (end of season, or EOS) [23]. It appears that mild drought can accelerate germination by triggering plants’ water stress response mechanisms, but extreme drought events can significantly delay both SOS and EOS [24,25].
Beyond the immediate effects of drought on vegetation phenology, cumulative or lagged drought effects can affect the vegetation performance in subsequent growing seasons. For example, drought may cause long-term damage to plant physiology, such as drought-induced root damage and xylem embolism, which remain after the drought is over and affect subsequent water uptake and transport [26,27,28]. Furthermore, vegetation consumes large amounts of non-structural carbohydrates (NSCs) during periods of drought, leading to a reduction in resource reserves and affecting subsequent growth and resilience [29,30]. Studies have increasingly pointed to the importance of both cumulative effects (potentially from multiple droughts over time) and lagged effects of previous droughts on vegetation phenology [31,32,33,34]. Specifically, cumulative effects involve the persistent impacts of drought that are gradually superimposed on the vegetation growth cycle during the year, while lagged effects manifest as the influence of previous periods of drought on the current characteristics of vegetation at a specific point in time [35]. These reflect two aspects of the legacy effects of water shortages on vegetation that can be highly variable across taxa in their lag time and manifestation [36]. However, previous studies on the cumulative and lagged effects of drought on vegetation growth have mostly focused on single vegetation types or on the overall vegetation NDVI, coverage, and productivity [35,37,38,39,40], overlooking possible differential responses to drought among vegetation phenological nodes and different vegetation types, such as forests composed of trees with different leaf forms (broadleaf and needleleaf), shrubs, and grasslands.
Different vegetation types have adopted different survival strategies (such as leaf form) and thus may display varying effects of drought on different phenological nodes (i.e., SOS and EOS). For example, the resource utilization strategies of trees are adapted to local thermal and moisture conditions [41,42]. Trees may be broadly categorized into four main types based on leaf form (needleleaf or broadleaf) and habit (evergreen or deciduous) [43]. These types represent different kinds of trade-offs between growth and survival [41]. In response to variations in water conditions, trees have developed different leaf forms and thus different growing season water use strategies: needle leaves often display a conservative adaptation strategy with cold- and drought-resistant structures as an adaptation to physiological drought, short growing seasons, and low temperatures [44], while broad leaves are less drought resistant, being distributed mainly in warmer and more humid climates [45]. Shrubs have longer leaf lifespan and higher biomass per unit leaf area for cold and drought resistance [41,46], and grasses generally exhibit a summer dormancy strategy that enhances their dehydration tolerance [47].
East Asia has unique climatic conditions and diverse vegetation types. In recent years, the severity of droughts in East Asia has shown a significant upward trend, and there is considerable unpredictability regarding changes in regional precipitation patterns accompanying global warming [48]. Frequent heatwaves and severe droughts occurring simultaneously in the region have exacerbated soil dryness, causing a detectable impact on vegetation growth [49]. Therefore, it is essential to conduct research on the relationship between drought and vegetation growth patterns, not only during the period of drought itself but also after the drought is alleviated.
Climate warming is changing global ecosystems by reshaping vegetation phenology rhythms [50]. Such changes not only threaten biodiversity but may also exacerbate the climate stress through carbon–water cycle feedbacks [51]. In a warming world, there is a need to better understand the cumulative and lagged effects of drought on the phenology of forests, shrubs, and grasslands, and among forests with different leaf forms. This study investigated the pattern of response to drought among different vegetation types in East Asia from 2001 to 2020. The objectives of this study were to determine (1) whether there were difference in response of phenology to cumulative and lagged effects of drought; (2) differences in effect of cumulative and lagged effects of drought on different phenological nodes; (3) differential effects of background moisture conditions on cumulative and lagged effects of drought; and (4) differences in dominant drought effects among shrublands, grasslands, and forests of different leaf forms in the study area. Understanding the mechanisms of drought-induced phenological changes among broadleaf and needleleaf forests, shrublands, and grasslands will be essential for better management of vegetation to maintain vital ecological functions.
2. Materials and Methods
2.1. Study Area
This study focuses on a region in the middle to high latitudes of northeast Asia (longitude 90–135°E, latitude 40–55°N), including portions of northern China and eastern Russia as well as most of Mongolia and a small part of the northern Korean peninsula [31]. The major geographical features of the study area include the Northeast China Plain, the Mongolian Plateau, the Greater and Lesser Khingan Mountains, and the Changbai Mountains (Figure 1a,b). The area’s topography descends from west to east. Precipitation varies seasonally and interannually with the summer and winter monsoons; moisture conditions range from semi-humid in the east to semi-arid and arid in the west, while thermal conditions range from the warm temperate zone in the south to the cold temperate zone in the north.
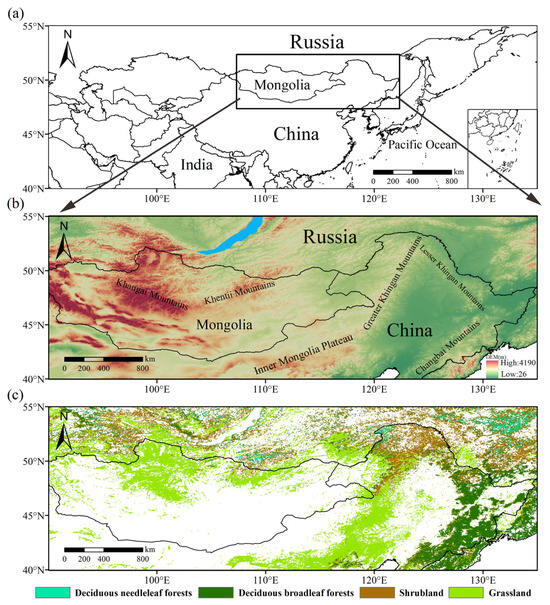
Figure 1.
(a) Location; (b) topography (STRM15+); (c) spatial distribution of vegetation types of the study area (IGBP MODIS).
Within the region, the main vegetation types consist of deciduous needleleaf forests (DNFs), deciduous broadleaf forests (DBFs), shrublands, and grasslands. DNFs are located in the northern parts of the Greater Khingan Mountains and southern and southeastern Siberia; DBFs are mainly found in northeast China; shrublands occur in southern Siberia and the Greater Khingan Mountains; and grasslands cover most of the Inner Mongolian Plateau in China as well as northern Mongolia (Figure 1c). Each of the vegetation types has different water use strategies during the growing season, such as leaf stomatal conductance and hydraulic safety margins, which may influence their phenological response to drought. (Other types of land cover, such as deserts and wetlands, are excluded from the analysis).
2.2. Datasets
2.2.1. Vegetation Type Data and Processing
We use the International Geosphere-Biosphere Program (IGBP) MODIS land cover classification scheme. MODIS land cover data were obtained from the MCD12Q1 data product (https://ladsweb.modaps.eosdis.nasa.gov (accessed on 1 November 2024)) with a spatial resolution of 500 m. Based on the main leaf form and habit of trees in the study area, vegetation types in the study area were reclassified into four categories: DNFs, DBFs, shrubland, and grassland. In order to minimize the disturbance of vegetation type changes, we extracted the area where vegetation types did not change during the study period [52,53] to use as the basis for the vegetation types in our analysis (Figure 1c). For reference, we also obtained STRM15+ V2.5.5 global topography data at a 15 arc-second resolution from the General Bathymetric Chart of the Oceans (https://gebco.net (accessed on 15 April 2025)).
2.2.2. SPEI Data Source and Processing
The standardized precipitation evapotranspiration index (SPEI) constructed by Vicente-Serrano et al. [54] can reflect regional drought situations in a more comprehensive way by considering the distribution of both potential evapotranspiration and precipitation [55]. The global SPEI dataset, SPEIbase v.2.8 (https://spei.csic.es/database.html (accessed on 1 October 2024)), identifies drought conditions at a time scale from 1 to 48 months and with a spatial resolution of 0.05° (approximately 3.5–5.5 km). We selected SPEI data for 1- to 12-month time scales to quantify drought occurrence in terms of intensity and length. We resampled the SPEI grids using a bilinear interpolation method to match the 500 m spatial resolution of the MODIS data [31,56,57,58,59].
2.3. Methods
2.3.1. Detecting Vegetation Phenology
The Normalized Difference Vegetation Index (NDVI) quantifies vegetation by measuring the difference between the reflected near-infrared and red light detected by remote sensors [60]. This index can effectively reflect vegetation activity and is the most commonly used indicator of the robustness of vegetation cover [61]. As the NDVI is highly sensitive to moisture conditions, it can be used to assess the drought status of vegetation, enabling a comparison with the SPEI drought index to reveal the relationship between vegetation activity and drought conditions. We use NDVI data for the period between January 2001 and December 2020 obtained from NASA MOD13A1 (https://ladsweb.modaps.eosdis.nasa.gov (accessed on 1 November 2024)), with a spatial resolution of 500 m and temporal resolution of 16 days [62].
Using MATLAB R2024a software, we applied the Savitzky–Golay filtering method to smooth the NDVI data, setting the window length at 9 and the smoothing polynomial degree at 3. This method effectively filled in the data gaps between the 16-day intervals, reconstructing the time series and removing the effects of outliers caused by clouds, aerosols, sensor noise, or other background noise [63]. We then calculated the dates of the vegetation phenological nodes, including the start of the growing season (SOS) and the end of the growing season (EOS), using the dynamic thresholding method [64]: based on the NDVI time series characteristics of the study area and prior research [65,66,67,68], we set the date when the NDVI rises to 30% of the annual NDVI amplitude as the SOS and set the date when the NDVI falls to 50% of the annual NDVI amplitude as the EOS. The workflow of this study is shown in Figure 2.
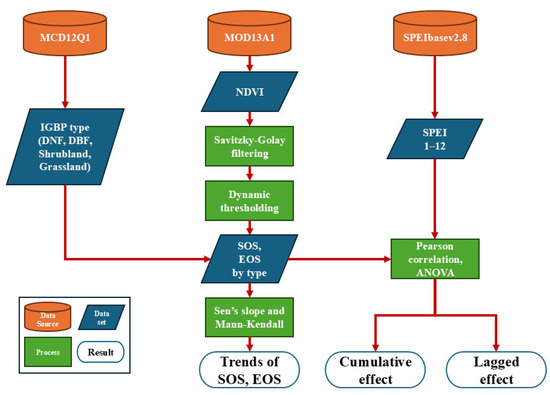
Figure 2.
Study workflow.
2.3.2. Trend Analysis
This study uses the non-parametric Sen’s slope and Mann–Kendall methods to calculate trend [69]. We implemented this equation in the R 4.4.1 programming language to analyze the regional-scale and pixel-scale SOS and EOS trends:
where xi and xj represent the values at times i and j, and n represents the length of the time series data. When the slope is positive, it indicates that SOS or EOS has been delayed; when the slope is negative, it indicates that SOS or EOS has advanced.
2.3.3. Detecting Cumulative and Lagged Effects of Drought
As the basis for comparing the cumulative and lagged effects of drought in terms of monthly time scales, we need to determine the starting months for SOS and EOS. If the average SOS date falls in the first half of the month, then we designate the previous month as the starting SOS month; if it falls in the second half of the month, then the current month is designated as the starting SOS month [70]. The same logic applies to the EOS month. For the vast majority of our study area from 2001 to 2020, the SOS falls in April and the EOS falls in September, so those are designated as the SOS and EOS starting months for comparison with the 1- to 12-month cumulative and lagged SPEI values [65].
Before performing a correlation analysis, we used a linearity test in SPSS 19.0 to verify that there is a linear relationship between phenology nodes and SPEI. Taking the phenology parameters and the cumulative SPEI (1 to 12 months) [32,71], we computed the Pearson correlation coefficient (r) to test the cumulative effect of drought on the phenology of different vegetation types in the study area [57,72,73]. Then, we used correlation analysis to calculate the correlation coefficients between vegetation phenology and the 1- to 12-month SPEI values, generating 12 correlation coefficients for both SOS and EOS from 2001 to 2020. We calculated the cumulative effect and flagged the cumulative month with the largest absolute value of the correlation coefficient to identify the time scale of the cumulative effect [53,73], as follows:
where ri is correlation coefficient between vegetation phenology and the SPEI for each cumulative month; x denotes the SOS or EOS; yi denotes the SPEI for cumulative month i; Rmax_cum is the maximum value of ri; and the cumulative response time is determined to be months corresponding with Rmax_cum.
The Pearson correlation coefficient (r) is used to test the lagged effect of drought on phenological parameters and 1-month SPEI01. Twelve correlation coefficients were generated by combining the 2001–2020 SOS and EOS time series with previous j-month SPEI series and calculating correlation coefficients between vegetation phenology and 1-month SPEI at different lagged times (1 12). Rmax_lag is the magnitude of the lagged effect and the month with the largest absolute value of the correlation coefficient is used as the time scale of the lagged effect. The drought lagged effect is calculated as follows:
where rj is the Pearson correlation coefficient between vegetation phenology and the SPEI for each lagged month; x denotes the SOS or EOS; yj denotes the SPEI for lagged j months; Rmax_lag is the maximum value of rj; and the lagged response time is determined to be the time corresponding with Rmax_lag.
In addition, we used one-way analysis of variance to test the significance of differences for Rmax between different vegetation types. To assess drought’s cumulative and lagged effects on vegetation phenology across moisture conditions, this study selected SPEI12 of December between 2001 and 2020 to represent annual average water conditions. The maximum correlation coefficient (Rmax_cum, Rmax_lag) and cumulative and lagged months were used to analyze changes in the effects of drought on vegetation phenology under different moisture conditions. The moisture gradient was determined by using the annual average SPEI at 0.1 intervals. To clarify the relative strengths of these correlations in terms of the areal extent affected by drought, we calculated the difference in magnitude between Rmax_cum and Rmax_lag:
where positive values indicate that the cumulative effect was more strongly correlated and negative values indicate that the lagged effect was more strongly correlated [31,53,74].
∆Rmax = |Rmax_cum| − |Rmax_lag|
3. Results
3.1. Phenology Changes by Vegetation Type
The spatial distributions of multiyear mean values of vegetation phenology in the study area from 2001 to 2020 are shown in Figure 3. The SOS was mainly concentrated between the 90th and 130th day of the year (DOY), that is, from early April to late May, accounting for 84.44% of the study area. From east to west, the SOS appeared earlier in eastern China and later in the northwest and southwest of the study area. Among the vegetation types, DNF, DBF, and shrub SOS were concentrated at DOY 90–125, while the grassland SOS was concentrated at DOY 95–150 (Figure 3a). Vegetation SOS in the northeast China, including the Greater Khingan Mountains, Lesser Khingan Mountains, and Changbai Mountains, showed a trend of advancement, with the DBF and eastern grassland SOS significantly advancing. The Khangai and Khentii Mountains in the northern part of the Mongolian Plateau and part of southwestern Inner Mongolia showed a trend of postponement, with the SOS significantly delayed mainly in the grasslands there. As shown in Figure 3c,e, the SOS advanced in 89.43% of the study area (13.32% passed a significance test, p < 0.05) while the SOS was delayed in 10.57% of the study area (1.50% significantly, p < 0.05).
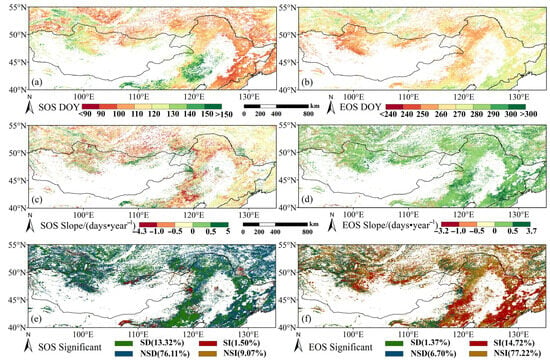
Figure 3.
Spatial distribution and change in average vegetation phenology between 2001 and 2020. (a) Mean SOS (day of year); (b) mean EOS; (c) SOS trends; (d) EOS trends; (e) SOS significance of trend; (f) EOS significance of trend. SD indicates significantly earlier, SI indicates significantly later, NSD and NSI indicate nonsignificant results; numbers in parentheses give the applicable percentage of the study area.
The time range of the EOS was from early September to late October, occurring from DOY 250 to 280 in 92.52% of the study area. The earliest EOS mainly appeared in the northwest and the latest EOS was concentrated in the southeast. The EOS of DNFs and DBFs was concentrated at DOY 257–287, the EOS of shrublands was concentrated at DOY 250–290, and the EOS of grassland was concentrated at DOY 243–275 (Figure 3b). Most of the study area showed a trend toward a delay in the EOS (91.94%, with 14.72% significant), especially in DBFs and a small portion of grasslands. An earlier (advancing) EOS was detected in parts of Inner Mongolia Plateau and a small portion of northwestern Mongolia (8.07% of the study area, with 1.37% significant) (see Figure 3d,f). Across the study area, grassland areas displayed both delays and advancements in SOS and EOS, while the other vegetation types saw more consistent trends of an advancing SOS and delayed EOS. In terms of the magnitude of change, advancements in the SOS and delays in the EOS were both higher in the southern ranges of the grasslands and DBFs.
3.2. Cumulative Effects of Drought on Phenology by Vegetation Type
3.2.1. Spatial Characteristics of Cumulative Effects
Figure 4a,b shows the maximum correlation coefficient Rmax_cum between the SOS and SPEI and the corresponding cumulative months, illustrating the spatial characteristics of the cumulative effect of drought on the vegetation SOS. Significant positive or negative Rmax_cum values indicate where phenology was affected by drought. The magnitude of the correlation coefficient indicates the extent to which phenology was affected by drought. The corresponding time scale indicates the response time of the sensitivity of phenology to drought [32].
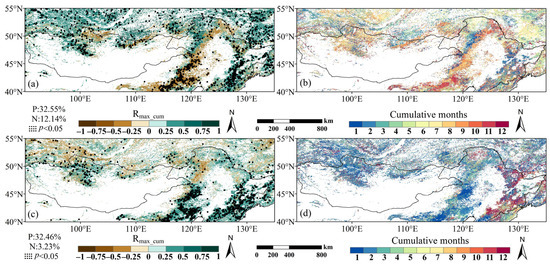
Figure 4.
Cumulative effects of drought on vegetation phenology: (a) maximum correlation coefficients (Rmax_cum) of multiyear SOS; (b) corresponding cumulative months for SOS; (c) maximum correlation coefficients of multiyear EOS; (d) corresponding cumulative months for EOS. Black dots indicate correlation coefficients passed 0.05 significance test; values in left lower corner indicate percentage of study area with significant positive (P) or negative (N) correlations.
The effects on the SOS of cumulative drought varied considerably in different regions. Positive significant correlations, indicating where drought induced advances in the SOS, accounted for a substantial percentage of the study area, around 32.55%, mainly in the Greater Khingan Mountains, the eastern part of Northeast China, the Khangai Mountains, and parts of southern Siberia. These are mostly areas with DNFs, DBFs, and shrub vegetation types. Negative significant correlations, indicating that drought contributed to a delayed SOS, appeared in 12.14% of the study area, mainly grasslands, including the western part of the Inner Mongolia Plateau. Drought led to a delayed SOS in these grassland ecosystems, counter to the trend seen in DNFs, DBFs, and shrublands (Figure 4a). There are significant differences between different vegetation types in the extent of the SOS affected by drought (Figure 5a). The distribution of cumulative months, indicating the length of the response time, showed no obvious difference among the time scale of 1 to 12 months, with a peak occurring at a length of 2 months (12.78%), followed by a length of 9 months (11.66%) (Figure 4b).
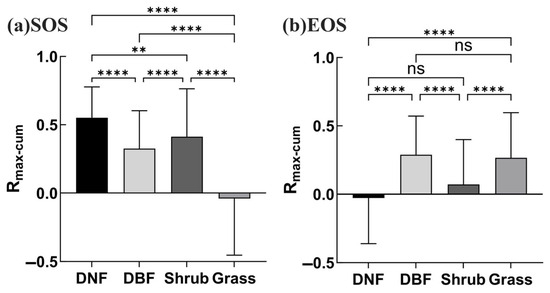
Figure 5.
Rmax_cum for different vegetation types in the study area: (a) for SOS, (b) for EOS. (Four asterisks indicate highly significant differences, p < 0.0001; two asterisks, p < 0.05; ns indicates no significant difference).
For the EOS, Rmax_cum showed positive significant correlations most obviously in the south—opposite to the spatial distribution of the SOS Rmax_cum (Figure 4c). Significantly positively correlated areas, indicating where drought induced advances in the EOS, accounted for 32.46% of the vegetation area, mainly in the southern part of northeastern China and the east-central part of Inner Mongolia, including areas of DNFs, DBFs, grassland, and shrub vegetation types. The EOS Rmax_cum displayed negative significant correlations, mainly in the south-central Greater Khingan Mountains and parts of southern Siberia, accounting for only 3.32% of the study area, predominantly DNFs. There were significant differences in the extent of the EOS affected by drought between DNFs and grasslands, as well as between DBFs and shrubs, but not between DNFs and shrubs or between DBFs and grasslands (Figure 5b). Time scales corresponding to the appearance of maximum correlation coefficients of the EOS vary across the study area, generally with longer response time scales in the east (Figure 4d). The peak cumulative months were mostly lower than for the SOS, with the 1-month scale having the largest proportion of affected area for the EOS (28.36%), followed by the 2-month scale (19.08%).
Overall, the area significantly affected by the Rmax_cum cumulative drought accounted for 44.69% of the vegetation distribution area for the SOS and 35.69% for the EOS. Results showed that the drought’s cumulative effect on vegetation phenology showed different response times, extents, and sensitivities for different vegetation types and different phenology nodes.
3.2.2. Time-Scale Characteristics of Cumulative Effects
As seen in Figure 6, there were obvious differences in the drought effects as superimposed over multiple time scales on the phenology of different vegetation types. (Bar graph results were extracted from the analysis of correlation between phenology and the SPEI at 12 cumulative time scales before selecting the maximum Rmax_cum [75,76]). The effect of drought on the DNF SOS was significant across most of the vegetation area (80.76% positive, 0.39% negative), with the peak occurring at 6 months of cumulative drought (69.52%) (Figure 6a). The drought effect on the DBF SOS was somewhat weaker, with 42.74% displaying significant correlations and the peak occurring at 2 months of cumulative drought (27.79%) (Figure 6b). The effect of drought on the shrub SOS was significant for 62.29% of its area, peaking at a cumulative 3 months (44.81%) and showing high sensitivity to drought at short time scales (Figure 6c). The effect of drought on grassland SOS achieved significance in 40.56% of that area, with a weak peak at a cumulative 7 months (14.87%) (Figure 6d). The positive effect of drought on the SOS in DNFs, DBFs, and shrublands was greater than the negative effect based on the proportions with significant correlations.
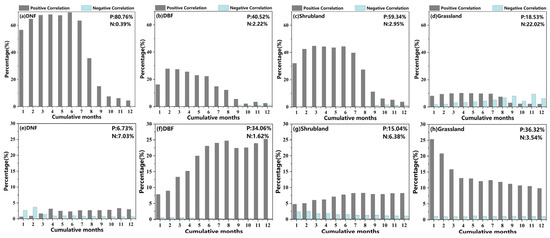
Figure 6.
Percentage of significant correlation coefficients between phenology and cumulative SPEI on a 1- to 12-month cumulative time scale for different vegetation types (p < 0.05). Above: (a–d) represent SOS. Below: (e–h) represent EOS. P and N indicate the percentage of vegetation area displaying positive and negative significant correlations with cumulative SPEI.
For EOS, the percentage of DNF area with significant correlations between cumulative drought and the EOS was small, only 13.76%, with little variation at different cumulative time scales (Figure 6e). By comparison, cumulative drought had a stronger effect on the DBF EOS (35.68%); the cumulative time scale was concentrated in the range of 6–12 months, with the peak occurring at 12 months (25.39%), indicating that prolonged drought had a stronger effect on the DBF EOS (Figure 6f). The effect of drought on the shrub EOS was relatively weak, with significant correlations in 21.42% of the distribution area and a peak occurring at 8 months (9.56%) (Figure 6g). The effect of drought on the grassland EOS is relatively stronger, with significant correlations in 39.86% of the distribution area and the peak occurring at 1 month (26.37%), indicating that grasslands were more susceptible to short-term drought stress (Figure 6h).
Overall, slightly more grassland area had significant negative correlations than significant positive correlations for the SOS, while significant positive correlations dominated in DNFs, DBFs, and shrublands. Cumulative drought had the greatest effect on the SOS in DNFs and the second in shrubs; cumulative drought had the greatest effect on the EOS in grasslands and the second in DBFs. The time scale of drought’s effect on grassland was shorter for the EOS, while the opposite was true for DBFs. These differences in the response to drought of the phenological nodes may relate to the adaptation strategies of different vegetation types, reflecting their physiological characteristics, water use efficiency, or preferred moisture and temperature conditions at different stages of growth and development.
3.2.3. Cumulative Effects Across Moisture Conditions
Figure 7 shows how peak drought’s cumulative months and Rmax_cum means vary with mean annual moisture conditions for the SOS and EOS of different vegetation types. For the SOS, drought’s cumulative month values for DNFs, DBFs, and shrublands were lower in relatively dry areas and higher in relatively humid areas (Figure 7a–c). R+max_cum (Rmax > 0) was significantly negatively correlated with the annual mean SPEI for DBFs and shrublands (Figure 7f,g). The opposite was true for grasslands, where the drought’s cumulative months gradually increased with decreasing moisture (Figure 7d). R−max_cum (Rmax < 0) was significantly positively correlated with the annual mean SPEI for grasslands (R2 = 0.602), with a greater effect in arid areas than in wet areas (Figure 7h).
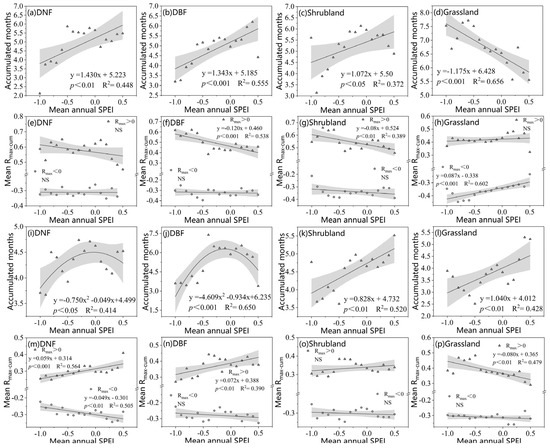
Figure 7.
Drought cumulative months (a–d) and Rmax_cum mean (e–h) on SOS along the moisture gradient. Drought cumulative months (i–l) and Rmax_cum mean (m–p) on EOS along the moisture gradient. Gray shading indicates 95% confidence interval. NS indicates not significant. The moisture gradient is represented in terms of annual average SPEI at 0.1 intervals.
For the EOS, drought’s cumulative months of DNFs and DBFs displayed nonlinear relationships with the annual mean SPEI. Drought cumulative months increase and then decrease with decreasing moisture (Figure 7i,j). Both shrub and grassland drought’s cumulative months were significantly positively correlated with the annual mean SPEI, suggesting that the shrub and grassland EOS respond faster to the cumulative effects of drought in dry regions and that increased water loss shortens the response time to drought. As shown in Figure 7m–p, for DNFs and DBFs, the annual average SPEI was significantly positively correlated with R+max_cum, but only DNFs’ annual average SPEI was significantly correlated with R−max_cum. Grasslands, by contrast, saw significantly negative correlations with R+max_cum, meaning that the cumulative effects of drought had a much smaller effect in areas with greater moisture. These variations reflected the adaptive adjustment of different vegetation types to drought under different moisture conditions, reflecting the complex relationship between vegetation and moisture dynamics.
3.3. Lagged Effects of Drought on Phenology by Vegetation Type
3.3.1. Spatial Characteristics of Lagged Effects
Figure 8 illustrates the spatial variation in Rmax_lag, the highest correlation coefficient between the SOS or EOS and monthly SPEI among the previous 12 months, and the corresponding lagged month. The distribution of the drought lagged effect on the SOS showed obvious spatial differences, with significant positive correlations accounting for 26.54% of the total area, mainly in eastern China and parts of southern Siberia, areas dominated by DNFs, DBFs, and shrublands. Significant negative correlations were mainly distributed in the Mongolian Plateau and western parts of Inner Mongolia, accounting for 23.19% of the total area, mostly grasslands (Figure 8a). There were no significant differences in the extent of SOS affected by drought between DNFs and shrubs in the study area, but there were significant differences between other vegetation types (Figure 9a). The largest percentage of drought impacts (14.32%) could be attributed to a lag of 1 month, followed by a lag of 10 months (10.70%) (Figure 8b).
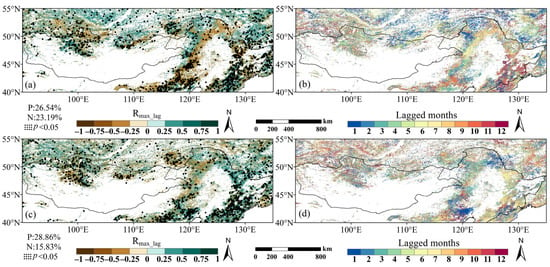
Figure 8.
Drought lagged effect on vegetation phenology: (a) maximum correlation coefficients (Rmax_lag) for multi-year SOS; (b) Rmax_lag corresponding lagged months for SOS; (c) Rmax_lag for EOS; (d) corresponding lagged months for EOS. Black dots indicate significant correlations (p < 0.05); values at the lower left indicate percentage of area with significant positive (P) or negative (N) Rmax_lag.
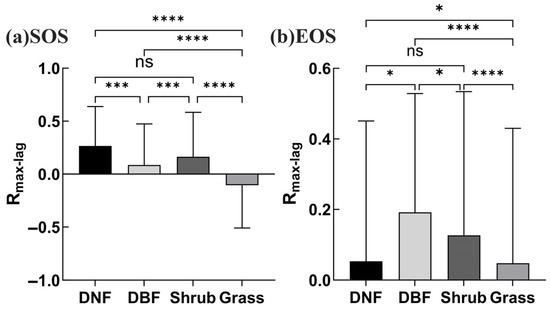
Figure 9.
Rmax_lag in study area in different vegetation types: (a) for SOS, (b) for EOS. (Four asterisks indicate highly significant differences, p < 0.0001; three asterisks, p < 0.001; one asterisk, p < 0.1; ns indicates no significant difference).
The drought lagged effect on the EOS also showed spatial differences, with 28.86% of the total area displaying significant positive correlations, concentrated in northeast China and across all four vegetation types. Significant negative correlations were found in central and western parts of Mongolia, accounting for 15.83% of the total area, mainly grasslands (Figure 8c). There are significant differences in the extent of the EOS affected by drought between different vegetation types, but no significant differences between DNFs and shrubs (Figure 9b). The drought effect of the first previous month of the EOS was the most widespread, with 13.61%, while the previous 5 months were the next most widespread, accounting for 10.48% of the total area (Figure 8d). Considering both positive and negative lagged effects, correlations were significant for 44.70% of the total area for the EOS, compared with 49.73% for the SOS. The variation in the direction and time scale of the lagged effect of drought on the phenology of different vegetation types appears to reflect their differentiated ecological adaptation strategies.
3.3.2. Time-Scale Characteristics of Lagged Effects
Figure 10 displays obvious differences among the lagged effects of drought on different vegetation types and phenological nodes. (The bar graph results were extracted from correlation analysis between phenology and SPEI at 12 lagged time scales before selecting the maximum Rmax_lag.) For SOS, the lagged effect of drought affected the largest area for DNFs (53.42% of the DNF area displayed a significant correlation), followed by grassland (50.82%), shrubland (50.03%), and then DBFs (47.93%). The lags affecting the DNF, DBF, and shrubland SOS were mainly concentrated in the time scales of 1–2 months, with a peak lagged effect for these three vegetation types occurring in the first month (23.34%, 16.49%, and 19.16% respectively). Thus, except for grassland, the SOS was mainly influenced by the lagged effect of drought in the previous month, and the closer the drought was to the phenological node, the greater the effect of drought. Only in grasslands did the proportion with negative significant correlations exceed that with positive correlations, and the lagged effects on grasslands did not vary much among the different time scales.
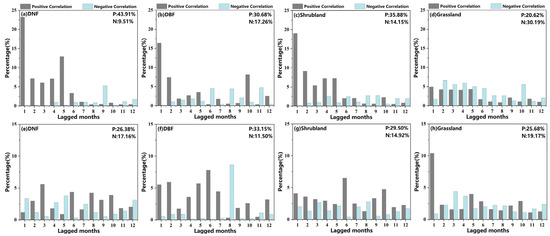
Figure 10.
Percentage of vegetation area with significant correlation coefficients between phenology and monthly SPEI on 1- to 12-month lagged time scales for different vegetation types (p < 0.05). Above: (a–d) represent SOS. Below: (e–h) represent EOS. P and N indicate the percentage of vegetation area displaying positive and negative significant correlations with SPEI.
For the EOS, the drought lagged effects on all four vegetation types displayed significant correlations on similar proportions of their spatial distribution, ranging from 44.85% for grasslands and 44.64% for DBFs to 44.41% for shrublands and 43.54% for DNFs. The correlations for DNFs, DBFs, and shrubland peaked at lags of 3 months (6.13%), 8 months (8.94%), and 6 months (6.91%), respectively; drought had a more rapid effect on grasslands. peaking at a 1-month lag (11.28%).
3.3.3. Lagged Effects Across Moisture Conditions
Both Rmax_lag and the corresponding number of lagged months were linked to moisture conditions (Figure 11). We find linear relationships for both the SOS and EOS of DBFs, shrubland, and grassland, but nonlinear relationships for DNFs. The number of drought effect lagged months for the DNF SOS was nonlinearly correlated with the annual mean SPEI (R2 = 0.621), first decreasing and then increasing with decreasing moisture (Figure 9a). The lagged months for DBFs, shrubland, and grassland SOS showed clear increases with increasing annual average SPEI, with shrubland having the largest response coefficient (Figure 11b–d). The annual mean SPEI of the four vegetation types was significantly negatively correlated with R+max_lag (Rmax > 0), and shrubland and grassland were significantly positively correlated with R−max_lag (Rmax < 0); the correlations were stronger in relatively dry areas than in relatively humid areas (Figure 11e–h).
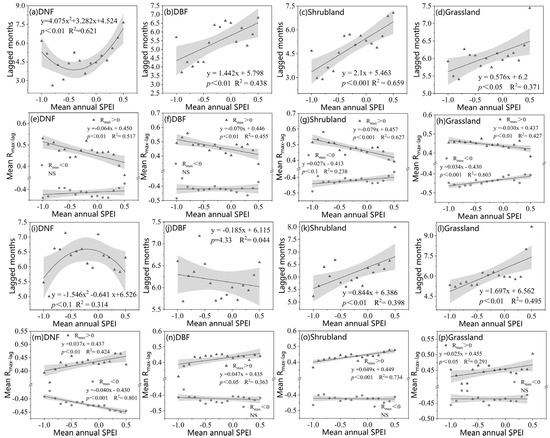
Figure 11.
Drought lagged months and mean Rmax_lag for positive and negative correlations along the moisture gradient: (a–h) SOS; (i–p) EOS. Gray shading indicates a 95% confidence interval. NS indicates not significant. The moisture gradient is represented in terms of annual average SPEI at 0.1 intervals.
For the EOS, the number of drought effect lagged months for DNFs was nonlinearly correlated with the annual mean SPEI (R2 = 0.314). The number of lagged months increased and then decreased with decreasing moisture conditions (Figure 11i). For DBFs, lagged months did not change obviously with changing moisture conditions. Both shrublands and grasslands displayed obvious increases in the number of lagged months with the increasing annual mean SPEI, meaning that decreases in water conditions shortened the response time to drought (Figure 11k,l). The correlation between the annual average SPEI and R+max_lag for all four vegetation types was positive, indicating that drought had a greater impact on the vegetation EOS in wetter areas. The correlation between the annual mean SPEI and R−max_lag for DNFs was negative (R2 = 0.804) (Figure 11m–p). In areas with more severe drought, moisture conditions show a weakened effect on the EOS.
3.4. Comparison of Lagged and Cumulative Effects
The difference between Rmax_cum and Rmax_lag (ΔRmax) indicates whether cumulative or lagged effects had a stronger statistical association with observed phenology. While this measure does not inherently indicate causal dominance, it may suggest avenues for further research. The ΔRmax values for both the SOS and EOS were largely concentrated in a narrow range between −0.15 and +0.15, indicating that the lagged and cumulative effects were close in strength (Figure 12a,b).
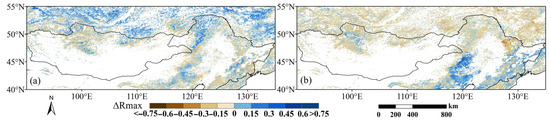
Figure 12.
Comparation of maximum correlations between cumulative and lagged effects for (a) SOS and (b) EOS. Positive values indicate that the cumulative effect of drought is more strongly correlated; negative values indicate that the lagged effect is more strongly correlated.
For the SOS, the lagged effect dominates 55.67% of the study area, mainly concentrated in eastern China and northern Mongolia; the cumulative effect prevailed in 44.33% of the area, mainly concentrated in the far north, including northern parts of the Greater Khingan Mountains, southern Siberia, and portions of southeast Inner Mongolia. For the EOS, the study area was also dominated by the lagged effect (61.55%), mainly concentrated in the north, including northern Mongolia, the Greater Khingan Mountains, and parts of southern Siberia; the cumulative effect prevailed in 38.45% of the total area, concentrated in the southeast part of northeast China and Inner Mongolia.
For different vegetation types, we found that the DNF SOS was mainly affected by the drought’s cumulative effect in 83.17% of the distribution area, with most of them having ΔRmax values between 0 to 0.3. In contrast, the DBF SOS was more strongly affected by the lagged effect of drought, accounting for 56.47% of the distribution area, with ΔRmax values mostly between −0.15 and 0. Shrublands, like DNFs, were mainly affected by drought’s cumulative effect, with a share of 62.71% of the distribution area and ΔRmax values mostly between 0 and 0.3. Grasslands, similar to DBFs, were strongly affected by drought’s lagged effect, with a share of 60.94% of the distribution area and ΔRmax values mostly between −0.15 and 0 (Figure 12a and Figure 13a). For the EOS, all four vegetation types were dominated by the lagged effect, accounting for 84.08%, 61.10%, 76.03%, and 57.63% of the distribution area of DNFs, DBFs, shrublands, and grasslands, respectively; among them, DNFs and shrublands had greater proportions of ΔRmax values between −0.3 and 0, while most of DBFs and grasslands had ΔRmax values between −0.15 and 0 (Figure 12b and Figure 13b).
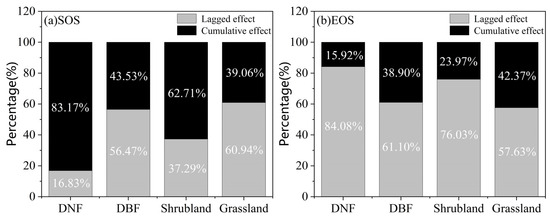
Figure 13.
Percentage of areas where phenology is more highly correlated with cumulative or lagged effects, by vegetation type: (a) SOS; (b) EOS.
4. Discussion
4.1. Drought’s Cumulative and Lagged Effects on the Phenology of Different Vegetation Types
We found that drought has cumulative and lagged effects on vegetation phenology and that there were significant differences in these effects between vegetation types with different trade-off strategies for growth and survival. Drought led to the delayed SOS in grasslands and advanced SOS in DNFs, DBFs, and shrublands in this study. This is in line with previous findings, where pre-seasonal drought events were found to cause the grassland SOS to be delayed and the forest SOS to be advanced in northeast China [77].
For perennial trees and shrubs, the SOS is a key physiological turning point in the transition from dormancy to active growth. Pre-SOS drought can increase temperatures by reducing evaporation energy consumption, thus promoting vegetation’s new leaf germination in spring [18,78]. In addition, deep-rooted forest trees are better able to utilize water in the deep soil to resist the effects of previous droughts on current vegetation growth, further reducing the negative effect of drought on the SOS [79]. Furthermore, precipitation moderates the effect of temperature on phenology in dry regions [80], when temperature is the main driver [6,81,82].
By comparison, grass root systems are shallow, so when drought depletes soil moisture quickly, grasses do not have enough available water for germination [83]. After drought conditions wane, grassland vegetation can respond rapidly to light rainfall events (<10 mm). This fast-growing, resource-accessing vegetation has the advantage of enhancing stability by engaging in drought escape or drought avoidance strategies [17,84,85], rather than the more conservative strategy of drought tolerance [86,87,88]. As a result, the germination of vegetation in grassland ecosystems under drought conditions occurs much later than under normal conditions, resulting in the delayed grassland SOS.
Prior studies proposed that the cumulative effects of drought affect a greater proportion of grassland areas compared with forests [32,89]. To the contrary, in this study, the results for the SOS showed that the percentage of grasslands affected by cumulative drought was lower than the percentage of DNFs affected. An important reason for this is that grassland ecosystems in the study area span arid, semiarid, and semihumid regions [77]—including areas of northern Mongolia and northeastern Inner Mongolia where precipitation is relatively high—and thus moisture is not a major constraint on grassland rejuvenation. In our study area, DNFs are mainly found in eastern Siberia and the Greater Khingan Mountains. DNFs that extend into permafrost regions tend to have shallow root systems and mainly utilize shallow soil moisture from winter snowmelt. With global warming, earlier snowmelt replenishes soil moisture in early spring and reduces the possible negative effects of drought [90]. However, drought can also promote increased temperatures [91], facilitating the earlier development of new leaves. As a result, prolonged cumulative drought has a greater impact on the advancement of the SOS in DNFs.
At the other end of the growing season, this study found that drought leads to an earlier EOS in DNFs, DBFs, shrublands, and grasslands. It has been found that summer and fall droughts significantly advance the vegetation EOS [92], which is consistent with our findings. Drought-induced premature senescence is another mitigation strategy that vegetation can adopt to cope with drought stress. When water stress coincides with natural senescence processes, plants optimize their survival strategies by accelerating senescence [93]. Continued summer warming, lower total precipitation, and higher evapotranspiration all promote vegetation leaf senescence [94]. Fall drought prompts leaves to close stomata, reducing transpiration and photosynthesis rates, but respiration is maintained at higher rates that accelerate carbon assimilation degradation, leading to earlier vegetation senescence [92]. Furthermore, the differences among vegetation types of the cumulative effects of drought are more obvious than among their lagged effects, which may be related to the fact that the cumulative effect builds on and deepens the lagged effect.
4.2. Effects on Phenology of Trees with Different Leaf Forms
This study found significant differences in the effects of drought on trees with different leaf forms (needleleaf or broadleaf). Drought had a greater effect on the SOS in DNFs, and on both the SOS and EOS in DBFs, and the character is more obvious in the cumulative aspect. This may stem from ecological adaptation strategies that tree species evolved to adapt to differential climates with different leaf forms [43,95,96]. These strategies are the main determinants of plants’ biomass allocation patterns [97]. Plant physiological traits and environmental adaptation strategies together shape phenological drought response patterns [5]. For example, conifer species typically exhibit conservative water use strategies [98], with lower stomatal conductance and lower photosynthetic rates [99]. Consequently, the SOS of DNFs reflects its low moisture requirement to initiate new shoot germination and photosynthesis. DBFs generally develop deep root systems, which reduce the negative effect of drought; their evapotranspiration requirements are low in the early stages of leaf development. Thus, drought may benefit the DBF and DNF SOS through the effect on thermal conditions.
At the other end of the growing season, there are obvious differences for trees with different leaf forms. This might be related to how the leaves of DNFs are highly adapted to drought and consume less water for photosynthesis [100], so drought only weakly regulates the processes of maturation to defoliation. In needleleaf forests, the needle-like leaves and conservative water-use strategy of DNFs make the EOS less sensitive to both short-term and long-term drought. By contrast, DBFs have a high leaf area, a high transpiration rate, and a greater water demand during the growing season [43], thus requiring large amounts of water to maintain transpiration and photosynthesis. The “opportunistic strategy” of DBFs tends to prioritize the allocation of limited water and nutrients to the root system or storage tissues rather than maintaining leaf function under drought stress [101,102]. When subjected to drought stress, these trees inhibit transpiration by decreasing stomatal conductance while accelerating leaf senescence signals (e.g., abscisic acid ABA accumulation), leading to chlorophyll degradation [103,104]. This resource redistribution drives early leaf abscission to reduce overall water consumption.
Consequently, this study found that the SOS of DNFs and DBFs are both highly affected by drought, mainly limited by tree water-use strategies and water availability, while the EOS of DBFs is also sensitive to drought, mainly stemming from hormonal regulation and resource redistribution to accelerate leaf senescence. These differences reflect the differentiation in phenological adaptation strategies among different leaf forms and functional types of plants. Future studies should incorporate multiscale observations (e.g., near-surface remote sensing, gene expression) to further validate the mechanisms behind these responses to drought.
4.3. Effects on Phenology Under Different Moisture Conditions
This study showed that the cumulative and lagged effects of drought on vegetation phenology also varied across different moisture conditions. The effect of drought on the vegetation SOS was stronger in relatively dry areas, intensifying with decreasing moisture. The effect on the vegetation EOS was stronger in relatively humid areas, which was consistent with a prior study [72]. This is because the vegetation SOS has a low resistance to moisture shortages, which tend to be more acute in dry regions, so drought’s influence on the SOS is stronger in dry regions. Vegetation that grows in wetter areas grows more foliage in the summer, accumulates more biomass, and consumes more water, and thus defoliation is more affected by fall drought [105]. Vegetation growing in arid areas, on the other hand, accumulates less biomass in summer and consumes less water, so leaf litter is relatively less affected by fall drought [18].
This study found that the time scales of drought’s cumulative effect on the DNF, DBF, and shrub SOS were shorter in response to drought in dry areas [106]. Conversely, the time scale of grassland’s response to drought is longer in arid regions [31]. This is mainly due to the fact that grasslands are more resilient and adaptive to shorter and more intense droughts in relatively arid regions, and are better able to self-regulate growth, morphology, and physiology to withstand drought stress on shorter time scales [107].
The drought’s cumulative effect response time scales showed a pattern of increasing and then decreasing with decreasing humidity for the DNF and DBF EOS, with different vegetation types exhibiting differentiated critical response thresholds. These patterns may be related to the differences in sensitivity to drought across vegetation types and need to be further explored in the future. The response time scales of drought’s cumulative effect were gradually shortened with decreasing humidity in the grassland and shrub EOS. We attribute this to the fact that vegetation growing in arid regions is frequently subjected to water stress, and in order to adapt quickly to water scarcity, plants in arid regions have evolved physiological, anatomical, and functional strategies to reduce their water loss, respiratory costs, photosynthetic activity, and growth rates [108].
For drought’s lagged effect, the lag time scales for the SOS in DNFs show a pattern of decrease and then increase with the intensification of drought, while for the EOS, the reverse pattern (increase and then decrease) was observed. The response time for the DBF SOS gradually shortened with the intensification of drought. The SOS and EOS of shrubs and grasslands responded faster to the lagged effect in arid regions: increased water loss shortened the response time to drought, and arid regions responded more rapidly to drought [106]. Arid regions have less soil moisture and generally poorer water-holding capacity, so vegetation growing in arid zones is usually sensitive to fluctuations in surface water resources [109]. In contrast, soil moisture content is more abundant in humid areas, so stable water reserves can continue to supply plant growth requirements even when precipitation is deficient [110].
The statistical strength of the association between drought effects and phenological nodes of different vegetation types was explored at the pixel scale. This study found that the lagged effect exceeded the cumulative effect in a larger proportion of the study area, which is consistent with the results of previous studies [31]. However, there were obvious differences in the strength of the correlations between drought and phenology nodes for different vegetation types: the DNF and shrub SOS was dominated by cumulative effects, while the DBF and grassland SOS was dominated by lagged effects. The EOS for all four vegetation types was dominated by lagged effects. While correlation strength is not sufficient to show a causal effect, these results suggest avenues for further research into the different growth strategies of different vegetation types. DNFs and shrubs focus on conservative growth strategies: their maximum photosynthetic rate is low and their photosynthetic and respiratory capacities are weak, making them more drought tolerant and resistant to prolonged cumulative drought [41]. For DBFs and grasslands, where resource-acquisitive strategies dominate, plants tend to close stomata earlier to show greater resistance to pre-existing droughts through drought escape and drought avoidance [88,102,111].
In addition, there are differences in water and nutrient requirements for vegetation growth at different phenological stages. Temperature and precipitation affect plant growth and ecosystem carbon cycling, which in turn affects the time of cyclical events in the plant life cycle [112]. As the climate warms and drought intensifies, the effects of drought on the phenological nodes of different vegetation types can be studied to “decode” the response of terrestrial ecosystems to climate stress. Its scientific value lies not only in revealing the micro-mechanisms of plant survival strategies, but also in providing decision support for macro-scale ecological risk management, the realization of carbon neutrality, and the sustainable development of human society.
5. Limitations and Prospects
This study used only the NDVI and SPEI datasets to investigate the effects of drought on the phenology of various types of vegetation. The relatively coarse spatial and temporal resolutions of these datasets and the lack of ground-based observational data for validation necessarily limit the applicability of the findings presented here. We rely on interpolation methods to fill gaps in the time series and to align data sources of different resolutions, but these methods cannot compensate for phenomena that are manifested at finer spatial or temporal resolutions. Furthermore, the study area extends into regions of permafrost, where the SPEI indicator may not capture the special hydrological processes dominated by snowmelt rather than incoming precipitation.
The interaction mechanism between climate change and vegetation phenology is relatively complex, and other potential factors such as land surface temperature or human activities will inevitably have a direct or indirect impact on vegetation phenology. Future studies should consider changes in the cumulative and lagged effects of intensified droughts in the context of climate change and incorporate multiscale observations (e.g., near-surface remote sensing, in situ validation, and gene expression) as well as more meteorological elements to further validate the mechanisms behind these responses to drought.
6. Conclusions
In this study, by analyzing vegetation phenology data and the multiscale SPEI index, we documented the differential phenological responses of shrubs, grasslands, and forests with different leaf forms to the cumulative and lagged effects of drought at multiple time scales from 2001 to 2020. The effect of drought on the vegetation SOS was generally more obvious than that on the EOS. There were differences in the cumulative and lagged effects of drought on grass, shrubs, and trees with different leaf forms. Our major findings include the following:
- (1)
- Drought led to a delayed SOS for grassland ecosystems, but an advanced SOS for DNFs, DBFs, and shrublands. Drought caused the advancement of the EOS in all four vegetation types. Drought had a large effect on the SOS in DNFs, and similar effects on the SOS and EOS in DBFs. The time scale for the grassland SOS response to drought was longer than that for the EOS, while the time scales for the DBF SOS response were shorter than for the EOS. Except for grasslands, the drought’s lagged effect on the SOS was mainly from the previous month, and the closer the drought to the phenological node, the greater the effect. The grassland EOS responded very rapidly to drought, mainly concentrated in the time scale of 1–2 months.
- (2)
- The effect of drought on the vegetation SOS was more obvious in relatively dry areas, while the effect of drought on the vegetation EOS was higher in relatively wet areas. There were differences in the change in the main drought response time scale to changes in average annual water availability. The cumulative time scale of the grassland SOS response to drought gradually increased with decreasing average annual water availability, while the cumulative time scale of the DNF, DBF, and shrub SOS response to drought gradually decreased with decreasing average annual water availability. The cumulative time scale of the grassland and shrub EOS response to drought gradually decreased, showing a pattern in DNFs and DBFs of first increasing and then decreasing with decreasing average annual water availability. In DNFs, with decreasing average annual water availability, the SOS drought lag time scales showed a decreasing and then increasing pattern, but an increasing and then decreasing pattern for the EOS. The lag gradually shortened in response to drought for the SOS in DBFs as well as for both the SOS and EOS in arid shrublands and grasslands.
- (3)
- The drought lagged effect on vegetation phenology exceeded that of the drought’s cumulative effect in 55.67% of the study area for the SOS and 61.55% for the EOS. The dominant drought effects varied among vegetation types and phenological nodes: For the SOS, drought’s cumulative effects dominated in DNFs (83.17%) and shrublands (62.71%), and lagged effects dominated in DBFs (56.47%) and grasslands (60.94%). For the EOS, the lagged effect was dominant in all four vegetation types, ranging from 84.08% of DNFs to 57.63% of grasslands.
Previous studies have focused more on cumulative and lagged effects of single vegetation types or the overall vegetation NDVI. The difference in cumulative and lagged effects of drought on different phenological nodes has not been fully studied and remains largely unknown, especially for trees with different leaf forms. This study found differences in the responses to drought of shrubs, grasses, and trees with different leaf forms, most significantly between grasslands and other vegetation types, especially for the SOS and less for the EOS. Differences in dominance effects across vegetation types reflect the differences between conservative growth strategies and resource-acquisitive strategies.
In summary, differences in vegetation survival strategies are the main reason for observed differences in the cumulative and lagged effects of drought. Therefore, a clear understanding of how different vegetation types and leaf forms respond to drought is essential for modeling the sensitivity of vegetation to future climate and moisture conditions. As drought intensifies, future empirical studies and models will need to focus on the varying effects of different growth strategies of vegetation as they pertain to different phenological nodes.
Author Contributions
Conceptualization, K.D. and B.L.; methodology, K.D.; software, K.D.; validation, K.D. and B.L.; formal analysis, K.D.; investigation, K.D., W.H., M.C., P.Z. and R.G.; resources, K.D.; data curation, K.D.; writing—original draft preparation, K.D.; writing—review and editing, B.L. and M.H.; visualization, K.D.; funding acquisition, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 41877416.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We appreciate the MODIS data support from NASA and the SPEI dataset from the Global SPEI database.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gong, Z.; Ge, W.; Guo, J.; Liu, J. Satellite remote sensing of vegetation phenology: Progress, challenges, and opportunities. ISPRS J. Photogramm. Remote Sens. 2024, 217, 149–164. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zohner, C.M.; Peñuelas, J.; Li, Y.; Wu, X.; Zhang, Y.; Liu, H.; Shen, P.; Jia, X.; et al. Declining precipitation frequency may drive earlier leaf senescence by intensifying drought stress and enhancing drought acclimation. Nat. Commun. 2025, 16, 910. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Zhang, H.; Zhang, Z.; Guo, X.; Wang, M. Detection and attribution of the start of the growing season changes in the Northern Hemisphere. Sci. Total Environ. 2023, 903, 166607. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, B.; Michaelides, K.; De Kauwe, M.G.; Wang, J.; Shen, M.; Zhang, W.; Wang, Y.; Xiao, X.; Qin, Y.; et al. Contrasting Trends in Onset of Spring Green-Up Between Grasslands and Forests in China. Earth’s Future 2025, 13, e2024EF005379. [Google Scholar] [CrossRef]
- Zhang, R.; Qi, J.; Leng, S.; Wang, Q. Long-Term Vegetation Phenology Changes and Responses to Preseason Temperature and Precipitation in Northern China. Remote Sens. 2022, 14, 1396. [Google Scholar] [CrossRef]
- Fu, Y.H.; Zhou, X.; Li, X.; Zhang, Y.; Geng, X.; Hao, F.; Zhang, X.; Hanninen, H.; Guo, Y.; De Boeck, H.J. Decreasing control of precipitation on grassland spring phenology in temperate China. Glob. Ecol. Biogeogr. 2021, 30, 490–499. [Google Scholar] [CrossRef]
- Zuccarini, P.; Delpierre, N.; Mariën, B.; Peñuelas, J.; Heinecke, T.; Campioli, M. Drivers and dynamics of foliar senescence in temperate deciduous forest trees at their southern limit of distribution in Europe. Agric. For. Meteorol. 2023, 342, 109716. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, N.; Chen, G.; Zhang, D.; Zheng, Z.; Fan, D. Divergent shifts and responses of plant autumn phenology to climate change on the Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2017, 239, 166–175. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, R.; Wen, Z.; Khalifa, M.; Zheng, C.; Ren, H.; Zhang, Z.; Wang, Z. Assessing the impacts of drought on net primary productivity of global land biomes in different climate zones. Ecol. Indic. 2021, 130, 108146. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Naumann, G.; Alfieri, L.; Wyser, K.; Mentaschi, L.; Betts, R.A.; Carrao, H.; Spinoni, J.; Vogt, J.; Feyen, L. Global Changes in Drought Conditions Under Different Levels of Warming. Geophys. Res. Lett. 2018, 45, 3285–3296. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, X.; Zhao, Y.; Shan, L.; Zhang, Y.; Sun, Z.; Zhang, P.; Si, S. Lagged and cumulative effects of drought on global vegetation greenness, coverage, and productivity. Environ. Impact Assess. Rev. 2025, 115, 108019. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, J.; Han, J.; Zhang, T.; Yang, S.; Wang, J.; Prodhan, F.A.; Yao, F. Projected Increases in Global Terrestrial Net Primary Productivity Loss Caused by Drought Under Climate Change. Earth’s Future 2022, 10, e2022EF002681. [Google Scholar] [CrossRef]
- Gebrechorkos, S.H.; Sheffield, J.; Vicente-Serrano, S.M.; Funk, C.; Miralles, D.G.; Peng, J.; Dyer, E.; Talib, J.; Beck, H.E.; Singer, M.B.; et al. Warming accelerates global drought severity. Nature 2025, 642, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhong, S. Advances in Optical and Thermal Remote Sensing of Vegetative Drought and Phenology. Remote Sens. 2024, 16, 4209. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Schwalm, C.R.; Gentine, P.; Smith, W.K.; Ciais, P.; Kimball, J.S.; Gazol, A.; Kannenberg, S.A.; Chen, A.; et al. Widespread spring phenology effects on drought recovery of Northern Hemisphere ecosystems. Nat. Clim. Change 2023, 13, 182–188. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, W.; Ge, Q.; Li, Z.; Wang, X.; Zhou, Y.; Zhang, Z.; Li, Y.; Huang, H.; Liu, G.; et al. Legacy effects of spring phenology on vegetation growth under preseason meteorological drought in the Northern Hemisphere. Agric. For. Meteorol. 2021, 310, 108630. [Google Scholar] [CrossRef]
- Lai, P.; Zhang, M.; Ge, Z.; Hao, B.; Song, Z.; Huang, J.; Ma, M.; Yang, H.; Han, X. Responses of Seasonal Indicators to Extreme Droughts in Southwest China. Remote Sens. 2020, 12, 818. [Google Scholar] [CrossRef]
- Peaucelle, M.; Janssens, I.A.; Stocker, B.D.; Descals Ferrando, A.; Fu, Y.H.; Molowny-Horas, R.; Ciais, P.; Peñuelas, J. Spatial variance of spring phenology in temperate deciduous forests is constrained by background climatic conditions. Nat. Commun. 2019, 10, 5388. [Google Scholar] [CrossRef]
- Chen, M.; Henderson, M.; Liu, B.; Zhou, W.; Ma, R.; Huang, W.; Dou, Z. Winter climate change mediates the sensitivity of vegetation leaf-out to spring warming in high latitudes in China. Front. Plant Sci. 2024, 15, 1476576. [Google Scholar] [CrossRef]
- Bernal, M.; Estiarte, M.; Peñuelas, J. Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora. Plant Biol. 2011, 13, 252–257. [Google Scholar] [CrossRef]
- Cui, T.; Martz, L.; Guo, X. Grassland Phenology Response to Drought in the Canadian Prairies. Remote Sens. 2017, 9, 1258. [Google Scholar] [CrossRef]
- Rihan, W.; Zhao, J.; Zhang, H.; Guo, X. Preseason drought controls on patterns of spring phenology in grasslands of the Mongolian Plateau. Sci. Total Environ. 2022, 838, 156018. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, R.; Chen, J.M. Responses of phenology to preseason drought and soil temperature for different land cover types on the Mongolian Plateau. Sci. Total Environ. 2024, 926, 171895. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Jiao, T.; Williams, C.A.; De Kauwe, M.G.; Schwalm, C.R.; Medlyn, B.E. Patterns of post-drought recovery are strongly influenced by drought duration, frequency, post-drought wetness, and bioclimatic setting. Glob. Change Biol. 2021, 27, 4630–4643. [Google Scholar] [CrossRef]
- Lu, M.; Sun, H.; Yang, Y.; Xue, J.; Ling, H.; Zhang, H.; Zhang, W. Assessing recovery time of ecosystems in China: Insights into flash drought impacts on gross primary productivity. Hydrol. Earth Syst. Sci. 2025, 29, 613–625. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Dynamics of nonstructural carbohydrates during drought and subsequent recovery: A global meta-analysis. Agric. For. Meteorol. 2025, 363, 110429. [Google Scholar] [CrossRef]
- Song, L.; Luo, W.; Griffin-Nolan, R.J.; Ma, W.; Cai, J.; Zuo, X.; Yu, Q.; Hartmann, H.; Li, M.-H.; Smith, M.D.; et al. Differential responses of grassland community nonstructural carbohydrate to experimental drought along a natural aridity gradient. Sci. Total Environ. 2022, 822, 153589. [Google Scholar] [CrossRef]
- Huang, W.; Henderson, M.; Liu, B.; Su, Y.; Zhou, W.; Ma, R.; Chen, M.; Zhang, Z. Cumulative and Lagged Effects: Seasonal Characteristics of Drought Effects on East Asian Grasslands. Remote Sens. 2024, 16, 3478. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Cui, M.; Lu, J.; Shi, H.; Ren, H.; Zhang, W.; Wen, Z. Global Assessment of Cumulative and Time-Lag Effects of Drought on Land Surface Phenology. GIScience Remote Sens. 2022, 59, 1918–1937. [Google Scholar] [CrossRef]
- Yuan, Y.; Bao, A.; Jiapaer, G.; Jiang, L.; De Maeyer, P. Phenology-based seasonal terrestrial vegetation growth response to climate variability with consideration of cumulative effect and biological carryover. Sci. Total Environ. 2022, 817, 152805. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Yan, S.; Chen, H.; Yang, J.; Wen, Y. Intra-Annual Cumulative Effects and Mechanisms of Climatic Factors on Global Vegetation Biomes’ Growth. Remote Sens. 2024, 16, 779. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Z.; Peng, S. Global analysis of time-lag and -accumulation effects of climate on vegetation growth. Int. J. Appl. Earth Obs. Geoinf. 2020, 92, 102179. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Schwalm, C.R.; Anderegg, W.R.L. Ghosts of the past: How drought legacy effects shape forest functioning and carbon cycling. Ecol. Lett. 2020, 23, 891–901. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, X.; Liang, S.; Zhou, T.; Huang, K.; Tang, B.; Zhao, W. Time-lag Effects of Global Vegetation Responses to Climate Change. Glob. Change Biol. 2015, 21, 3520–3531. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Q.; Liu, R.; Zhao, Y.; Zhang, D. Effects of climate change and human activities on vegetation coverage change in northern China considering extreme climate and time-lag and -accumulation effects. Sci. Total Environ. 2023, 860, 160527. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Liu, S.; Jiang, L.; Mao, J.; Jia, X.; Zha, T.; Zhang, K.; Wu, Y.; Zhou, J. Time-Lag Effect of Climate Conditions on Vegetation Productivity in a Temperate Forest–Grassland Ecotone. Forests 2022, 13, 1024. [Google Scholar] [CrossRef]
- Marsh, H.; Zhang, W. Direct and Legacy Effects of Spring Temperature Anomalies on Seasonal Productivity in Northern Ecosystems. Remote Sens. 2022, 14, 2007. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Ma, H.; Crowther, T.; Mo, L.; Maynard, D.; Renner, S.; Hoogen, J.; Zou, Y.; Liang, J.; Miguel, S.D.; Nabuurs, G.-J.; et al. The global biogeography of tree leaf form and habit. Nat. Plants 2023, 9, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Bond, W.J. The tortoise and the hare: Ecology of angiosperm dominance and gymnosperm persistence. Biol. J. Linn. Soc. 1989, 36, 227–249. [Google Scholar] [CrossRef]
- Ye, Y.; Kitayama, K.; Onoda, Y. A cost–benefit analysis of leaf carbon economy with consideration of seasonal changes in leaf traits for sympatric deciduous and evergreen congeners: Implications for their coexistence. New Phytol. 2022, 234, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, H.; He, Z.; Ma, D.; Sun, W.; Xu, X.; Tian, Q. Effects of Drought Stress on Leaf Functional Traits and Biomass Characteristics of Atriplex canescens. Plants 2024, 13, 2006. [Google Scholar] [CrossRef]
- Bristiel, P.; Roumet, C.; Violle, C.; Volaire, F. Coping with drought: Root trait variability within the perennial grass Dactylis glomerata captures a trade-off between dehydration avoidance and dehydration tolerance. Plant Soil 2019, 434, 327–342. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, L.; Liu, Y.; Hao, Z.; Zhang, J. Changes in the drought condition over northern East Asia and the connections with extreme temperature and precipitation indices. Glob. Planet. Change 2021, 207, 103645. [Google Scholar] [CrossRef]
- Zhang, P.; Jeong, J.-H.; Yoon, J.-H.; Kim, H.; Wang, S.-Y.S.; Linderholm, H.W.; Fang, K.; Wu, X.; Chen, D. Abrupt shift to hotter and drier climate over inner East Asia beyond the tipping point. Science 2020, 370, 1095–1099. [Google Scholar] [CrossRef]
- Fu, Y.H.; Zhao, H.; Piao, S.; Peaucelle, M.; Peng, S.; Zhou, G.; Ciais, P.; Huang, M.; Menzel, A.; Peñuelas, J.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef]
- Peñuelas, J.; Rutishauser, T.; Filella, I. Phenology Feedbacks on Climate Change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef]
- Yang, M.; Zou, J.; Ding, J.; Zou, W.; Yahefujiang, H. Stronger Cumulative than Lagged Effects of Drought on Vegetation in Central Asia. Forests 2023, 14, 2142. [Google Scholar] [CrossRef]
- Wei, X.; He, W.; Zhou, Y.; Ju, W.; Xiao, J.; Li, X.; Liu, Y.; Xu, S.; Bi, W.; Zhang, X.; et al. Global assessment of lagged and cumulative effects of drought on grassland gross primary production. Ecol. Indic. 2022, 136, 108646. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Gouveia, C.; Camarero, J.J.; Beguería, S.; Trigo, R.; López-Moreno, J.I.; Azorín-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J.; et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Guo, E.; Yin, S.; Wang, Y.; Na, R.; Wan, Z. Assessment of the Cumulative and Lagging Effects of Drought on Vegetation Growth in Inner Mongolia. Acta Agrestia Sin. 2021, 29, 1301–1310. [Google Scholar] [CrossRef]
- Wei, W.; Liu, T.; Zhou, L.; Wang, J.; Yan, P.; Xie, B.; Zhou, J. Drought-Related Spatiotemporal Cumulative and Time-Lag Effects on Terrestrial Vegetation across China. Remote Sens. 2023, 15, 4362. [Google Scholar] [CrossRef]
- Shen, X.; Shen, M.; Wu, C.; Peñuelas, J.; Ciais, P.; Zhang, J.; Freeman, C.; Palmer, P.I.; Liu, B.; Henderson, M.; et al. Critical role of water conditions in the responses of autumn phenology of marsh wetlands to climate change on the Tibetan Plateau. Glob. Change Biol. 2024, 30, e17097. [Google Scholar] [CrossRef]
- Mei, L.; Bao, G.; Tong, S.; Yin, S.; Bao, Y.; Jiang, K.; Hong, Y.; Tuya, A.; Huang, X. Elevation-dependent response of spring phenology to climate and its legacy effect on vegetation growth in the mountains of northwest Mongolia. Ecol. Indic. 2021, 126, 107640. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Chen, J.; Chen, Y.; Li, J.; Yan, G.; Zhang, X. Analysis of NDVI and scaled difference vegetation index retrievals of vegetation fraction. Remote Sens. Environ. 2006, 101, 366–378. [Google Scholar] [CrossRef]
- Ding, Y.; He, X.; Zhou, Z.; Hu, J.; Cai, H.; Wang, X.; Li, L.; Xu, J.; Shi, H. Response of vegetation to drought and yield monitoring based on NDVI and SIF. CATENA 2022, 219, 106328. [Google Scholar] [CrossRef]
- Chen, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A simple method for reconstructing a high-quality NDVI time-series data set based on the Savitzky–Golay filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Jonsson, P.; Eklundh, L. Seasonality extraction by function fitting to time-series of satellite sensor data. IEEE Trans. Geosci. Remote Sens. 2002, 40, 1824–1832. [Google Scholar] [CrossRef]
- He, X.H.; Liu, A.Q.; Tian, Z.H.; Wu, L.L.; Zhou, G.S. Response of Vegetation Phenology to Climate Change on the Tibetan Plateau Considering Time-Lag and Cumulative Effects. Remote Sens. 2024, 16, 49. [Google Scholar] [CrossRef]
- Peng, J.; Wu, C.; Zhang, X.; Wang, X.; Gonsamo, A. Satellite detection of cumulative and lagged effects of drought on autumn leaf senescence over the Northern Hemisphere. Glob. Change Biol. 2019, 25, 2174–2188. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Zhang, H.; Zhang, Z.; Guo, X.; Zhang, T.; Wu, R. Detecting the response characteristics and thresholds of grassland spring phenology to climatic factors in the Mongolian Plateau. Ecol. Indic. 2023, 153, 110440. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Zhang, Z.; Guo, X.; Li, X.; Chen, C. Spatial and Temporal Changes in Vegetation Phenology at Middle and High Latitudes of the Northern Hemisphere over the Past Three Decades. Remote Sens. 2015, 7, 10973–10995. [Google Scholar] [CrossRef]
- Sen, P.K. Estimates of the Regression Coefficient Based on Kendall’s Tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, N.; Shen, M.; Guo, L. Effect of preseason diurnal temperature range on the start of vegetation growing season in the Northern Hemisphere. Ecol. Indic. 2020, 112, 106161. [Google Scholar] [CrossRef]
- Xu, H.-J.; Wang, X.-P.; Zhao, C.-Y.; Yang, X.-M. Diverse responses of vegetation growth to meteorological drought across climate zones and land biomes in northern China from 1981 to 2014. Agric. For. Meteorol. 2018, 262, 1–13. [Google Scholar] [CrossRef]
- Qiao, L.; Xia, H.; Zhao, X.; Yang, J.; Song, H.; Liu, Y. Divergent impacts of drought on autumn phenology in China. Ecol. Indic. 2024, 160, 111770. [Google Scholar] [CrossRef]
- Zhan, C.; Liang, C.; Zhao, L.; Jiang, S.; Niu, K.; Zhang, Y. Drought-related cumulative and time-lag effects on vegetation dynamics across the Yellow River Basin, China. Ecol. Indic. 2022, 143, 109409. [Google Scholar] [CrossRef]
- Liu, L.; Guan, J.; Zheng, J.; Wang, Y.; Han, W.; Liu, Y. Cumulative effects of drought have an impact on net primary productivity stability in Central Asian grasslands. J. Environ. Manag. 2023, 344, 118734. [Google Scholar] [CrossRef]
- Gu, X.; Guo, E.; Yin, S.; Wang, Y.; Mandula, N.; Wan, Z.; Yun, X.; Li, H.; Bao, Y. Differentiating cumulative and lagged effects of drought on vegetation growth over the Mongolian Plateau. Ecosphere 2022, 13, e4289. [Google Scholar] [CrossRef]
- Zhao, A.; Yu, Q.; Feng, L.; Zhang, A.; Pei, T. Evaluating the cumulative and time-lag effects of drought on grassland vegetation: A case study in the Chinese Loess Plateau. J. Environ. Manag. 2020, 261, 110214. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, L.; Lin, A.; Wang, L.; Li, Q.; She, D.; Qu, S. Impacts of preseason drought on vegetation spring phenology across the Northeast China Transect. Sci. Total Environ. 2020, 738, 140297. [Google Scholar] [CrossRef]
- Hu, H.; Xue, P.; Huang, S.; Wang, Z.; Xiong, N.; Shi, L.; Liang, B.; Wang, J. The impact of drought on forest spring phenology in northern China. Ecol. Indic. 2025, 170, 113022. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Li, X.; Ciais, P.; Babst, F.; Guo, W.; Zhang, C.; Magliulo, V.; Pavelka, M.; Liu, S.; et al. Differentiating drought legacy effects on vegetation growth over the temperate Northern Hemisphere. Glob. Change Biol. 2018, 24, 504–516. [Google Scholar] [CrossRef]
- Shen, M.; Piao, S.; Cong, N.; Zhang, G.; Jassens, I.A. Precipitation impacts on vegetation spring phenology on the Tibetan Plateau. Glob. Change Biol. 2015, 21, 3647–3656. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, J.; Li, X.; Cheng, G.; Ma, M.; Zhu, G.; Altaf Arain, M.; Andrew Black, T.; Jassal, R.S. No trends in spring and autumn phenology during the global warming hiatus. Nat. Commun. 2019, 10, 2389. [Google Scholar] [CrossRef]
- Cong, N.; Wang, T.; Nan, H.; Ma, Y.; Wang, X.; Myneni, R.B.; Piao, S. Changes in satellite-derived spring vegetation green-up date and its linkage to climate in China from 1982 to 2010: A multimethod analysis. Glob. Change Biol. 2013, 19, 881–891. [Google Scholar] [CrossRef]
- Finger Higgens, R.A.; Hoover, D.L.; Knight, A.C.; Schlaepfer, D.R.; Duniway, M.C. Flexible Phenology of a C4 Grass Linked to Resiliency to Seasonal and Multiyear Drought Events in the American Southwest. Ecol. Evol. 2025, 15, e71435. [Google Scholar] [CrossRef]
- Franks, S.J. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol. 2011, 190, 249–257. [Google Scholar] [CrossRef]
- Jentsch, A.; Kreyling, J.; Boettcher-Treschkow, J.; Beierkuhnlein, C. Beyond gradual warming: Extreme weather events alter flower phenology of European grassland and heath species. Glob. Change Biol. 2009, 15, 837–849. [Google Scholar] [CrossRef]
- Post, A.K.; Knapp, A.K. The importance of extreme rainfall events and their timing in a semi-arid grassland. J. Ecol. 2020, 108, 2431–2443. [Google Scholar] [CrossRef]
- Fay, P.A.; Kaufman, D.M.; Nippert, J.B.; Carlisle, J.D.; Harper, C.W. Changes in grassland ecosystem function due to extreme rainfall events: Implications for responses to climate change. Glob. Change Biol. 2008, 14, 1600–1608. [Google Scholar] [CrossRef]
- Yan, P.; He, N.; Fernández-Martínez, M.; Yang, X.; Zuo, Y.; Zhang, H.; Wang, J.; Chen, S.; Song, J.; Li, G.; et al. Plant Acquisitive Strategies Promote Resistance and Temporal Stability of Semiarid Grasslands. Ecol. Lett. 2025, 28, e70110. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Wang, X.; Chen, X.; Duan, G.; Han, P.; Lin, Z.; Shi, H.; Wen, Z. Evaluating the effect of Multi-Scale droughts on autumn phenology of global land biomes with satellite observation. J. Hydrol. 2024, 639, 131547. [Google Scholar] [CrossRef]
- Wang, J.; Ouyang, W.; Liu, X.; Wang, L. Monitoring hydrological changes with satellite data: Spring thaw’s effect on soil moisture and groundwater in seasonal Freezing-Thawing zones. J. Hydrol. 2023, 626, 130365. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, W.; Li, Y.; Huang, C.; Zhang, X.; Peñuelas, J.; Zhang, Y.; Gentine, P.; Li, Z.; Wang, X.; et al. Increasing meteorological drought under climate change reduces terrestrial ecosystem productivity and carbon storage. One Earth 2023, 6, 1326–1339. [Google Scholar] [CrossRef]
- Kang, W.; Wang, T.; Liu, S. The Response of Vegetation Phenology and Productivity to Drought in Semi-Arid Regions of Northern China. Remote Sens. 2018, 10, 727. [Google Scholar] [CrossRef]
- Anjum, S.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirb. Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Lukasová, V.; Vido, J.; Škvareninová, J.; Bičárová, S.; Hlavatá, H.; Borsányi, P.; Škvarenina, J. Autumn Phenological Response of European Beech to Summer Drought and Heat. Water 2020, 12, 2610. [Google Scholar] [CrossRef]
- Yang, J.; Spicer, R.A.; Spicer, T.E.V.; Arens, N.C.; Jacques, F.M.B.; Su, T.; Kennedy, E.M.; Herman, A.B.; Steart, D.C.; Srivastava, G.; et al. Leaf form–climate relationships on the global stage: An ensemble of characters. Glob. Ecol. Biogeogr. 2015, 24, 1113–1125. [Google Scholar] [CrossRef]
- Lauder, J.D.; Moran, E.V.; Hart, S.C. Fight or flight? Potential tradeoffs between drought defense and reproduction in conifers. Tree Physiol. 2019, 39, 1071–1085. [Google Scholar] [CrossRef]
- Puglielli, G.; Laanisto, L.; Poorter, H.; Niinemets, Ü. Global patterns of biomass allocation in woody species with different tolerances of shade and drought: Evidence for multiple strategies. New Phytol. 2021, 229, 308–322. [Google Scholar] [CrossRef]
- Flo, V.; Martínez-Vilalta, J.; Mencuccini, M.; Granda, V.; Anderegg, W.R.L.; Poyatos, R. Climate and functional traits jointly mediate tree water-use strategies. New Phytol. 2021, 231, 617–630. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Medlyn, B.E.; Duursma, R.A.; Prentice, I.C.; Wang, H.; Baig, S.; Eamus, D.; de Dios, V.R.; Mitchell, P.; Ellsworth, D.S.; et al. Optimal stomatal behaviour around the world. Nat. Clim. Change 2015, 5, 459–464. [Google Scholar] [CrossRef]
- Song, Y.; Sterck, F.; Zhou, X.; Liu, Q.; Kruijt, B.; Poorter, L. Drought resilience of conifer species is driven by leaf lifespan but not by hydraulic traits. New Phytol. 2022, 235, 978–992. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef]
- Körner, C.; Basler, D. Phenology Under Global Warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef]
- Asad, M.A.U.; Zakari, S.A.; Zhao, Q.; Zhou, L.; Ye, Y.; Cheng, F. Abiotic Stresses Intervene with ABA Signaling to Induce Destructive Metabolic Pathways Leading to Death: Premature Leaf Senescence in Plants. Int. J. Mol. Sci. 2019, 20, 256. [Google Scholar] [CrossRef]
- Abhilasha, A.; Roy Choudhury, S. Molecular and Physiological Perspectives of Abscisic Acid Mediated Drought Adjustment Strategies. Plants 2021, 10, 2769. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Huang, J.; Fu, L.; Han, D.; Guan, X.; Zhang, B. Divergent sensitivity of vegetation to aridity between drylands and humid regions. Sci. Total Environ. 2023, 884, 163910. [Google Scholar] [CrossRef]
- Xu, H.-J.; Wang, X.-P.; Zhao, C.-Y. Drought sensitivity of vegetation photosynthesis along the aridity gradient in northern China. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102418. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Vilagrosa, A.; Alonso-Forn, D.; Ferrio, J.P.; Sancho-Knapik, D.; Gil-Pelegrín, E. Living in Drylands: Functional Adaptations of Trees and Shrubs to Cope with High Temperatures and Water Scarcity. Forests 2020, 11, 1028. [Google Scholar] [CrossRef]
- Chaves, M.; Maroco, J.; Pereira, J. Understanding plant responses to drough—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Sun, S.; Du, W.; Song, Z.; Zhang, D.; Wu, X.; Chen, B.; Wu, Y. Response of Gross Primary Productivity to Drought Time-Scales Across China. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005953. [Google Scholar] [CrossRef]
- Barnes, M.L.; Moran, M.S.; Scott, R.L.; Kolb, T.E.; Ponce-Campos, G.E.; Moore, D.J.P.; Ross, M.A.; Mitra, B.; Dore, S. Vegetation productivity responds to sub-annual climate conditions across semiarid biomes. Ecosphere 2016, 7, e01339. [Google Scholar] [CrossRef]
- Lubbe, F.; Bitomsky, M.; Hájek, T.; Bello, F.; Dolezal, J.; Jandová, V.; Janeček, S.; Bartušková, A.; Lanta, V.; Klimešová, J. A tale of two grasslands: How belowground storage organs coordinate their traits with water-use traits. Plant Soil 2021, 465, 533–548. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Regnier, P.; Yuan, W. New insights on plant phenological response to temperature revealed from long-term widespread observations in China. Glob. Change Biol. 2018, 24, 2066–2078. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).