Evaluating Remote Sensing Products for Pasture Composition and Yield Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Experimental Site and Flow Chart
2.2. Plant Material and Sample Design
2.3. Evaluation of Variables
2.3.1. Percentage of Grasses
2.3.2. Percentage of Legumes
2.4. Laboratory Analysis
2.4.1. Soil Organic Matter Analysis
2.4.2. Nutritional Quality
2.5. Spectral Data Collection
Indices Calculation
2.6. Capturing Aerial Images with UAVs for Field Testing
2.6.1. Planning Photogrammetric Flights
2.6.2. Flight Parameters
2.6.3. Flight Execution
2.6.4. Photogrammetric Process
2.6.5. Calibration of Images Obtained from the Parrot Camera
2.6.6. Calibration of Orthomosaics Generated by the Mapir Camera
2.6.7. Generation of Spectral Indices for Images Obtained by UAVs
2.6.8. Extracting Index Values Generated in SNAP
2.7. Statistical Analysis
3. Results
3.1. Botanical Composition
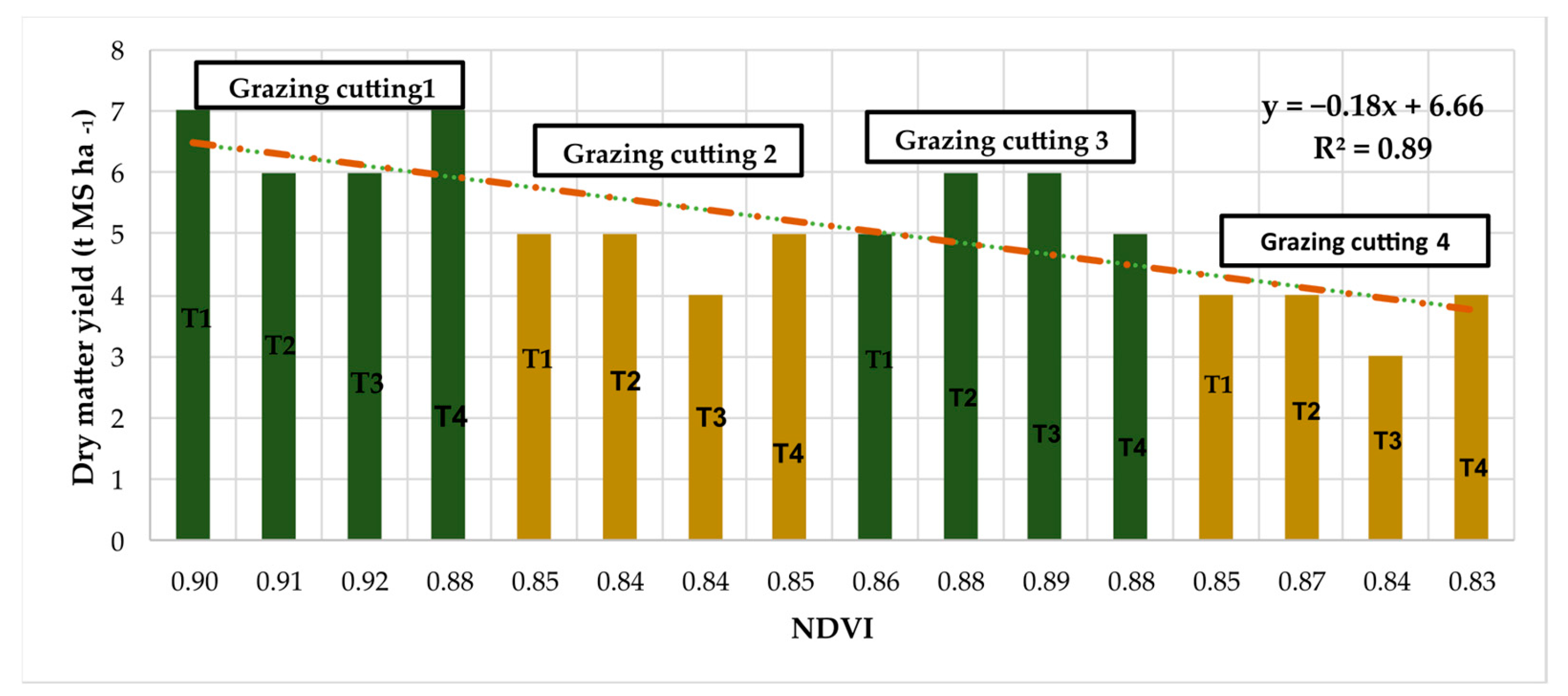
3.2. Performance
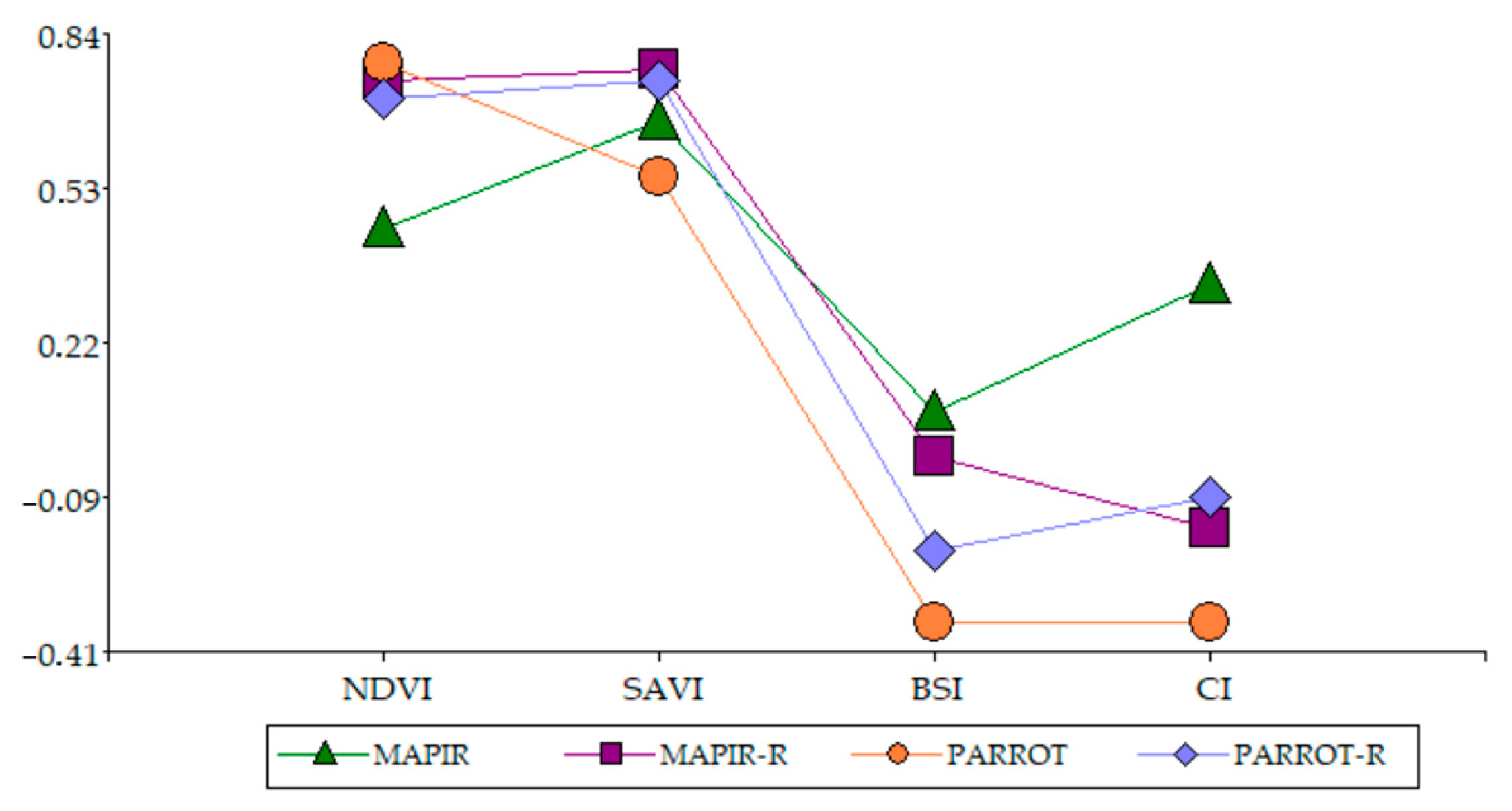
3.3. Spectral Indices
3.3.1. Vegetation Indices
- Normalized Difference Vegetation Index (NDVI)
- B.
- Soil-Adjusted Vegetation Index (SAVI)
3.3.2. Soil Indices
- Bare Soil Index (BSI)
- B.
- Color Index (CI)
3.4. Relationship Between Spectral Indices
3.5. Relationship Between Sensors
3.6. Sensor Validation and Yield and Botanical Composition Estimation
4. Discussion
4.1. Performance Comparison
4.2. Vegetation Index Behavior
4.3. Soil Index Behavior and Index Comparison
4.4. Yield and Botanical Composition Estimation with Indices
5. Conclusions
- (1)
- Yield estimation and species contribution
- (2)
- Spectral index behavior and sensor performance
- (3)
- Sensor comparison, correlation analysis and spectral limitations
- (4)
- Importance of NDVI for yield prediction
- (5)
- Practical implications
- (6)
- Limitations and future research
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sensor | Cutting | NDVI |
|---|---|---|
| Parrot | 1 | 0.90 ± 0.01 a |
| Parrot | 3 | 0.90 ± 0.01 a |
| Parrot | 5 | 0.88 ± 0.01 a |
| Parrot | 7 | 0.88 ± 0.01 a |
| Parrot-R | 3 | 0.88 ± 0.01 ab |
| Parrot-R | 1 | 0.87 ± 0.01 ab |
| Parrot-R | 7 | 0.86 ± 0.01 ab |
| Parrot-R | 5 | 0.85 ± 0.01 ab |
| Mapir-R | 1 | 0.85 ± 0.01 b |
| Mapir-R | 3 | 0.84 ± 0.01 b |
| Mapir-R | 5 | 0.80 ± 0.01 b |
| Mapir-R | 7 | 0.78 ± 0.01 b |
| Parrot | 2 | 0.72 ± 0.01 c |
| Parrot | 6 | 0.71 ± 0.01 c |
| Parrot | 4 | 0.70 ± 0.01 c |
| Parrot-R | 8 | 0.70 ± 0.01 c |
| Parrot-R | 2 | 0.69 ± 0.01 c |
| Parrot-R | 4 | 0.65 ± 0.01 c |
| Parrot-R | 6 | 0.65 ± 0.01 c |
| Parrot | 8 | 0.65 ± 0.01 c |
| Mapir-R | 2 | 0.64 ± 0.01 c |
| Mapir | 1 | 0.59 ± 0.01 d |
| Mapir | 3 | 0.57 ± 0.01 d |
| Mapir | 5 | 0.54 ± 0.01 d |
| Mapir | 7 | 0.48 ± 0.01 d |
| Mapir | 2 | 0.47 ± 0.01 d |
| Mapir | 4 | 0.47 ± 0.01 d |
| Mapir-R | 4 | 0.45 ± 0.01 d |
| Mapir-R | 6 | 0.43 ± 0.01 d |
| Mapir-R | 8 | 0.41 ± 0.01 d |
| Mapir | 8 | 0.32 ± 0.01 d |
| Mapir | 6 | 0.31 ± 0.01 d |
| Sensor | Cutting | SAVI |
|---|---|---|
| Mapir-R | 5 | 0.89 ± 0.02 a |
| Parrot-R | 5 | 0.87 ± 0.02 a |
| Mapir-R | 3 | 0.86 ± 0.02 a |
| Mapir-R | 7 | 0.86 ± 0.02 a |
| Parrot-R | 3 | 0.86 ± 0.02 a |
| Parrot-R | 7 | 0.84 ± 0.02 a |
| Mapir-R | 1 | 0.84 ± 0.02 a |
| Parrot-R | 1 | 0.80 ± 0.02 a |
| Mapir-R | 6 | 0.79± 0.02 b |
| Mapir-R | 4 | 0.79 ± 0.02 b |
| Parrot-R | 4 | 0.77 ± 0.02 b |
| Mapir-R | 2 | 0.76 ± 0.02 b |
| Parrot-R | 6 | 0.74 ± 0.02 b |
| Parrot-R | 2 | 0.70 ± 0.02 b |
| Mapir | 1 | 0.70 ± 0.02 c |
| Mapir | 7 | 0.68 ± 0.02 c |
| Parrot | 5 | 0.68 ± 0.02 c |
| Mapir | 5 | 0.67 ± 0.02 c |
| Mapir | 3 | 0.67 ± 0.02 c |
| Parrot | 3 | 0.64 ± 0.02 c |
| Mapir | 4 | 0.63 ± 0.02 c |
| Parrot-R | 8 | 0.63 ± 0.02 c |
| Mapir | 2 | 0.61 ± 0.02 d |
| Mapir-R | 8 | 0.60 ± 0.02 d |
| Parrot | 1 | 0.60 ± 0.02 d |
| Parrot | 7 | 0.58 ± 0.02 d |
| Parrot | 6 | 0.48 ± 0.02 e |
| Mapir | 8 | 0.47 ± 0.02 e |
| Mapir | 6 | 0.46 ± 0.02 e |
| Parrot | 2 | 0.46 ± 0.02 e |
| Parrot | 8 | 0.44 ± 0.02 e |
| Parrot | 4 | 0.40 ± 0.02 e |
| Sensor | Cutting | BSI |
|---|---|---|
| Mapir | 5 | 0.12 ± 0.01 a |
| Mapir | 8 | 0.10 ± 0.01 a |
| Mapir | 3 | 0.08 ± 0.01 a |
| Mapir | 6 | 0.07 ± 0.01 a |
| Mapir | 7 | 0.06 ± 0.01 a |
| Mapir | 4 | 0.06 ± 0.01 a |
| Mapir | 1 | 0.06 ± 0.01 a |
| Mapir | 2 | 0.06 ± 0.01 a |
| Mapir-R | 8 | 0.05 ± 0.01 ab |
| Mapir-R | 6 | 0.05 ± 0.01 ab |
| Mapir-R | 2 | −0.02 ± 0.01 b |
| Mapir-R | 5 | −0.02 ± 0.01 b |
| Mapir-R | 7 | −0.02 ± 0.01 b |
| Mapir-R | 3 | −0.02 ± 0.01 b |
| Mapir-R | 1 | −0.02 ± 0.01 b |
| Mapir-R | 4 | −0.02 ± 0.01 b |
| Parrot-R | 8 | −0.09 ± 0.01 c |
| Parrot-R | 2 | −0.12 ± 0.01 c |
| Parrot | 8 | −0.14 ± 0.01 c |
| Parrot-R | 6 | −0.16 ± 0.01 c |
| Parrot | 7 | −0.19 ± 0.01 c |
| Parrot-R | 4 | −0.20 ± 0.01 d |
| Parrot-R | 1 | −0.21 ± 0.01 d |
| Parrot | 4 | −0.22 ± 0.01 d |
| Parrot | 2 | −0.25 ± 0.01 d |
| Parrot | 6 | −0.25 ± 0.01 d |
| Parrot-R | 3 | −0.26 ± 0.01 d |
| Parrot-R | 7 | −0.27 ± 0.01 d |
| Sensor | Cutting | CI |
|---|---|---|
| Mapir | 5 | 0.44 ± 0.01 a |
| Mapir | 7 | 0.36 ± 0.01 a |
| Mapir | 6 | 0.35 ± 0.01 a |
| Mapir | 3 | 0.35 ± 0.01 a |
| Mapir | 8 | 0.34 ± 0.01 a |
| Mapir | 1 | 0.32 ± 0.01 a |
| Mapir | 4 | 0.28 ± 0.01 a |
| Mapir | 2 | 0.23 ± 0.01 a |
| Parrot-R | 6 | 0.04 ± 0.01 b |
| Parrot-R | 8 | 0.02 ± 0.01 b |
| Mapir-R | 8 | 0.02 ± 0.01 b |
| Mapir-R | 6 | −0.03 ± 0.01 c |
| Parrot-R | 2 | −0.03 ± 0.01 c |
| Mapir-R | 2 | −0.08 ± 0.01 c |
| Parrot-R | 4 | −0.13 ± 0.01 d |
| Parrot-R | 1 | −0.13 ± 0.01 d |
| Parrot-R | 7 | −0.17 ± 0.01 d |
| Parrot-R | 3 | −0.17 ± 0.01 de |
| Parrot-R | 5 | −0.18 ± 0.01 de |
| Parrot | 4 | −0.19 ± 0.01 e |
| Mapir-R | 1 | −0.19 ± 0.01 f |
| Parrot | 6 | −0.19 ± 0.01 f |
| Mapir-R | 4 | −0.19 ± 0.01 f |
| Mapir-R | 7 | −0.25 ± 0.01 g |
| Mapir-R | 3 | −0.26 ± 0.01 g |
| Mapir-R | 5 | −0.27 ± 0.01 g |
| Parrot | 8 | −0.28 ± 0.01 g |
| Parrot | 2 | −0.28 ± 0.01 g |
| Parrot | 1 | −0.43 ± 0.01 h |
| Parrot | 7 | −0.44 ± 0.01 h |
| Parrot | 3 | −0.46 ± 0.01 h |
| Parrot | 5 | −0.52 ± 0.01 h |
References
- Vandehaar, M.J. Efficiency of nutrient use and relationship to profitability on dairy farms. J. Dairy Sci. 1998, 81, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Rotz, C.A.; Satter, L.D.; Mertens, D.R.; Muck, R.E. Feeding strategy, nitrogen cycling, and profitability of dairy farms. J. Dairy Sci. 1999, 82, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Tey, Y.S.; Brindal, M. Factors influencing farm profitability. Sustain. Agric. Rev. 2015, 15, 235–255. [Google Scholar]
- Hanrahan, L.; McHugh, N.; Hennessy, T.; Moran, B.; Kearney, R.; Wallace, M.; Shalloo, L. Factors associated with profitability in pasture-based systems of milk production. J. Dairy Sci. 2018, 101, 5474–5485. [Google Scholar] [CrossRef]
- Balehegn, M.; Duncan, A.; Tolera, A.; Ayantunde, A.A.; Issa, S.; Karimou, M.; Adesogan, A.T. Improving adoption of technologies and interventions for increasing supply of quality livestock feed in low-and middle-income countries. Glob. Food Secur. 2020, 26, 100372. [Google Scholar] [CrossRef]
- Torres, B.; Cayambe, J.; Paz, S.; Ayerve, K.; Heredia-R, M.; Torres, E.; García, A. Livelihood capitals, income inequality, and the perception of climate change: A case study of small-scale cattle farmers in the Ecuadorian Andes. Sustainability 2022, 14, 5028. [Google Scholar] [CrossRef]
- Wachendorf, C.; Stuelpnagel, R.; Wachendorf, M. Influence of land use and tillage depth on dynamics of soil microbial properties, soil carbon fractions and crop yield after conversion of short-rotation coppices. Soil. Use Manag. 2017, 33, 379–388. [Google Scholar] [CrossRef]
- Wachendorf, M.; Fricke, T.; Möckel, T. Remote sensing as a tool to assess botanical composition, structure, quantity and quality of temperate grasslands. Grass Forage Sci. 2018, 73, 1–14. [Google Scholar] [CrossRef]
- Cope, J.S.; Corney, D.; Clark, J.Y.; Remagnino, P.; Wilkin, P. Plant species identification using digital morphometrics: A review. Expert Syst. Appl. 2012, 39, 7562–7573. [Google Scholar] [CrossRef]
- Heberling, J.M. Herbaria as big data sources of plant traits. Int. J. Plant Sci. 2022, 183, 87–118. [Google Scholar] [CrossRef]
- Monteiro, A.; Santos, S.; Gonçalves, P. Precision agriculture for crop and livestock farming—Brief review. Animals 2021, 11, 2345. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of remote sensing in precision agriculture: A review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Liaghat, S.; Balasundram, S.K. A review: The role of remote sensing in precision agriculture. Am. J. Agric. Biol. Sci. 2010, 5, 50–55. [Google Scholar] [CrossRef]
- Sabir, R.M.; Mehmood, K.; Sarwar, A.; Safdar, M.; Muhammad, N.E.; Gul, N.; Akram, H.M.B. Remote Sensing and Precision Agriculture: A Sustainable Future. In Transforming Agricultural Management for a Sustainable Future: Climate Change and Machine Learning Perspectives; Springer Nature: Cham, Switzerland, 2024; pp. 75–103. [Google Scholar]
- Seelan, S.K.; Laguette, S.; Casady, G.M.; Seielstad, G.A. Remote sensing applications for precision agriculture: A learning community approach. Remote Sens. Environ. 2003, 88, 157–169. [Google Scholar] [CrossRef]
- Aquilani, C.; Confessore, A.; Bozzi, R.; Sirtori, F.; Pugliese, C. Precision Livestock Farming technologies in pasture-based livestock systems. Animal 2022, 16, 100429. [Google Scholar] [CrossRef]
- Shanmugapriya, P.; Rathika, S.; Ramesh, T.; Janaki, P. Applications of remote sensing in agriculture—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2270–2283. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.L. Scale issues in remote sensing: A review on analysis, processing and modeling. Sensors 2009, 9, 1768–1793. [Google Scholar] [CrossRef]
- Chi, M.; Plaza, A.; Benediktsson, J.A.; Sun, Z.; Shen, J.; Zhu, Y. Big data for remote sensing: Challenges and opportunities. Proc. IEEE 2016, 104, 2207–2219. [Google Scholar] [CrossRef]
- Liu, P. A survey of remote-sensing big data. Front. Environ. Sci. 2015, 3, 45. [Google Scholar] [CrossRef]
- Ali, I.; Cawkwell, F.; Dwyer, E.; Barrett, B.; Green, S. Satellite remote sensing of grasslands: From observation to management. J. Plant Ecol. 2016, 9, 649–671. [Google Scholar] [CrossRef]
- Bégué, A.; Arvor, D.; Bellon, B.; Betbeder, J.; De Abelleyra, D.; PD Ferraz, R.; Verón, S.R. Remote sensing and cropping practices: A review. Remote Sens. 2018, 10, 99. [Google Scholar] [CrossRef]
- Reinermann, S.; Asam, S.; Kuenzer, C. Remote sensing of grassland production and management—A review. Remote Sens. 2020, 12, 1949. [Google Scholar] [CrossRef]
- Subhashree, S.N.; Igathinathane, C.; Akyuz, A.; Borhan, M.; Hendrickson, J.; Archer, D.; Halvorson, J. Tools for predicting forage growth in rangelands and economic analyses—A systematic review. Agriculture 2023, 13, 455. [Google Scholar] [CrossRef]
- Pinter, P.J., Jr.; Hatfield, J.L.; Schepers, J.S.; Barnes, E.M.; Moran, M.S.; Daughtry, C.S.; Upchurch, D.R. Remote sensing for crop management. Photogramm. Eng. Remote Sens. 2003, 69, 647–664. [Google Scholar] [CrossRef]
- Cevallos, L.N.M.; García, J.L.R.; Suárez, B.I.A.; González, C.A.L.; González, I.S.; Campoverde, J.A.Y.; Toulkeridis, T. A NDVI analysis contrasting different spectrum data methodologies applied in pasture crops previous grazing—A case study from Ecuador. In Proceedings of the 2018 International Conference on eDemocracy & eGovernment (ICEDEG), Ambato, Ecuador, 4–6 April 2018; IEEE: New York, NY, USA, 2018; pp. 126–135. [Google Scholar]
- Curatola Fernández, G.F.; Obermeier, W.A.; Gerique, A.; Lopez Sandoval, M.F.; Lehnert, L.W.; Thies, B.; Bendix, J. Land cover change in the Andes of Southern Ecuador—Patterns and drivers. Remote Sens. 2015, 7, 2509–2542. [Google Scholar] [CrossRef]
- García, V.J.; Márquez, C.O.; Rodríguez, M.V.; Orozco, J.J.; Aguilar, C.D.; Ríos, A.C. Páramo ecosystems in Ecuador’s southern region: Conservation state and restoration. Agronomy 2020, 10, 1922. [Google Scholar] [CrossRef]
- Sönmez, N.; Emekli, Y.; Sari, M.; Baştuğ, R. Relationship between spectral reflectance water stress conditions of Bermuda grass. N. Z. J. Agric. Res. 2010, 51, 223. [Google Scholar] [CrossRef]
- Jiang, Y.; Carrow, R.N. Broadband spectral reflectance models of turfgrass species and cultivars to drought stress. Crop Sci. 2007, 47, 1611–1618. [Google Scholar] [CrossRef]
- Radoglou-Grammatikis, P.; Sarigiannidis, P.; Lagkas, T.; Moscholios, I. A compilation of UAV applications for precision agriculture. Comput. Netw. 2020, 172, 107148. [Google Scholar] [CrossRef]
- Tsouros, D.C.; Bibi, S.; Sarigiannidis, P.G. A review on UAV-based applications for precision agriculture. Information 2019, 10, 349. [Google Scholar] [CrossRef]
- Velusamy, P.; Rajendran, S.; Mahendran, R.K.; Naseer, S.; Shafiq, M.; Choi, J.G. Unmanned Aerial Vehicles (UAV) in precision agriculture: Applications and challenges. Energies 2021, 15, 217. [Google Scholar] [CrossRef]
- Primicerio, J.; Di Gennaro, S.F.; Fiorillo, E.; Genesio, L.; Lugato, E.; Matese, A.; Vaccari, F.P. A flexible unmanned aerial vehicle for precision agriculture. Precis. Agric. 2012, 13, 517–523. [Google Scholar] [CrossRef]
- Messina, G.; Modica, G. Applications of UAV thermal imagery in precision agriculture: State of the art and future research outlook. Remote Sens. 2020, 12, 1491. [Google Scholar] [CrossRef]
- Srivastava, K.; Bhutoria, A.J.; Sharma, J.K.; Sinha, A.; Pandey, P.C. UAVs technology for the development of GUI based application for precision agriculture and environmental research. Remote Sens. Appl. Soc. Environ. 2019, 16, 100258. [Google Scholar] [CrossRef]
- Sani, J.; Tierra, A.; Toulkeridis, T.; Padilla, O. Evaluation of Horizontal and Vertical Positions Obtained from an Unmanned Aircraft Vehicle Applied to Large Scale Cartography of Infrastructure Loss Due to the Earthquake of April 2016 in Ecuador. In International Conference on Applied Technologies; Springer Nature: Cham, Switzerland, 2022; pp. 60–73. [Google Scholar]
- Shakhatreh, H.; Sawalmeh, A.H.; Al-Fuqaha, A.; Dou, Z.; Almaita, E.; Khalil, I.; Guizani, M. Unmanned aerial vehicles (UAVs): A survey on civil applications and key research challenges. IEEE Access 2019, 7, 48572–48634. [Google Scholar] [CrossRef]
- Mohsan, S.A.H.; Khan, M.A.; Noor, F.; Ullah, I.; Alsharif, M.H. Towards the unmanned aerial vehicles (UAVs): A comprehensive review. Drones 2022, 6, 147. [Google Scholar] [CrossRef]
- Pajares, G. Overview and current status of remote sensing applications based on unmanned aerial vehicles (UAVs). Photogramm. Eng. Remote Sens. 2015, 81, 281–330. [Google Scholar] [CrossRef]
- Asadzadeh, S.; de Oliveira, W.J.; de Souza Filho, C.R. UAV-based remote sensing for the petroleum industry and environmental monitoring: State-of-the-art and perspectives. J. Pet. Sci. Eng. 2022, 208, 109633. [Google Scholar] [CrossRef]
- Yao, H.; Qin, R.; Chen, X. Unmanned aerial vehicle for remote sensing applications—A review. Remote Sens. 2019, 11, 1443. [Google Scholar] [CrossRef]
- O’Connor, J.; Smith, M.J.; James, M.R. Cameras and settings for aerial surveys in the geosciences: Optimising image data. Prog. Progress. Phys. Geogr. 2017, 41, 325–344. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, X.; Dedman, S.; Rosso, M.; Zhu, J.; Yang, J.; Wang, J. UAV remote sensing applications in marine monitoring: Knowledge visualization and review. Sci. Total Environ. 2022, 838, 155939. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Glenn, E.P.; Huete, A.R.; Nagler, P.L.; Nelson, S.G. Relationship between remotely-sensed vegetation indices, canopy attributes and plant physiological processes: What vegetation indices can and cannot tell us about the landscape. Sensors 2008, 8, 2136–2160. [Google Scholar] [CrossRef] [PubMed]
- Huete, A.R. Vegetation indices, remote sensing and forest monitoring. Geogr. Compass 2012, 6, 513–532. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rosell, J.R.; Sanz, R. A review of methods and applications of the geometric characterization of tree crops in agricultural activities. Comput. Electron. Agric. 2012, 81, 124–141. [Google Scholar] [CrossRef]
- Lee, W.S.; Alchanatis, V.; Yang, C.; Hirafuji, M.; Moshou, D.; Li, C. Sensing technologies for precision specialty crop production. Comput. Electron. Agric. 2010, 74, 2–33. [Google Scholar] [CrossRef]
- Chlingaryan, A.; Sukkarieh, S.; Whelan, B. Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: A review. Comput. Electron. Agric. 2018, 151, 61–69. [Google Scholar] [CrossRef]
- Yengoh, G.T.; Dent, D.; Olsson, L.; Tengberg, A.E.; Tucker, C.J., III. Use of the Normalized Difference Vegetation Index (NDVI) to Assess Land Degradation at Multiple Scales: Current Status, Future Trends, and Practical Considerations; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Adar, S.; Sternberg, M.; Argaman, E.; Henkin, Z.; Dovrat, G.; Zaady, E.; Paz-Kagan, T. Testing a novel pasture quality index using remote sensing tools in semiarid and Mediterranean grasslands. Agric. Ecosyst. Environ. 2023, 357, 108674. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Näsi, R.; Korhonen, P.; Mustonen, A.; Niemeläinen, O.; Koivumäki, N.; Honkavaara, E. High-precision estimation of grass quality and quantity using UAS-based VNIR and SWIR hyperspectral cameras and machine learning. Precis. Agric. 2024, 25, 186–220. [Google Scholar] [CrossRef]
- Nicola, F.; Miguel, R.C.J.; Intrigliolo, D.S.; Giuseppe, T.; Sabina, F. Remote Sensing for Pasture Biomass Quantity and Quality Assessment: Challenges and Future Prospects. Smart Agric. Technol. 2025, 12, 101057. [Google Scholar] [CrossRef]
- Shahi, T.B.; Balasubramaniam, T.; Sabir, K.; Nayak, R. Pasture monitoring using remote sensing and machine learning: A review of methods and applications. Remote Sens. Appl. Soc. Environ. 2025, 37, 101459. [Google Scholar] [CrossRef]
- Shorten, P.R.; Trolove, M.R. UAV-based prediction of ryegrass dry matter yield. Int. J. Remote Sens. 2022, 43, 2393–2409. [Google Scholar] [CrossRef]
- De Rosa, D.; Basso, B.; Fasiolo, M.; Friedl, J.; Fulkerson, B.; Grace, P.R.; Rowlings, D.W. Predicting pasture biomass using a statistical model and machine learning algorithm implemented with remotely sensed imagery. Comput. Electron. Agric. 2021, 180, 105880. [Google Scholar] [CrossRef]
- Freitas, R.G.; Pereira, F.R.; Dos Reis, A.A.; Magalhães, P.S.; Figueiredo, G.K.; do Amaral, L.R. Estimating pasture aboveground biomass under an integrated crop-livestock system based on spectral and texture measures derived from UAV images. Comput. Electron. Agric. 2022, 198, 107122. [Google Scholar] [CrossRef]
- Pranga, J.; Borra-Serrano, I.; Aper, J.; De Swaef, T.; Ghesquiere, A.; Quataert, P.; Lootens, P. Improving accuracy of herbage yield predictions in perennial ryegrass with UAV-based structural and spectral data fusion and machine learning. Remote Sens. 2021, 13, 3459. [Google Scholar] [CrossRef]
- Sinde-González, I.; Gil-Docampo, M.; Arza-García, M.; Grefa-Sánchez, J.; Yánez-Simba, D.; Pérez-Guerrero, P.; Abril-Porras, V. Biomass estimation of pasture plots with multitemporal UAV-based photogrammetric surveys. Int. J. Appl. Earth Obs. Geoinf. 2021, 101, 102355. [Google Scholar] [CrossRef]
- Fernandez-Figueroa, E.G.; Wilson, A.E.; Rogers, S.R. Commercially available unoccupied aerial systems for monitoring harmful algal blooms: A comparative study. Limnol. Oceanogr. Methods 2022, 20, 146–158. [Google Scholar] [CrossRef]
- Hall, M.L.; Samaniego, P.; Le Pennec, J.L.; Johnson, J.B. Ecuadorian Andes volcanism: A review of Late Pliocene to present activity. J. Volcanol. Geotherm. Res. 2008, 176, 1–6. [Google Scholar] [CrossRef]
- Toulkeridis, T.; Zach, I. Wind directions of volcanic ash-charged clouds in Ecuador–implications for the public and flight safety. Geomat. Nat. Hazards Risk 2017, 8, 242–256. [Google Scholar] [CrossRef]
- INAMHI Instituto Nacional de Meteorología e Hidrología. Estadística Climatológica. Datos Meteorológicos Correspondientes al Periodo Enero–Diciembre 2019; Estación Izobamba: Mejía, Ecuador, 2019. [Google Scholar]
- Burdon, J.J. Trifolium repens L. J. Ecol. 1983, 71, 307–330. [Google Scholar] [CrossRef]
- Turkington, R.; Harper, J.L. The growth, distribution and neighbour relationships of Trifolium repens in a permanent pasture: IV. Fine-scale biotic differentiation. J. Ecol. 1979, 67, 245–254. [Google Scholar] [CrossRef]
- Vásquez, H.V.; Valqui, L.; Bobadilla, L.G.; Meseth, E.; Trigoso, M.J.; Zagaceta, L.H.; Maicelo, J.L. Agronomic and Nutritional Evaluation of INIA 910—Kumymarca Ryegrass (Lolium multiflorum Lam.): An Alternative for Sustainable Forage Production in Department of Amazonas (NW Peru). Agronomy 2025, 15, 100. [Google Scholar] [CrossRef]
- Lee, J.M.; Matthew, C.; Thom, E.R.; Chapman, D.F. Perennial ryegrass breeding in New Zealand: A dairy industry perspective. Crop Pasture Sci. 2012, 63, 107–127. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Carelli, M. Origin of Ladino white clover as inferred from patterns of molecular and morphophysiological diversity. Crop Sci. 2014, 54, 2696–2706. [Google Scholar] [CrossRef]
- Nicolau, S.T.; Matzger, A.J. An evaluation of resolution, accuracy, and precision in FT-IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 319, 124545. [Google Scholar] [CrossRef]
- Akin, D.E.; Burdick, D. Percentage of Tissue Types in Tropical and Temperate Grass Leaf Blades and Degradation of Tissues by Rumen Microorganisms 1. Crop Sci. 1975, 15, 661–668. [Google Scholar] [CrossRef]
- León, R.; Bonifaz, N.; Gutiérrez, F. Pastos Y Forrajes del Ecuador: Siembra Y Producción De pasturas, 3rd ed.; Editorial Universitaria Abya-Yala: Quito, Ecuador, 2018. [Google Scholar]
- Muñoz, E.C.; Andriamandroso, A.L.H.; Beckers, Y.; Ron, L.; Montufar, C.; da Silva Neto, G.F.; Bindelle, J. Analysis of the nutritional and productive behaviour of dairy cows under three rotation bands of pastures, Pichincha, Ecuador. J. Agric. Rural Dev. Trop. Subtrop. 2021, 122, 289–298. [Google Scholar]
- Velasco, L.; Condolo, L.; Vaca, M.; Santos, C. Nutritional Contribution of Cultivated Pastures in the Eastern Part of Ecuador. J. Surv. Fish. Sci. 2023, 1, 214–221. [Google Scholar]
- Sanderson, M.A.; Soder, K.J.; Muller, L.D.; Klement, K.D.; Skinner, R.H.; Goslee, S.C. Forage mixture productivity and botanical composition in pastures grazed by dairy cattle. Agron. J. 2005, 97, 1465–1471. [Google Scholar] [CrossRef]
- Trotter, M. PA Innovations in livestock, grazing systems and rangeland management to improve landscape productivity and sustainability. Agric. Sci. 2013, 25, 27–31. [Google Scholar]
- INIAP. Laboratorio de Suelos Y Calidad Nutricional: Toma de Muestras Para Análisis de Suelos, Aguas Y Plantas; Instituto Nacional Autónomo de Investigaciones Agropecuarias (INIAP): Quito, Ecuador, 2018; p. 2. [Google Scholar]
- Franzini, M.; Ronchetti, G.; Sona, G.; Casella, V. Geometric and radiometric consistency of parrot sequoia multispectral imagery for precision agriculture applications. Appl. Sci. 2019, 9, 5314. [Google Scholar] [CrossRef]
- Moriarty, C.; Cowley, D.C.; Wade, T.; Nichol, C.J. Deploying multispectral remote sensing for multi-temporal analysis of archaeological crop stress at Ravenshall, Fife, Scotland. Archaeological Prospection 2019, 26, 33–46. [Google Scholar] [CrossRef]
- MAPIR. Survey3W Camera—Red+Green+NIR (RGN, NDVI). MAPIR CAMERA. 2021. Available online: https://www.mapir.camera/products/survey3w-camera-red-green-nir-rgn-ndvi (accessed on 17 July 2025).
- Reeves, M.; Fulkerson, W.J.; Kellaway, R.C. Forage quality of kikuyu (Pennisetum clandestinum): The effect of time of defoliation and nitrogen fertiliser application and in comparison with perennial ryegrass (Lolium perenne). Aust. J. Agric. Res. 1996, 47, 1349–1359. [Google Scholar] [CrossRef]
- Pauly, K. Applying conventional vegetation vigor indices to UAS-derived orthomosaics: Issues and considerations. In Proceedings of the 12th International Conference for Precision Agriculture, Sacramento, CA, USA, 20–23 July 2014; pp. 20–23. [Google Scholar]
- Escadafal, R. Remote sensing of arid soil surface color with Landsat thematic mapper. Adv. Space Res. 1989, 9, 159–163. [Google Scholar] [CrossRef]
- Arciniegas-Ortega, S.; Molina, I.; Garcia-Aranda, C. Soil order-land use index using field-satellite spectroradiometry in the Ecuadorian Andean Territory for modeling soil quality. Sustainability 2022, 14, 7426. [Google Scholar] [CrossRef]
- Castro, R.; Hernández, A.; Vaquera, H.; Hernández, J.; Quero, A.; Enríquez, J.; Martínez, P. Comportamiento productivo de asociaciones de gramíneas con leguminosas en pastoreo. Rev. Fitotec. Mex. 2012, 35, 87. [Google Scholar] [CrossRef]
- Villalobos, L.; Sánchez, J. Evaluación agronómica y nutricional del pasto Rye grass Perenne Tetraploide (Lolium perenne) producido en lecherías de las zonas altas de Costa Rica. II. Valor nutricional. Agron. Costarric. 2016, 34, 43–52. [Google Scholar]
- Dronova, I.; Taddeo, S. Remote sensing of phenology: Towards the comprehensive indicators of plant community dynamics from species to regional scales. J. Ecol. 2022, 110, 1460–1484. [Google Scholar] [CrossRef]
- Hamada, Y.; Stow, D.A.; Roberts, D.A. Estimating life-form cover fractions in California sage scrub communities using multispectral remote sensing. Remote Sens. Environ. 2011, 115, 3056–3068. [Google Scholar] [CrossRef]
- Ruiz, D.A.C.; Villacís, M.G.M.; Kirby, E.; Guzmán, J.A.M.; Toulkeridis, T. Correlation of NDVI obtained by different methodologies of spectral data collection in a commercial crop of Quinoa (Chenopodium Quinoa) in central Ecuador. In Proceedings of the 2020 Seventh International Conference on eDemocracy & eGovernment (ICEDEG), Buenos Aires, Argentina, 22–24 April 2020; IEEE: New York, NY, USA, 2020; pp. 208–215. [Google Scholar]
- Villacís, M.G.M.; Ruiz, D.A.C.; Powney, E.P.K.; Guzmán, J.A.M.; Toulkeridis, T. Index relationship of vegetation with the development of a Quinoa Crop (Chenopodium quinoa) in its first phenological stages in central Ecuador based on GIS techniques. In Proceedings of the 2020 Seventh International Conference on eDemocracy & eGovernment (ICEDEG), Buenos Aires, Argentina, 22–24 April 2020; IEEE: New York, NY, USA, 2020; pp. 191–200. [Google Scholar]
- Marais, J.P. Factors affecting the nutritive value of kikuyu grass (Pennisetum clandestinum)—A review. Trop. Grassl. 2001, 35, 65–84. [Google Scholar]
- Kumar, P.; Pandey, P.; Singh, B.; Katiyar, S.; Mandal, V.; Rani, M.; Tomar, V.; Patairiya, S. Estimation of accumulated soil organic carbon stock in tropical forest using geospatial strategy. Egypt. J. Remote Sens. Space Sci. 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Sinde, I.; Yánez, D.; Grefa, J.; Arza, M.; Gil, M. Estimación del rendimiento del pasto mediante NDVI con imágenes multiespectrales de vehículos aéreos no tripulados (UAV). Rev. Geoespacial 2020, 17, 14. [Google Scholar]
- Aper, J.; Borra-Serrano, I.; Ghesquiere, A.; De Swaef, T.; Roldán-Ruiz, I.; Lootens, P.; Baert, J. Yield estimation of perennial ryegrass plots in breeding trials using UAV images. In Improving Sown Grasslands Through Breeding and Management; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 312–314. [Google Scholar]














| Sources of Variation | Degrees of Freedom |
|---|---|
| Total | 11 |
| Blocks | 2 |
| Treatment | 3 |
| Experimental error | 6 |
| Bands | Center Band (nm) | Bandwidth (nm) | Min | Max |
|---|---|---|---|---|
| Parrot Sequoia | ||||
| Green | 550 | 40 | 510 | 590 |
| Red | 660 | 40 | 620 | 700 |
| Red edge | 735 | 10 | 725 | 745 |
| Near infrared | 790 | 40 | 750 | 830 |
| Survey 3W-Red+Green+NIR | ||||
| Green | 550 | 30 | 520 | 580 |
| Red | 660 | 30 | 630 | 690 |
| Near infrared | 850 | 50 | 800 | 900 |
| Sampling | Grazing | Season | DAS |
|---|---|---|---|
| First | Pre-grazing | Rainy | 84 |
| Second | Post-grazing | Rainy | 89 |
| Third | Pre-grazing | Rainy | 112 |
| Fourth | Post-grazing | Rainy | 120 |
| Fifth | Pre-grazing | Dry | 216 |
| Sixth | Post-grazing | Dry | 223 |
| Seventh | Pre-grazing | Dry | 254 |
| Eighth | Post-grazing | Dry | 264 |
| Flight Parameters | Unit | |
|---|---|---|
| Phantom 4 with Parrot Sequoia multispectral camera (G+R+RE+NIR) | Flight height | 30 m |
| Total terrain area | 20 m × 20 m | |
| Vertical overlap | 75% | |
| Horizontal overlap | 75% | |
| Flight lines | 5 | |
| Number of photographs | 656 | |
| Mavic Pro with a Survey 3W-Red+Green+NIR camera | Flight height | 30 m |
| Total terrain area | 20 m × 20 m | |
| Vertical overlap | 75% | |
| Horizontal overlap | 75% | |
| Flight lines | 8 | |
| Number of photographs | 271 |
| Band | Reflectance Factor |
|---|---|
| Green | 0.73 |
| Red | 0.73 |
| Red edge | 0.68 |
| NIR | 0.71 |
| Associations (Perennial Ryegrass/White Clover; %) | |||||||
|---|---|---|---|---|---|---|---|
| Cut | 100:0 | 90:10 | 80:20 | 70:30 | StDv | Avg | Sig. |
| 1 | 7.00 Aa | 6.00 Aa | 6.23 Ba | 6.63 Aa | 0.52 | 6.47 a | * |
| 2 | 5.47 Ab | 4.97 ABc | 6.03 Bb | 5.50 ABb | 0.54 | 5.49 b | * |
| 3 | 4.53 Ac | 5.13 Bb | 4.23 Ac | 4.57 ABc | 0.47 | 4.62 c | * |
| 4 | 3.93 Ac | 4.37 ABc | 3.83 Bc | 3.43 ABc | 0.26 | 3.89 c | * |
| Avg. | 5.23 A | 5.12 AB | 5.08 AB | 5.03 AB | |||
| StDv | 0.29 | 0.22 | 0.19 | 0.21 | |||
| Sig. | NS | NS | NS | NS | |||
| Tot.yield | 20.93 | 20.47 | 20.32 | 20.13 | |||
| Associations (Perennial Ryegrass/White Clover; %) | |||||||
|---|---|---|---|---|---|---|---|
| Cut | 100:0 | 90:10 | 80:20 | 70:30 | StDv | Avg | Sig. |
| Rye grass perenne (t MS ha−1) | |||||||
| 1 | 7.00 Aa | 5.53 Aa | 5.23 Ba | 5.03 Ca | 0.47 | 5.70 a | * |
| 2 | 5.47 Ab | 4.57 Bb | 5.10 Bb | 4.23 Cb | 0.44 | 4.84 b | * |
| 3 | 4.53 Ab | 4.80 Ab | 3.63 Bc | 3.57 Cb | 0.47 | 4.13 bc | * |
| 4 | 3.93 Ac | 4.00 Bc | 3.27 BC | 2.60 Cc | 0.23 | 3.45 c | * |
| Avg. | 5.23 A | 4.73 B | 4.31 B | 3.86 C | |||
| StDv | 0.20 | 0.19 | 0.17 | 0.19 | |||
| Sig. | * | * | * | * | |||
| Tot.yield | 20.93 | 18.9 | 17.23 | 15.43 | |||
| White clover (t MS ha−1) | |||||||
| 1 | - | 0.43 Aa | 1.00 Ba | 1.63 Ca | 0.06 | 0.77 a | * |
| 2 | - | 0.40 Aa | 0.93 Ba | 1.23 Ca | 0.05 | 0.64 a | * |
| 3 | - | 0.37 Ab | 0.63 Ab | 1.03 Bb | 0.07 | 0.51 b | * |
| 4 | - | 0.30 Ab | 0.57 Bb | 0.80 Cbc | 0.04 | 0.42 bc | * |
| Avg. | - | 0.38 A | 0.78 B | 1.17 C | |||
| StDv | 0.05 | 0.05 | 0.04 | ||||
| Sig. | * | * | * | ||||
| Tot.yield | - | 1.50 | 3.13 | 4.69 | |||
| Sensor | n | Mean | Minimum | Maximum | Average |
|---|---|---|---|---|---|
| Mapir | 32 | 0.45 | 0.30 | 0.59 | 0.47 |
| Mapir-R | 32 | 0.74 | 0.43 | 0.91 | 0.78 |
| Parrot | 32 | 0.78 | 0.64 | 0.92 | 0.79 |
| Parrot-R | 32 | 0.71 | 0.39 | 0.89 | 0.72 |
| Sensor | n | Mean | Minimum | Maximum | Average |
|---|---|---|---|---|---|
| Mapir | 32 | 0.66 | 0.44 | 0.88 | 0.68 |
| Mapir-R | 32 | 1.09 | 0.58 | 1.36 | 1.13 |
| Parrot | 32 | 0.55 | 0.37 | 0.77 | 0.54 |
| Parrot-R | 32 | 1.05 | 0.63 | 1.33 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albacura-Campues, K.M.; Sinde-González, I.; Maiguashca, J.; Herrera, M.; Zapata, J.; Toulkeridis, T. Evaluating Remote Sensing Products for Pasture Composition and Yield Prediction. Remote Sens. 2025, 17, 2561. https://doi.org/10.3390/rs17152561
Albacura-Campues KM, Sinde-González I, Maiguashca J, Herrera M, Zapata J, Toulkeridis T. Evaluating Remote Sensing Products for Pasture Composition and Yield Prediction. Remote Sensing. 2025; 17(15):2561. https://doi.org/10.3390/rs17152561
Chicago/Turabian StyleAlbacura-Campues, Karen Melissa, Izar Sinde-González, Javier Maiguashca, Myrian Herrera, Judith Zapata, and Theofilos Toulkeridis. 2025. "Evaluating Remote Sensing Products for Pasture Composition and Yield Prediction" Remote Sensing 17, no. 15: 2561. https://doi.org/10.3390/rs17152561
APA StyleAlbacura-Campues, K. M., Sinde-González, I., Maiguashca, J., Herrera, M., Zapata, J., & Toulkeridis, T. (2025). Evaluating Remote Sensing Products for Pasture Composition and Yield Prediction. Remote Sensing, 17(15), 2561. https://doi.org/10.3390/rs17152561








