Abstract
Understanding the geographic distribution of mammal species is essential for informed conservation planning, maintaining local ecosystem stability, and addressing research gaps, particularly in data-deficient regions. This study investigated the distribution and richness of 20 mammal species within Nyerere National Park (NNP), a large and understudied protected area in Southern Tanzania. We applied species distribution models (SDMs) using presence data collected through ground surveys between 2022 and 2024, combined with environmental variables derived from remote sensing, including land surface temperature, vegetation indices, soil moisture, elevation, and proximity to water sources and human infrastructure. Models were constructed using the Maximum Entropy (MaxEnt) algorithm, and performance was evaluated using the Area Under the Curve (AUC) metric, yielding high accuracy ranging from 0.81 to 0.97. Temperature (32.3%) and vegetation indices (23.4%) emerged as the most influential predictors of species distributions, followed by elevation (21.7%) and proximity to water (14.5%). Species richness, estimated using a stacked SDM approach, was highest in the northern and riparian zones of the park, identifying potential biodiversity hotspots. This study presents the first fine-scale SDMs for mammal species in Nyerere National Park, offering a valuable ecological baseline to support conservation planning and promote sustainable ecotourism development in Tanzania’s southern protected areas.
1. Introduction
Global mammalian populations have declined by 68% between 1970 and 2016 [1], largely due to anthropogenic pressures such as land-use change [2] and wildlife exploitation [3]. Despite the international conservation frameworks like the Convention on Biological Diversity (CBD) [4], there is still a pressing need for improved monitoring and assessment of mammalian species’ conservation status worldwide [5].
Africa harbors exceptional mammalian diversity [6], shaped by its wide range of geographical and climatic conditions [7]. Understanding the spatial distribution and richness of mammalian species, a commonly used measure of biodiversity, is essential for conservation and ecosystem management [8]. Mammalian richness also serves as an indicator of ecosystem health, often correlating with ecological balance and functionality [9]. Mammals play critical ecological roles [10], contribute to ecotourism [11], and hold significant cultural value [12]. In Tanzania, this richness underpins a vital tourism industry that contributes approximately 17.2% to the national GDP (Gross Domestic Product) [13], supported by the allocation of around 36% of the country’s land to conservation [14]. While Northern Tanzania’s protected areas have been the focus of extensive ecological research, leading to robust infrastructure and conservation strategies, the southern areas, such as Nyerere National Park (NNP), remain comparatively understudied. Research in NNP has so far focused primarily on governance issues [15], local extinction risks (e.g., cheetahs) [16], tourism potential [17], the impacts of illegal bushmeat hunting [18], and small mammals distribution [19], with limited attention to biodiversity patterns or mammal distribution.
Species distribution models (SDMs) provide a cost-effective approach to address this knowledge gap, especially in areas where biodiversity data are sparse or unevenly collected. By linking known species occurrences with environmental variables, SDMs can identify potential suitable habitats and inform conservation planning at multiple spatial scales [20]. This makes them particularly useful for establishing ecological baselines in NNP.
A recent systematic review underscored the importance of remote sensing (RS)-derived environmental variables in SDMs to improve predictive accuracy and ecological relevance [21]. In Tanzania, several studies have successfully applied SDMs and RS data to map species distributions and habitat suitability across various ecosystems. For instance, [22] mapped habitat suitability for black rhinoceros in Ngorongoro using Sentinel-2 and PlanetScope imagery and machine learning algorithms. MaxEnt models were used in [23] to predict small-mammal distributions in the Selous (Nyerere) ecosystem, using Landsat-derived NDVI, land cover, and WorldClim variables [24]. Stacked species distribution models (SSDM) were developed for seven ungulates between Lake Manyara and Tarangire [25], and in [26], multiple elephant records were integrated with ~5 m land cover metrics for connectivity across Tanzania. While these studies demonstrate the growing use of RS and ensemble modeling approaches in Tanzanian conservation research, none have combined a full SSDM framework with species-specific, high-resolution field data within the NNP. This limitation is particularly important given that most historical wildlife monitoring in NNP has relied on aerial surveys, which are prone to detection and spatial biases [27]. Ground-based observations remain scarce, limiting the ecological precision of existing distribution assessments. Although the application of SDMs and SSDMs is expanding across Africa, their implementation remains uneven and is often constrained by data availability and methodological inconsistencies.
To overcome current data limitations in NNP, this study applies a novel approach that combines presence-only SDMs (using MaxEnt), satellite-derived environmental predictors, and systematically collected ground-based species records. By stacking species-specific models, we produced a fine-scale, community-level habitat suitability map for 20 mammal species, extending previous efforts in the Selous ecosystem [23] through a more comprehensive and spatially explicit SSDM framework.
This study has three objectives: (i) to model the spatial distribution of selected mammal species using SDMs and remote sensing-derived environmental variables; (ii) to identify key environmental factors shaping species distributions at a local scale; and (iii) to estimate mammalian species richness through an SDM framework. By achieving these aims, this study provides essential ecological baselines to inform targeted conservation planning and support sustainable ecotourism development in Southern Tanzania, in line with national biodiversity strategies and economic diversification priorities [28].
2. Materials and Methods
2.1. Study Area
The NNP is a UNESCO World Heritage Site, located between 7.75°–10.5°S and 36.0°–38.7°E [18]. It is the largest national park in Africa [17,29], covering an area of 30,893 km2 (Figure 1). The park was established in 2019, and incorporated within its boundaries is a region that formerly belonged to the Selous Game Reserve, with the following upgrade in conservation status, affecting both consumptive and non-consumptive uses [30,31].
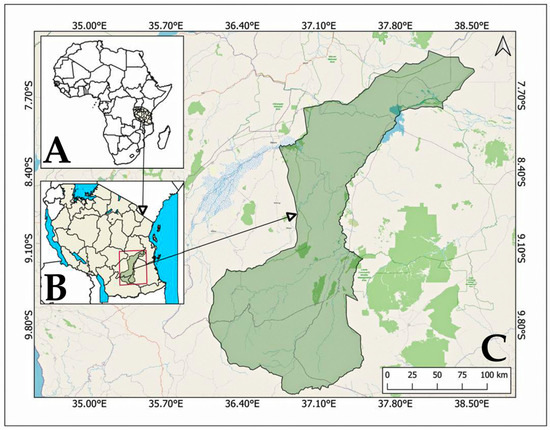
Figure 1.
Africa with Tanzania highlighted (A), the geographical context of Nyerere National Park (NNP) in Southern Tanzania by a red frame (B), and a subsequent zoom into NNP’s boundaries (C) with arrows showing zoom directions. Different colors represent administrative boundaries and landscape features.
The NNP hosts a diverse assemblage of mammal species, including threatened species such as the endangered African elephant (Loxodonta africana), the near–threatened African Cape buffalo (Syncerus caffer), and the vulnerable hippopotamus (Hippopotamus amphibius) [32]. The park also supports a range of large carnivores such as the African lion (Panthera leo), leopard (Panthera pardus), African wild dog (Lycaon pictus), and spotted hyena (Crocuta crocuta) [31].
The park encompasses a mosaic of habitats, such as Miombo woodlands and Acacia-dominated grasslands [33], interspersed with permanent water sources such as the Mbarang’andu, Lukimwa, and Rufiji rivers [34]. It experiences two main seasons: a dry season from May to October and a wet season from November to April, with an average annual precipitation of approximately 1400 mm [35]. This environmental heterogeneity underpins the park’s high ecological value [18] and highlights its importance for regional biodiversity conservation and nature-based tourism.
2.2. Distribution Models
The modeling procedure involved five main stages (Figure 2). First, species presence data were collected through systematic field surveys conducted between 2022 and 2024, covering a range of habitats across the park. Next, a set of 12 environmental predictors was selected based on ecological relevance and the recent literature. These variables were then processed and standardized to a spatial resolution of 30 m, with highly correlated variables filtered out using Pearson correlation analysis. To account for alternative combinations, nine uncorrelated variable sets were constructed for model evaluation. Species distribution models were developed using MaxEnt, applying a target-group background approach to correct for sampling bias. Each model was assessed using performance metrics, including the Area Under the Curve (AUC), omission rates, and corrected Akaike Information Criterion (dAIC), with the best-performing model selected for each species. Finally, the binary presence outputs of all species models were stacked to generate a spatially explicit map of predicted species richness, providing an overview of potential biodiversity hotspots within the park.
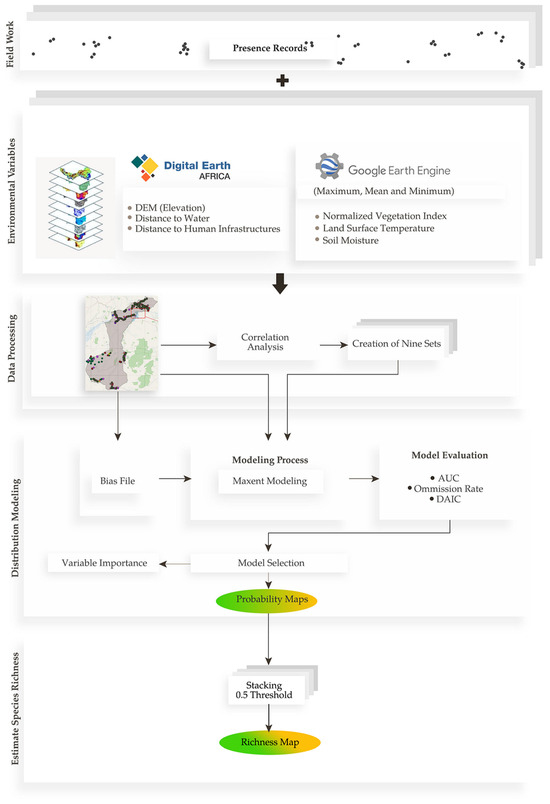
Figure 2.
Workflow for modeling species distributions and estimating richness in NNP. Arrows indicate the sequence of analytical steps and data flow between components.
2.2.1. Presence and Background Records
Data were collected between 2022 and 2024 using ground-based surveys in both dry and wet seasons. A combination of vehicle- and walking-based transects was used, following the standardized protocol described in [36]. Vehicle transects were conducted along primary administrative and patrol roads, where two observers were seated on each side of the rear, and a third acted as data recorder. To optimize detectability, the vehicle was driven at a speed not exceeding 20 km/h. Every 5 km, a 2 km gap was left before the next transect. In addition, 1 km walking transects were conducted perpendicular to the roads (alternating left and right) to detect indirect mammal signs and complement direct sightings.
All species encountered during surveys were recorded to ensure consistent detection effort across habitats. However, species varied in detectability and encounter frequency. We prioritized species with ≥20 independent occurrences, the minimum recommended for presence-only modeling [37], although most species in our dataset exceeded 30 records. Species with fewer records (e.g., dik-dik, waterbuck, banded mongoose, klipspringer) were excluded. Similarly, large carnivores such as lions, leopards, hyenas, and jackals, though observed, were not included due to their strong reliance on prey availability and biotic interactions, which are not adequately captured by the abiotic predictors used in this study [38,39].
A total of 2334 presence records were collected, corresponding to the 20 mammal species listed in Table 1. Small-sized mammals included bushbuck (Tragelaphus scriptus), African civet (Civettictis civetta), bush pig (Potamochoerus larvatus), and African savanna hare (Lepus victoriae). Medium-sized species comprised yellow baboon (Papio cynocephalus), aardvark (Orycteropus afer), warthog (Phacochoerus africanus), common duiker (Sylvicapra grimmia), Lichtenstein’s hartebeest (Alcelaphus lichtensteinii), zebra (Equus quagga), impala (Aepyceros melampus), and lesser kudu (Tragelaphus imberbis). Large-sized mammals were represented by hippopotamus (Hippopotamus amphibius), giraffe (Giraffa camelopardalis tippelskirchi), African buffalo (Syncerus caffer), wildebeest (Connochaetes taurinus), sable antelope (Hippotragus niger), common eland (Tragelaphus oryx), greater kudu (Tragelaphus strepsiceros), and African elephant (Loxodonta africana). The selected species represent a range of functional guilds (grazers, browsers, and omnivores) and are among the most consistently observed mammals in NNP. Collectively, they provide an ecologically representative basis for examining habitat use and informing spatial conservation planning across the landscape.

Table 1.
Mammal Species and Presence Records from Field Surveys (2022–2024).
Despite efforts to standardize the surveys, logistical constraints during the wet season, such as flooded roads and dense vegetation, limited access, and detectability in some areas. As a result, temporal sampling was uneven: 78% of the records were collected in the dry season and only 22% during the wet season. Although seasonal variation in habitat use has been shown to significantly influence species distributions in savanna ecosystems [40,41], all presence data were pooled to avoid unreliable model estimates from small seasonal samples.
Similarly, a spatial imbalance was observed, of the 2334 records, 1338 (57.32%) originated from the northern section of NNP, while 996 (42.68%) came from the southern part (Figure 3).
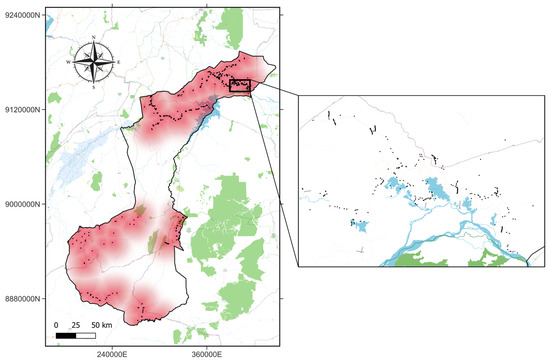
Figure 3.
Spatial distribution of presence records in NNP. Burgundy shades represent sampling effort. The inset map provides a zoomed-in view, highlighting individual presence records (black dots). Base map colors represent landscape features.
Although relatively modest, this asymmetry could introduce spatial autocorrelation. To mitigate this, a binary mask was created using a minimum convex polygon around all presence points, which was then rasterized at the analysis resolution [42]. This bias layer, approximating the effective survey extent, was used to guide background point selection in MaxEnt (i.e., a target-group background approach), with sampling weighted toward areas reflecting actual survey effort.
2.2.2. Description of Environmental Variables
A total of 12 environmental variables known to influence the distribution of mammal species were selected based on the recent literature [22,43,44]. These variables were derived from RS images and geospatial datasets to ensure ecologically relevant predictors with adequate spatial and temporal resolution (Table 2).

Table 2.
Environmental variables.
Sources and Derivation of Environmental Predictors
Three dynamic variables, Land Surface Temperature (LST), Normalized Difference Vegetation Index (NDVI), and Soil Moisture, were obtained from Google Earth Engine. For each, minimum, mean, and maximum values were calculated for the period 2021–2023 to reflect seasonal variability.
LST was derived from the MOD11A2 V6.1 dataset, which provides 8-day average LST and Emissivity at 1 km resolution globally, derived from Terra MODIS (Moderate Resolution Imaging Spectroradiometer) data. Each pixel represents a simple average of all corresponding daily LST observations over the 8-day period (https://developers.google.com/earth-engine/datasets/catalog/MODIS_061_MOD11A2?hl=es-419, accessed on 7 April 2025).
NDVI was derived from the Landsat 8 Collection 2, Tier 1 Level-2 data, providing surface reflectance and thermal data at 30 m resolution with a 16-day revisit cycle. NDVI was calculated from the red (Band 4) and near-infrared (Band 5) reflectance (https://developers.google.com/earth-engine/datasets/catalog/LANDSAT_LC08_C02_T1_L2?hl=es-419, accessed on 7 April 2025).
Following a similar approach from [49], we used the SMAP L4 Global Surface and Root Zone Soil Moisture product SPL4SMGP.007 to calibrate surface soil moisture derived from Sentinel-1 VV backscatter (https://developers.google.com/earth-engine/datasets/catalog/COPERNICUS_S1_GRD?hl=es-419, accessed on 7 April 2025). The Sentinel-1 SAR GRD dataset provides C-band Synthetic Aperture Radar (SAR) imagery from the Sentinel-1 mission. This collection includes Ground Range Detected (GRD) scenes that have been processed to generate calibrated, ortho-corrected products. The data offers multiple polarizations (VV, HH, VH, HV) and resolutions (10 m, 25 m, 40 m), making it suitable for applications like land cover classification, soil moisture estimation, and flood monitoring. The SPL4SMGP.007 product provides surface soil moisture estimates by integrating SMAP L-band brightness temperature observations with land surface modeling. It offers volumetric soil moisture data for both surface (0–5 cm) and root zone (0–100 cm) layers, updated every 3 h on a global 9 km grid (https://developers.google.com/earth-engine/datasets/catalog/NASA_SMAP_SPL4SMGP_007?hl=es-419, accessed on 17 April 2025). Sentinel-1 VV values were spatially averaged to match the SMAP grid and temporally paired with the closest available SMAP acquisition. A linear regression was then applied to estimate the coefficients (a, b) of the empirical model (Soil Moisture = a × VV + b), producing a region-specific radar-based soil moisture estimator for NNP. This variable spatial resolution was calculated using the 10 m resolution VV data.
In addition, three topographic and anthropogenic variables were sourced from the Digital Earth Africa (DE Africa) Sandbox platform (https://www.digitalearthafrica.org/, accessed on 7 April 2025): elevation, distance to water sources, and distance to infrastructures (used as a proxy for human disturbance).
Distance to water bodies was derived from the DE Africa Waterbodies Historical Extent from the Waterbodies Monitoring Service, used to identify and characterize surface water bodies across the study area. This service leverages satellite observations from the Landsat archive (1984–present) to map the Historical Extent areas where water has been observed in at least 5% of clear-sky observations. These products enable the detection of persistent and seasonal water bodies at a continental scale using calibrated, pixel-based water classification from the Water Observations from Space (WOfS) algorithm [50]. Euclidean distance to water bodies was calculated.
Elevation data was obtained from the Copernicus 30 m Digital Elevation Model (DEM) derived from the European Space Agency’s Copernicus Program. This DEM provides bare-earth elevation at a spatial resolution of 30 m, generated through radar interferometry using data from the TanDEM-X and TerraSAR-X satellite missions. The product represents ground elevation independent of vegetation or built structures, making it suitable for terrain-based ecological and climatic modeling. In this study, elevation was extracted as a static topographic variable to assess its influence on species distribution.
Distance to human infrastructures was calculated as the Euclidean distance to the nearest mapped feature using data from OpenStreetMap (OSM), a global, community-driven geospatial database that provides openly licensed vector data for roads, buildings, and other anthropogenic structures. Infrastructure features were extracted using the “osmnx” Python v. 1.9.4 package and processed to generate continuous distance rasters representing proximity to human-modified areas. These layers served as proxies for anthropogenic pressure in the species distribution modeling framework.
The four topographic and anthropogenic variables were calculated at 30 m × 30 m resolution, and the RS-derived variables were resampled at the same spatial resolution.
Variable Selection and Multicollinearity Analysis
To reduce the risk of multicollinearity and improve model interpretability, a pairwise Pearson correlation analysis was performed using the corr package in R 4.4.1 [51]. A threshold of 0.8 was applied, following established practices [52,53,54], to identify and exclude highly correlated variables within the same predictor set. Based on this analysis, nine uncorrelated variable sets were constructed (Table 3), ensuring that each set included ecologically relevant predictors while avoiding collinearity. These sets were used to generate multiple candidate models per species, allowing for a robust comparison of model performance across different environmental configurations.

Table 3.
Variable set selection. Crosses identify a variable’s membership in each set.
2.2.3. Model Fitting
For each species, nine models were constructed using the variable sets described in Section 2.2.2, resulting in 180 models (9 environmental sets × 20 species). Species distribution modeling was conducted using the Maximum Entropy algorithm (MaxEnt v.3.4.2), which is widely recognized for its high accuracy in predicting species distributions using presence-only data [55], especially with limited sample sizes [56]. Each model was run with a maximum of 5000 iterations to ensure model stability [57], and a randomly selected set of 10,000 background points is often considered sufficient for reliable species distribution models [58,59]. A regularization multiplier of 1 was applied to balance model complexity and prevent overfitting [55], while feature types were set to Auto to allow MaxEnt to automatically select the most appropriate transformations based on the sample size [60]. To assess model robustness, a bootstrap resampling method with 10 replicates per model was employed to address the variability [61]. The importance of individual variables was evaluated using the built-in Jackknife test [62], which quantifies each variable’s importance and combined contribution to the model.
2.2.4. Model Evaluation
The performance and predictive capabilities of all nine models generated per species were evaluated using three key metrics: (i) AUC, (ii) omission rates, and (iii) dAICc. These metrics were calculated using a combination of R packages: “dismo” [63], “ENMeval” [64], “maxnet” [65], “pROC” [66], and “AICcmodavg [67]. The optimal model per species was selected based on a combination of the highest AUC and the lowest dAIC, prioritizing models with strong discriminatory performance and optimal model fit.
2.2.5. Staked Species Distribution Model
We estimated species richness using SSDM, following the approach described by [68]. Distribution maps generated with MaxEnt from the selected optimal models were combined into a single stack using the “raster” package [69] in R 4.4.1. A habitat suitability threshold of 0.5 was applied, a commonly used midpoint value in the literature for binary classification of presence and absence [70]. Pixels with predicted suitability values ≥ 0.5 were considered presence locations for each species. By summing the number of species predicted to be present per pixel, we calculated the species richness across NNP. The resulting output provided a spatially explicit richness map, highlighting areas for potential biodiversity hotspots.
3. Results
3.1. Distribution Models Selection
A single model was selected for each species among all nine models, based on its evaluation metrics. For each of the species, Table 4 presents the Area Under the Curve (AUC), omission rate, and dAIC values, along with the final set of environmental predictors retained in the selected model.

Table 4.
Evaluation metrics and selected variables set for each species.
All models achieved AUC scores above 0.8, denoting good to excellent model discrimination. AUC values ranged from 0.81 to 0.97, with a mean of 0.88. The hippopotamus (0.97) and bush pig (0.96) models had the highest values, while the sable antelope model showed the lowest (0.81).
The dAIC values had a mean of 1.01, ranging from 0.82 to 1.35, reflecting generally well-optimized models with minimal overfitting. The lowest dAIC scores were observed for hippopotamus (0.82), bush pig (0.84), and wildebeest (0.90), suggesting efficient balance between model complexity and fit. The highest was obtained for greater kudu (1.35).
Omission rates averaged 0.09, with values ranging from 0.03 to 0.14. The bush pig (0.03) and hippopotamus (0.05) again showed the strongest performance with the lowest omission errors, whereas African buffalo, Hartebeest, and Wildebeest models showed the highest omission rates (0.14), though still within acceptable error margins. These low omission rates indicate a high overall predictive capability.
The importance values of the four most influential environmental variables for each species, ranked by permutation importance, are presented in Table 5. A complete list of variable importance scores for all predictors included in each model is provided in Table S1 in the Supplementary Material.

Table 5.
Ranked variables’ importance (%) of the four most influential environmental variables for each species.
Species distribution models revealed clear ecological groupings based on the most influential environmental predictors, with top-ranking variables often aligning with known habitat requirements and physiological constraints.
DEM was the leading predictor for several species, including lesser kudu, impala, giraffe, bushbuck, African savanna hare, and African buffalo. Importance values for DEM ranged from 34.9% to 75%, confirming that topographic gradients are highly influential in determining species distributions.
Distance to water (DIST_WAT) was the most important predictor for aquatic and semi-aquatic species, particularly the hippopotamus, where it accounted for 63% of model importance. DIST_WAT also ranked highly, although with lower relative importance, for terrestrial species such as the bush pig and the common duiker.
Land surface temperature (MEAN_LST or MAX_LST) emerged as the top predictor for the common warthog, sable antelope, African elephant, and greater kudu. For these species, LST variables contributed between 38% and 57% to overall model performance.
Vegetation indices (NDVI), particularly mean or maximum NDVI, were the primary predictors for species such as the African civet, yellow baboon, African buffalo, and common eland, with variable importance ranging up to 53.3%.
A smaller subset of species responded most strongly to less conventional environmental predictors. The aardvark was associated with soil moisture, and proximity to human settlements (POP_DIST) was an important predictor for species like the common duiker and bush pig.
3.2. Species Distribution Maps
Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8 present the modeled spatial distribution of all species, expressed in terms of habitat suitability, ranging from 0 (completely unsuitable) to 1 (completely suitable).
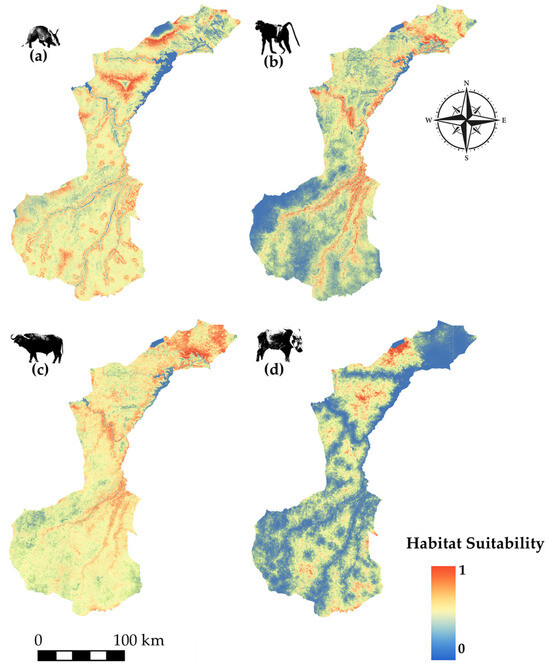
Figure 4.
Species modeled spatial distribution for (a) aardvark (Orycteropus afer), (b) yellow baboon (Papio cynocephalus), (c) African buffalo (Syncerus caffer), and (d) bush pig (Potamochoerus larvatus).
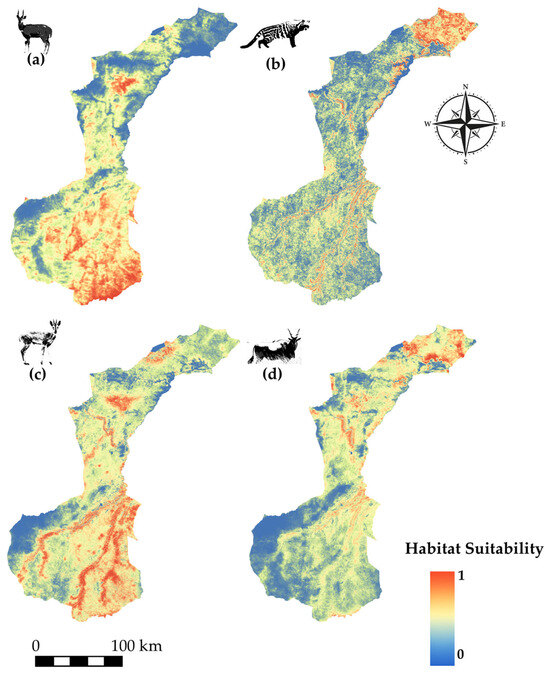
Figure 5.
Species modeled spatial distribution for (a) bush buck (Tragelaphus scriptus), (b) African civet (Civettictis civetta), (c) common duiker (Sylvicapra grimmia), and (d) common eland (Tragelaphus oryx).
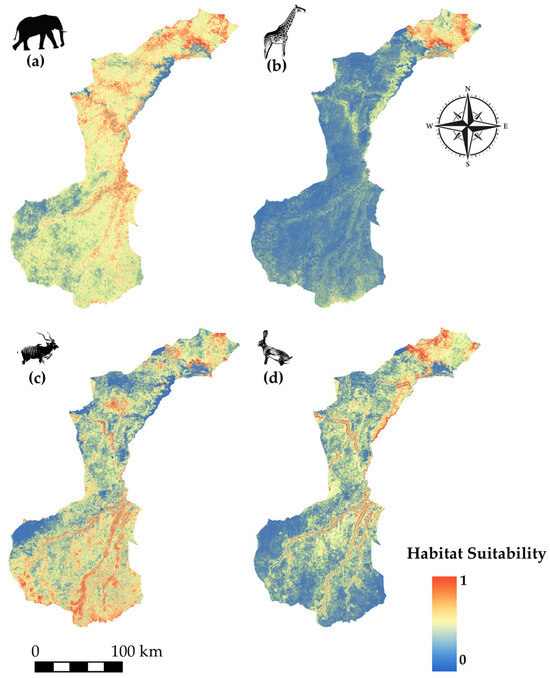
Figure 6.
Species modeled spatial distribution for (a) African elephant (Loxodonta africana), (b) giraffe (Giraffa camelopardalis tippelskirchi), (c) greater kudu (Tragelaphus strepsiceros), and (d) African savanna hare (Lepus victoriae).
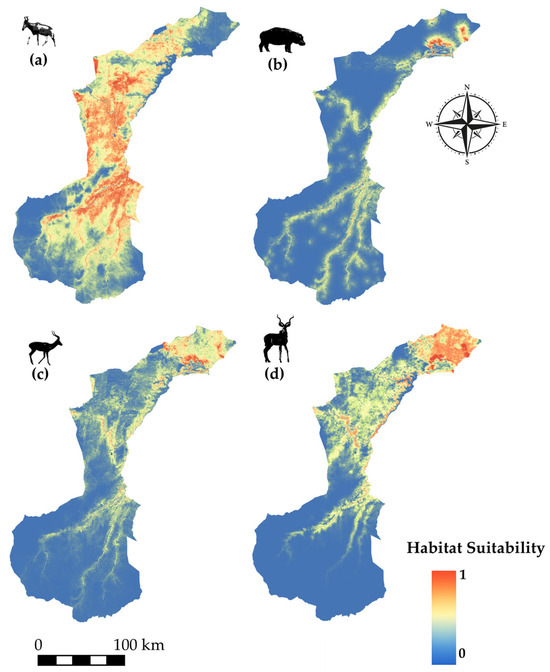
Figure 7.
Species modeled spatial distribution for (a) Lichtenstein’s hartebeest (Alcelaphus lichtensteinii), (b) hippopotamus (Hippopotamus amphibius), (c) impala (Aepyceros melampus), and (d) lesser kudu (Tragelaphus imberbis).
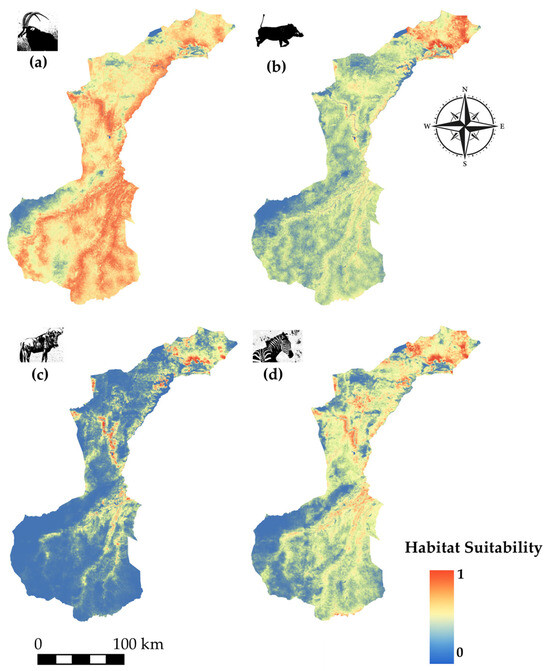
Figure 8.
Species modeled spatial distribution for (a) sable antelope (Hippotragus niger), (b) common warthog (Phacochoerus africanus), (c) wildebeest (Connochaetes taurinus), and (d) zebra (Equus quagga).
Aardvark (Figure 4a) and African buffalo (Figure 4c) show strong suitability in the northern areas, with the buffalo’s range extending slightly into central zones. Bush pig (Figure 4d) also shows fragmented suitability in both the north and far south, while the yellow baboon (Figure 4b) exhibits widespread suitability, with higher concentrations near watercourses.
Bushbuck (Figure 5a) displays high suitability in the southern and southeastern parts of NNP, with smaller suitable pockets extending northward. African civet (Figure 5b) is strongly associated with the northern section of the park, particularly along the northeastern boundary. Common duiker’s distribution (Figure 5c) is centred in the southeast, with isolated patches in the northwest, indicating a fragmented range. Common eland (Figure 5d) exhibits core suitability in the north, particularly the northwest, with habitat extending south-eastward in a patchy but continuous gradient.
African elephant (Figure 6a) exhibits broad suitability across much of the park, extending from the north through the centre and into the southeast, indicating a wide-ranging habitat preference. Giraffe (Figure 6b) shows a concentration of suitable habitat primarily in the northern and central areas, closely associated with open landscapes. The greater kudu (Figure 6c) displays strong suitability in the southeast, with smaller patches extending into central and northeastern regions. African savanna hare (Figure 6d) shows core suitability in the north, particularly in the northwest, with habitat suitability gradually decreasing toward the southeastern part of the park.
Lichtenstein’s hartebeest (Figure 7a) displays a distinct concentration of suitable habitat in the central region of NNP, with smaller extensions into the northern and southern zones, forming a north–south corridor-like pattern. Hippopotamus (Figure 7b) shows a strong affinity for riparian areas, with suitability tightly following the course of the main river system, particularly pronounced in the northern floodplain. Impala (Figure 7c) and lesser kudu (Figure 7d) exhibit high suitability in the northern and northeastern parts of the park, aligned with well-vegetated areas and river networks, with decreasing suitability toward the centre and south.
Sable antelope (Figure 8a) exhibits widespread habitat suitability across most of NNP, with the exception of the southwestern corner, where suitability is notably lower. Common warthog (Figure 8b) displays strong suitability in the northern and northeastern zones, with patchier suitability extending southward along open and drier habitats. Wildebeest suitability (Figure 8c) is highest along the main river corridors from north to central NNP, forming a discontinuous but directional pattern, while the southwestern region shows minimal suitability. Zebra (Figure 8d) distribution follows a similar riverine pattern, with high suitability from the north into the central part of the park, likely reflecting preference for open grasslands near water sources.
3.3. Species Richness
Figure 9 represents the modeled species richness across NNP. It was produced by stacking the continuous habitat suitability maps, resulting in a composite where higher values indicate areas predicted to be suitable for a greater number of species. This spatial overlay reveals clear geographic gradients in mammalian richness within NNP.
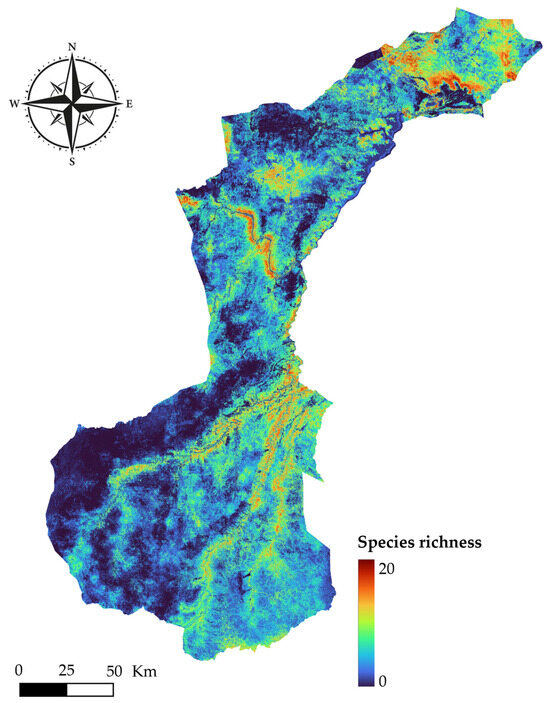
Figure 9.
Predicted species richness across NNP based on ensemble habitat suitability models for 20 mammal species.
The highest richness values are concentrated in the northern section of the park, particularly in the northwest quadrant and along the Rufiji River, which aligns with areas of greater water availability and vegetation productivity. The riparian corridors and wetland zones exhibit dense richness hotspots, consistent with known mammalian reliance on permanent water sources in savanna ecosystems.
The southern and southwestern sectors of NNP display lower modeled richness. These zones are characterized by more homogeneous vegetation cover, lower topographic variation, and reduced accessibility to permanent water bodies, which may limit species diversity.
4. Discussion
This study represents the first comprehensive assessment of mammalian species distribution and richness within NNP, using environmental predictors derived from remote sensing data and presence records collected entirely through field surveys. The integration of SSDMs and ground-based presence records provided valuable ecological insights, offering an essential baseline for conservation planning, management, and tourism in one of Africa’s most biologically significant protected areas. This approach aligns with previous research that has successfully applied SDMs in similar contexts across Tanzania, for instance, in modeling carnivore distributions in Ruaha [71], assessing the effects of climate change on forest ecosystems [72], and mapping habitat suitability for species of conservation concern, such as the pancake tortoise [73] and the black rhinoceros in Ngorongoro [22]. Our results expand on these efforts by focusing on a broader mammalian community in NNP, illustrating the utility of SSDMs for biodiversity monitoring in data-limited regions.
4.1. Field Surveys and Presence Data
The reliability of SDMs is highly dependent on the quality of the presence data. In this study, all 2334 presence records were obtained through systematic, ground-based surveys, ensuring spatial accuracy and ecological relevance. This contrasts with many SDM applications that rely heavily on secondary sources, such as GBIF, which often suffer from temporal heterogeneity and sampling bias [74,75]. By basing our models on field-verified occurrences, we minimized these limitations and improved the biological relevance of the predicted distributions.
Although some modeled species had relatively few presence points, the predictive performance remained robust, consistent with previous findings that MaxEnt performs well even with small datasets [62,76]. Nonetheless, it is well established that larger sample sizes tend to improve model reliability and reduce uncertainty [77,78]. Additional records, particularly for less frequently observed species, would likely reduce errors of omission. As highlighted by [79], high-quality, systematically collected presence data are particularly critical in data-poor regions like Southern Tanzania, where remote areas remain undersampled. Our study demonstrates that well-designed field surveys not only strengthen SDM outcomes but also serve as a foundation for ongoing biodiversity monitoring in protected areas.
4.2. Integration of Remote Sensing and Environmental Predictors in SDMs
This study leveraged remotely sensed environmental variables with high spatial and temporal resolution to better capture the heterogeneity of the NNP landscape. Unlike conventional SDMs that often rely on coarse-resolution climatic layers, we prioritized satellite-derived predictors such as NDVI, LST, and soil moisture. These variables provide a more dynamic and ecologically relevant representation of habitat conditions, particularly in tropical savanna ecosystems where vegetation and surface temperature fluctuate markedly over short time scales.
To further account for environmental complexity, topographic (DEM) and anthropogenic variables (distance to infrastructures), as well as hydrological proximity (distance to water), were also integrated, all of which are known to shape species distributions. Variable selection was guided by ecological relevance and statistical independence (after multicollinearity filtering), ensuring model robustness.
Although land use and land cover (LULC) variables have been shown to improve SDM performance [80,81,82], they were deliberately excluded due to the absence of locally validated, thematically detailed classifications. Global LULC products (e.g., USGS GLC, GLC2000) offer limited habitat categories and poor thematic resolution for African savannas [83,84,85], potentially introducing classification bias [86,87]. Integrating such data would have required extensive ground-truthing, an effort beyond the scope of this study but critical for future work.
Similarly, although bioclimatic variables from WorldClim are widely used in SDMs, their utility in local-scale modeling is constrained by coarse spatial resolution and temporal averaging [88,89,90,91,92]. Our approach aligns with a growing body of the literature, advocating for the use of remote sensing products in fine-scale ecological modeling [22,43], particularly in data-limited yet ecologically complex landscapes like NNP.
4.3. Model Fitting and Evaluation
The models achieved consistently high predictive performance, with AUC values ranging from 0.81 to 0.97 (Table 4). According to established thresholds [93,94], all models can be considered at least “good,” with several classified as “very good” (AUC > 0.9). These values indicate that the models effectively captured the ecological signals underlying species distributions across Nyerere National Park. Moreover, omission rates were generally low and negatively correlated with AUC, suggesting that models with stronger predictive ability also minimized false absences.
The results are consistent with previous studies that employed presence-only SDMs for African mammals. For example, ref. [95] reported AUCs above 0.9 for species such as buffalo, wildebeest, and zebra using similar modeling techniques, and ref. [96] obtained AUCs above 0.8 in elephant distribution models. These studies emphasize the value of carefully selected environmental predictors and field-verified presence data in improving model accuracy, principles that also underpin the present analysis.
While the lack of prior SDM research specific to NNP limits direct comparisons, our findings align well with performance benchmarks reported for large mammals in other Tanzanian savanna ecosystems. Thus, despite regional data gaps, the use of widely accepted evaluation metrics (e.g., AUC, omission rate, dAIC) provides a robust framework for assessing model performance and ensuring reliability for conservation applications.
4.4. Species Distribution Patterns and Environmental Drivers
Understanding species-specific distribution patterns is essential for interpreting ecological dynamics and informing conservation strategies. Each species responds uniquely to environmental gradients, shaped by its ecological niche, habitat preferences, and mobility.
The models’ results align closely with known ecological traits of the study species, revealing coherent patterns of environmental dependence. Elevation (DEM), temperature-related variables (LST), vegetation indices (NDVI), and distance to water (DIST_WAT) emerged as dominant predictors, each associated with distinct ecological strategies (Table S1).
DEM was the top predictor for a range of species, including lesser kudu, impala, giraffe, bushbuck, African savanna hare, and African buffalo. While this could suggest altitudinal preferences, it is more likely that elevation serves as a spatial filter integrating multiple landscape-level factors such as vegetation type, microclimate, and terrain complexity. For wide-ranging species such as giraffe and impala, elevation may reflect preferences for flat, open habitats, while for more habitat-specific taxa like bushbuck or hare, it may correspond to restricted ecological niches or reduced human accessibility at certain altitudes. In the case of bushbuck, which occurs across a broad elevational gradient, DEM likely captures associated environmental transitions (e.g., vegetation density or anthropogenic pressure), rather than elevation per se.
Land surface temperature was particularly influential for species occupying open, sun-exposed savannas. MAX_LST and MEAN_LST were dominant predictors for warthog, sable antelope, African elephant, greater kudu, and zebra, supporting earlier observations of heat-sensitive behaviors in ungulates. These thermal predictors may not only reflect physiological stress thresholds but also habitat patterns in response to exposure and shade availability. In elephants, MEAN_LST may additionally capture broader shifts in water and forage availability across temperature gradients.
Mean and maximum NDVI were primary drivers for species such as the African civet, yellow baboon, common eland, and African buffalo. These species rely on consistent vegetation structure for foraging, concealment, and social interactions. NDVI-based predictors effectively represent spatial and seasonal variability in plant productivity, and their importance underscores species’ vulnerability to climate-induced vegetation change or anthropogenic degradation. For grazers such as eland and buffalo, this dependency may reflect minimum biomass thresholds required to support large-bodied herbivory, while for omnivores like baboons and civets, persistent vegetative cover ensures foraging diversity and protection.
Distance to water was the most important predictor for obligate aquatic species, particularly the hippopotamus, where it accounted for over 60% of variable importance. This result is consistent with their strong behavioral dependence on water bodies for thermoregulation, safety, and reproduction. Interestingly, DIST_WAT also emerged as a top predictor for more terrestrial species such as the sable antelope, bush pig, and duiker. This suggests that proximity to water may serve both as a direct requirement and as a proxy for riparian vegetation or microclimatic refugia during the dry season. The sable antelope, for example, exhibited a strong temperature signal (MAX_LST: 52.5%) but also retained DIST_WAT among its top variables (14.4%), reinforcing the need to interpret variable rankings in an integrated ecological context.
Some results were less expected. Both the African elephant and buffalo, which are known to be water-dependent, had models dominated by temperature and elevation, respectively. These outcomes may reflect spatial correlations between water availability and other environmental gradients within the landscape, or potential limitations in predictor resolution and sample coverage. In the case of elephants, thermal stress may act as a more immediate constraint than water per se, particularly in areas where surface water is widespread or ephemeral.
Finally, species-specific predictors such as soil moisture and distance to human settlements (POP_DIST) highlight the diversity of ecological strategies among the savanna mammal community. The aardvark’s association with soil moisture likely reflects its fossorial behavior and dependence on diggable substrates. Similarly, the prominence of POP_DIST in species such as bush pig and common duiker suggests behavioral tolerance or opportunism in response to human-altered environments. However, these patterns may also indicate increased risk under future land-use intensification.
4.5. Species Richness Patterns in NNP: Insights from SSDMs
The species richness map, derived from stacking individual binary SDMs, revealed clear spatial gradients across Nyerere National Park. Richness peaked in the northern and riverine zones and declined toward central and southwestern areas. These hotspots coincide with ecologically favourable conditions, moderate temperatures, proximity to water, suitable elevation, and higher vegetation cover, which support more diverse mammal assemblages.
While bias in sampling effort is known to influence species richness estimates, often inflating richness in heavily surveyed areas [97], SSDM outputs suggest independence from sampling efforts. Although survey effort was concentrated in the northern section of NNP, and richness was indeed high there, the southwestern sector, which also received substantial sampling, exhibited relatively low species richness. Moreover, we identified a notable richness patch in the central part of the park, where sampling effort was minimal. This further underscores that the SSDM outputs are capturing genuine ecological gradients, not merely reflecting the distribution of effort. Together, these patterns emphasize the strong alignment between modeled richness and environmental features, such as elevation, vegetation heterogeneity, and riparian proximity.
This is particularly evident in the role of water availability, which, despite its limited influence in single-species models, emerges as a key driver of richness patterns at the community level, as richness was clearly higher near rivers. This likely reflects species-specific habitat preferences: while not all species are directly water-dependent, many benefit indirectly from riparian environments for food, cover, or connectivity. Thus, variables with modest influence at the species level may exert a stronger cumulative effect at the community level. A similar pattern was observed by [44] in South Korea, where water-related variables were weak predictors for individual species but key for richness models.
Despite its limitations, such as assuming species independence, the SSDM approach remains a robust tool for identifying biodiversity hotspots, particularly in data-poor regions. In NNP, modeled richness gradients aligned with observed wildlife concentrations reported by the 2022 TAWIRI (Tanzania Wildlife Research Institute) aerial census [32], especially in the northern floodplains and along the Rufiji River. This spatial agreement reinforces the ecological validity of the SSDM outputs and underscores the value of integrating remote sensing and field data.
Finally, our results are consistent with those of SSDM applications in other Tanzanian ecosystems, such as Ngorongoro [22], and support calls for the broader adoption of ensemble-based, ground-validated SDMs in national conservation planning [37,98,99].
4.6. Climate Change Vulnerability
Our models underscore that NNP’s mammals are tightly constrained by local climate, implying significant vulnerability to global warming. Continental projections suggest up to 10–15% of African mammal ranges may be lost by 2050 under warming scenarios [100]. In NNP, hotter, drier future conditions will likely push species upslope or into protected mesic refugia (e.g., along rivers and upland forests [101].
These considerations should be integrated into park management. For example, regional policies already emphasize transboundary ecosystem management by 2030 [102]. In practice, managers should identify and secure potential microrefugia, such as evergreen riparian thickets and high-elevation woodlands, that can buffer heat stress [103]. Incorporating climate projections into zoning and planning (e.g., water resource maps, future land-use scenarios) will help anticipate and mitigate climate impacts on wildlife.
4.7. Landscape Connectivity and Conservation Implications
NNP lies at the core of the globally significant Selous ecosystem, a landscape that supports diverse and wide-ranging mammal populations, including elephants, buffalo, and endangered carnivores. Ensuring landscape connectivity, particularly the protection of wildlife corridors, is essential for sustaining these populations [104]. However, many key ecological linkages are increasingly threatened. Recent national assessments indicate that ~30 historic wildlife corridors across Tanzania are now highly degraded or obstructed due to agricultural expansion and settlement. Notably, the Udzungwa–Selous and Wami–Mbiki–Nyerere corridors have suffered severe fragmentation due to deforestation and land conversion [105].
In this context, a major policy development occurred in June 2025, when the Tanzanian government approved the reassignment of 1617 km2 from NNP back to Selous Game Reserve, resolving long-standing boundary disputes. This adjustment, intended to reduce human-protected area conflicts and strengthen coexistence with local communities and Wildlife Management Areas (WMAs), also has potential implications for ecological connectivity. According to official statements, the move seeks to secure a key corridor between NNP and Selous and to preserve the steady flow of the Rufiji River, which is critical both for biodiversity and national infrastructure such as the Julius Nyerere Hydropower Project (JNHPP).
This decision reflects a growing recognition that conservation boundaries must align with ecological functions, a principle supported by our habitat models. Reabsorbing strategic buffer zones into the Selous could, if properly managed, help maintain core–corridor–core connectivity and ensure movement pathways for migratory species. However, successful implementation requires coordinated governance and ecological planning.
At the landscape level, we recommend the establishment of multi-stakeholder “Corridor Planning Committees” to harmonize land use across WMAs, village lands, and protected areas [106]. These committees could guide interventions such as joint land-use planning to prevent habitat fragmentation, incentive schemes for corridor conservation, and strategic expansion of WMAs to protect high-suitability areas identified in this study.
Our findings also highlight the need to integrate habitat maps into development and infrastructure planning. For example, fire management in miombo woodlands, through early-dry-season burns, can reduce destructive fires and support habitat quality [107]. Similarly, any expansion of roads, pipelines, or tourism facilities must avoid the high-suitability areas and movement corridors predicted by our models. In particular, infrastructure around the Rufiji River, NNP’s ecological backbone, should be designed to maintain environmental flows, a point of concern given the downstream impact of the JNHPP [108].
Beyond highlighting areas of high conservation value, our results underscore the importance of spatially nuanced management strategies. The alignment between richness patterns and environmental heterogeneity, particularly in riparian corridors, the northern plateau, and parts of central NNP, suggests that these zones may function as ecological refugia under increasing climate and anthropogenic pressure. Given their potential to support diverse assemblages even in areas with low sampling effort, such as the central zones, these landscapes warrant additional attention in future monitoring and zoning plans. Importantly, the species-specific differences in environmental drivers revealed by our SDMs caution against uniform conservation strategies. Instead, integrating fine-scale habitat preferences into corridor design, anti-poaching patrol planning, and ecotourism development could enhance the ecological effectiveness of ongoing landscape-scale initiatives.
By aligning conservation planning with updated ecological data and recent territorial adjustments, Tanzania can strengthen its ecosystem-based management approach and support both biodiversity and rural livelihoods in the broader Selous–Nyerere landscape.
4.8. Limitations and Future Directions
This study represents a first step toward fine-scale, ground-based mammal distribution modeling in NNP; however, several limitations should be acknowledged to guide future research.
First, seasonality was not explicitly modeled due to the limited availability of wet season presence records, largely a result of restricted field access during those months. This may have constrained our ability to detect seasonal shifts in habitat use, particularly for species such as hippopotamus, bush pig, and warthog, whose spatial behavior varies markedly across seasons. Although we pooled records from both wet and dry periods to maximize sample size, this approach may have masked important intra-annual variation. Indeed, 8 of the 20 modeled species lacked sufficient wet season records to support season-specific models, representing a clear trade-off between ecological resolution and statistical feasibility.
Second, the spatial distribution of records was uneven, with greater sampling success in northern NNP. While this pattern partly reflects logistical challenges during the wet season, it may also have introduced bias into the species richness map, potentially overstating richness in better-sampled areas. We opted not to apply spatial thinning due to the clustered nature of presence records in ecologically diverse microhabitats and the risk of losing valuable data for rare or range-restricted species. This decision prioritizes ecological representativeness over strict spatial independence, reflecting a balance commonly adopted in other studies across large, heterogeneous landscapes [109].
Third, while we integrated a suite of high-resolution environmental predictors, some variables (particularly MODIS-derived layers) required resampling from coarser resolutions. This may introduce spatial uncertainty, especially in habitats with fine-scale variation. Similarly, LULC data were excluded due to the lack of locally validated, thematically detailed maps, limiting insights into habitat quality and anthropogenic pressures. These limitations, discussed earlier, highlight the need for improved local environmental datasets.
Future research should prioritize season-specific surveys, particularly during the wet season, to better capture dynamic species–environment relationships. Incorporating fine-resolution, locally validated LULC and climate data would enhance model realism and ecological inference. In addition, expanding sampling into underrepresented southern zones of NNP could improve the spatial balance of presence data and the robustness of community-level richness estimates.
Despite these challenges, our study provides an important ecological baseline for NNP. The detection and modeling of 20 mammal species, many of them of conservation or touristic value, underscores the park’s biodiversity and its potential for nature-based tourism. As the first SDM-based assessment grounded in systematically collected field data, this work lays the foundation for evidence-based conservation planning and opens new avenues for ecological monitoring in one of Tanzania’s most important protected areas.
5. Conclusions
This study provides the first fine-scale, ground-based assessment of mammal distributions in Nyerere National Park, demonstrating how presence-only SDMs integrated with high-resolution remote sensing data can overcome data limitations in complex, undersampled landscapes. By adopting a stacked SDM framework, we mapped habitat suitability and species richness across the park, offering critical spatial insights for conservation efforts.
Our results reveal clear patterns of mammal richness linked to ecological gradients, particularly in riparian and northern zones, and underscore the importance of integrating dynamic environmental variables beyond traditional climatic layers. This approach complements existing aerial surveys and offers a replicable model for other data-poor tropical systems. While limitations such as seasonal data gaps and environmental data resolution persist, the methodology lays a strong foundation for future temporal analyses, climate vulnerability assessments, and corridor planning.
Ultimately, this work supports more targeted and adaptive management strategies in NNP, aligning with national priorities for biodiversity conservation, sustainable tourism, and landscape connectivity. As climate and land-use pressures intensify, scalable tools like those applied here will be essential to safeguard biodiversity in Tanzania’s rapidly changing savanna ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/rs17142504/s1, Table S1: Variables’ permutation importance, minimum, and maximum suitable values for each species.
Author Contributions
Conceptualization, G.M. and E.C.; methodology, G.M. and E.C.; validation, W.M. (Wilfred Marealle), T.I.M., W.M. (Willard Mbewe) and R.L.; formal analysis, G.M. and E.C.; data curation, G.M. and E.C.; writing—original draft preparation, G.M., E.C. and M.A.; writing—review and editing, G.M., E.C., W.M. (Wilfred Marealle), W.M. (Willard Mbewe), R.L., T.I.M. and M.A.; supervision, W.M. (Willard Mbewe), E.C. and M.A.; project administration, W.M. (Wilfred Marealle), and R.L.; funding acquisition, W.M. (Wilfred Marealle), R.L. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the World Bank under the soft grant number P150523.
Data Availability Statement
The data used in this study included freely available satellite images available from GEE and DE Africa. Field survey data are available upon request to the main author (goodluck.massawe@tawiri.or.tz).
Acknowledgments
Thanks to Malawi University of Science and Technology (MUST) and the JRS Biodiversity Foundation for the MSc scholarship in Biodiversity Informatics (Grant No. 60902). We also express our sincere gratitude to the Ministry of Natural Resources and Tourism (MNRT) for funding and facilitating this study through the REGROW Project Coordination Unit (PCU-MNRT). We also acknowledge the World Bank for its valuable technical support. Special appreciation goes to TAWIRI and COSTECH for granting the necessary research permits. We are grateful to TANAPA for granting access and facilitating our work within their protected areas. We also acknowledge the invaluable technical assistance provided during data collection from Deusdedith Bwenge, Felix Shayo, Grayson Mwakalebe, and Damian Nguma. Special thanks to our drivers, Dickson Lukumay, Joram Milio, and Athumani Kipehi, for their dedicated support throughout the fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WWF. Living Planet Report 2020; WWF: Gland, Switzerland, 2020; ISBN 9782940529995. [Google Scholar]
- Brink, A.B.; Bodart, C.; Brodsky, L.; Defourney, P.; Ernst, C.; Donney, F.; Lupi, A.; Tuckova, K. Anthropogenic Pressure in East Africa-Monitoring 20 Years of Land Cover Changes by Means of Medium Resolution Satellite Data. Int. J. Appl. Earth Obs. Geoinf. 2014, 28, 60–69. [Google Scholar] [CrossRef]
- Rija, A.A.; Critchlow, R.; Thomas, C.D.; Beale, C.M. Global Extent and Drivers of Mammal Population Declines in Protected Areas under Illegal Hunting Pressure. PLoS ONE 2020, 15, e0227163. [Google Scholar] [CrossRef]
- CBD. Global Biodiversity Outlook 3; A Report by the Secretariat of the Convention on Biological Diversity, Montréal; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2010; ISBN 9292252208. [Google Scholar]
- Leadley, P.; Gonzalez, A.; Obura, D.; Krug, C.B.; Londoño-Murcia, M.C.; Millette, K.L.; Radulovici, A.; Rankovic, A.; Shannon, L.J.; Archer, E.; et al. Achieving Global Biodiversity Goals by 2050 Requires Urgent and Integrated Actions. One Earth 2022, 5, 597–603. [Google Scholar] [CrossRef]
- Bezeng, B.S.; Ameka, G.; Angui, C.M.V.; Atuah, L.; Azihou, F.; Bouchenak-Khelladi, Y.; Carlisle, F.; Doubi, B.T.S.; Gaoue, O.G.; Gatarabirwa, W.; et al. An African Perspective to Biodiversity Conservation in the Twenty-First Century. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2025, 380, 20230443. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.; Hortal, J.; Martínez-Maza, C.; Morales, J.; Ortiz-Jaureguizar, E.; Pelaez-Campomanes, P.; Pickford, M.; Prado, J.L.; Rodríguez, J.; Senut, B.; et al. Historical Determinants of Mammal Diversity in Africa: Evolution of Mammalian Body Mass Distribution in Africa and South America During Neogene and Quarternary Times. In African Biodiversity; Springer: Boston, MA, USA, 2007; pp. 287–295. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Jarnevich, C.S.; Pearse, I.S.; Smyth, R.L.; Auer, S.; Cook, G.L.; Edwards, T.C.; Guala, G.F.; Howard, T.G.; Morisette, J.T.; et al. Development and Delivery of Species Distribution Models to Inform Decision-Making. Bioscience 2019, 69, 544–557. [Google Scholar] [CrossRef]
- Murwendo, T.; Murwira, A.; Masocha, M. Modelling and Predicting Mammalian Wildlife Abundance and Distribution in Semi-Arid Gonarezhou National Park, South Eastern Zimbabwe. Ecofeminism Clim. Chang. 2020, 1, 151–163. [Google Scholar] [CrossRef]
- Lacher, T.E.; Davidson, A.D.; Fleming, T.H.; Gómez-Ruiz, E.P.; McCracken, G.F.; Owen-Smith, N.; Peres, C.A.; Vander Wall, S.B. The Functional Roles of Mammals in Ecosystems. J. Mammal. 2019, 100, 942–964. [Google Scholar] [CrossRef]
- Okello, M.M.; Manka, S.G.; D’aMour, D.E. The Relative Importance of Large Mammal Species for Tourism in Amboseli The Relative Importance of Large Mammal Species for Tourism in Amboseli National Park, Kenya. Tour. Manag. 2008, 29, 751–760. [Google Scholar] [CrossRef]
- Kideghesho, J.R. The Potentials of Traditional African Cultural Practices in Mitigating Overexploitation of Wildlife Species and Habitat Loss: Experience of Tanzania. Int. J. Biodivers. Sci. Manag. 2009, 5, 83–94. [Google Scholar] [CrossRef]
- Catherine, A.M.; Revocatus, M.; Hussein, S. Will Ngorongoro Conservation Area Remain a World Heritage Site amidst Increasing Human Footprint? Int. J. Biodivers. Conserv. 2015, 7, 394–407. [Google Scholar] [CrossRef]
- Gizachew, B.; Rizzi, J.; Shirima, D.D.; Zahabu, E. Deforestation and Connectivity among Protected Areas of Tanzania. Forests 2020, 11, 170. [Google Scholar] [CrossRef]
- Ma, L.; Wu, J.; Zhang, H.; Lobora, A.; Hou, Y.; Wen, Y. Conflict Governance between Protected Areas and Surrounding Communities: Willingness and Behaviors of Communities—Empirical Evidence from Tanzania. Diversity 2024, 16, 278. [Google Scholar] [CrossRef]
- Searle, C.E.; Strampelli, P.; Haule, L.; Parsais, S.N.; Olesyapa, K.; Salum, N.D.; Ikanda, D.; Mtoka, S.; Hape, G.; Mathayo, D.; et al. Cheetahs in Tanzania’s Selous-Nyerere Ecosystem: Lack of Evidence for Current Persistence, and Reflections on Historical Status. Oryx 2023, 58, 532–536. [Google Scholar] [CrossRef]
- Mkwizu, K.H. Experiences and Enjoyment of National Parks: Study of Nyerere National Park in Tanzania. Int. Hosp. Rev. 2023, 38, 355–375. [Google Scholar] [CrossRef]
- Foya, Y.R.; Mgeni, C.P.; Kadigi, R.M.J.; Kimaro, M.H.; Hassan, S.N. Do Communities Understand the Impacts of Unlawful Bushmeat Hunting and Trade? Insights from Villagers Bordering Western Nyerere National Park Tanzania. Glob. Ecol. Conserv. 2023, 46, e02626. [Google Scholar] [CrossRef]
- Saanya, A.E.; Mulungu, L.S.; Massawe, A.W.; Makundi, R.H. Current and Potential Future Distribution of Small Mammals in the Selous Ecosystem, Tanzania. J. East Afr. Nat. Hist. 2023, 112, 47–62. [Google Scholar] [CrossRef]
- Grenié, M.; Violle, C.; Munoz, F. Is Prediction of Species Richness from Stacked Species Distribution Models Biased by Habitat Saturation? Ecol. Indic. 2020, 111, 105970. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Y.; Zong, J.; Wang, Y.; Cao, X.; Wang, Y.; Cheng, X.; Zhang, W. Remote Sensing Application in Ecological Restoration Monitoring: A Systematic Review. Remote Sens. 2024, 16, 2204. [Google Scholar] [CrossRef]
- Borges, J.; Symeonakis, E.; Higginbottom, T.P.; Jones, M.; Cain, B.; Kisingo, A.; Maige, D.; Oliver, O.; Lobora, A.L. Assessing Habitat Suitability: The Case of Black Rhino in the Ngorongoro Conservation Area. Remote Sens. 2024, 16, 2855. [Google Scholar] [CrossRef]
- Saanya, A.; Mulungu, L.; Sabuni, C.; Massawe, A.; Makundi, R. Effects of Prescribed Burning on Rodents in an East African Woodland Ecosystem. Afr. J. Ecol. 2023, 61, 583–594. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Riggio, J.; Foreman, K.; Freedman, E.; Gottlieb, B.; Hendler, D.; Radomille, D.; Rodriguez, R.; Yamashita, T.; Kioko, J.; Kiffner, C. Predicting Wildlife Corridors for Multiple Species in an East African Ungulate Community. PLoS ONE 2022, 17, e0265136. [Google Scholar] [CrossRef]
- Song, L.; Frazier, A.E.; Estes, A.B.; Estes, L.D. A Multi-Scale Approach for Integrating Species Distribution Models with Landscape Connectivity to Identify Critical Linkage Zones for African Savanna Elephants (Loxodonta Africana). Ecol. Modell. 2025, 507, 111198. [Google Scholar] [CrossRef]
- Lamprey, R.; Ochanda, D.; Brett, R.; Tumwesigye, C.; Douglas-Hamilton, I. Cameras Replace Human Observers in Multi-Species Aerial Counts in Murchison Falls, Uganda. Remote Sens. Ecol. Conserv. 2020, 6, 529–545. [Google Scholar] [CrossRef]
- COWI Tanzania Ltd. Environmental and Social Management Framework for the Resilient Natural Resources Management for Tourism and Growth Project (P150523-PPA-C-07); Prepared for the Ministry of Natural Resources and Tourism, Tanzania; COWI Tanzania Ltd.: Dar es Salaam, Tanzania, 3 August 2017. [Google Scholar]
- Foya, Y.R.; Mgeni, C.P.; Kadigi, R.M.J.; Kimaro, M.H.; Hassan, S.N. The Knowledge about the Potential Health Risks of Illegal Bushmeat Activities among Local Communities Adjacent to Western Nyerere National Park, Tanzania. Open J. Ecol. 2023, 13, 22–36. [Google Scholar] [CrossRef]
- Bigirwa, D.; Roden Msese, L.; Rwakalaza, R.; Bilame, O. Measuring the Economic Use Values of Recreation Resources in Protected Areas, Evidence from Nyerere National Park in Tanzania. Am. J. Environ. Resour. Econ. 2021, 6, 54–65. [Google Scholar] [CrossRef]
- Searle, C.E.; Strampelli, P.; Hape, G.; Elisa, M.; Mathayo, D.; Ikanda, D.; Mtoka, S.; Lobora, A.L. Spatially Explicit Camera Trap-Based Lion Monitoring in Tanzania ’ s Selous–Nyerere Landscape. J. Zool. 2025, 1–12. [Google Scholar] [CrossRef]
- TAWIRI. Aerial Wildlife Survey of Large Animals and Human Activities in the NyerereSelousMikumi Ecosystem, Dry Season 2022; Tanzania Wildlife Research Institute: Arusha, Tanzania, 2023. [Google Scholar]
- Baldus, R.D.; Hahn, R. The Selous–Niassa Wildlife Corridor in Tanzania: Biodiversity Conservation from the Grassroots. Practical Experiences and Lessons from Integrating Local Communities into Trans-Bounday Natural Resources Management; CIC Technical Series Publication No.6; International Council for Game and Wildlife Conservation: Budakeszi, Hungary; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; 48p. [Google Scholar]
- Mpanduji, D. Distribution and Movements of Elephants and Other Wildlife in the Selous-Niassa Wildlife Corridor, Tanzania; Tropical Ecology Support Programme, GTZ: Eschborn, Germany, 2004. [Google Scholar]
- Growcott, J.; Lobora, A.; Markham, A.; Searle, C.E.; Wahlström, J.; Wijers, M.; Simmons, B.I. The Secret Acoustic World of Leopards: A Paired Camera Trap and Bioacoustics Survey Facilitates the Individual Identification of Leopards via Their Roars. Remote Sens. Ecol. Conserv. 2024, 1–14. [Google Scholar] [CrossRef]
- Norton-Griffiths, M. Counting Animals. Handbook No.1; African Wildlife Foundation: Nairobi, Kenya, 1978. [Google Scholar]
- Benavides Rios, E.; Sadler, J.; Graham, L.; Matthews, T.J. Species Distribution Models and Island Biogeography: Challenges and Prospects. Glob. Ecol. Conserv. 2024, 51, e02943. [Google Scholar] [CrossRef]
- Dormann, C.F.; Bobrowski, M.; Dehling, D.M.; Harris, D.J.; Hartig, F.; Lischke, H.; Moretti, M.D.; Pagel, J.; Pinkert, S.; Schleuning, M.; et al. Biotic Interactions in Species Distribution Modelling: 10 Questions to Guide Interpretation and Avoid False Conclusions. Glob. Ecol. Biogeogr. 2018, 27, 1004–1016. [Google Scholar] [CrossRef]
- Fern, R.R.; Morrison, M.L.; Wang, H.H.; Grant, W.E.; Campbell, T.A. Incorporating Biotic Relationships Improves Species Distribution Models: Modeling the Temporal Influence of Competition in Conspecific Nesting Birds. Ecol. Modell. 2019, 408, 108743. [Google Scholar] [CrossRef]
- Jeza, G.T.; Bekele, A. Seasonal Distribution Model of African Elephants (Loxodonta Africana) under a Changing Environment and Land Use in Omo National Park, Ethiopia. J. Wildl. Biodivers. 2023, 7, 96–117. [Google Scholar] [CrossRef]
- Roug, A.; Muse, E.A.; Clifford, D.L.; Larsen, R.; Paul, G.; Mathayo, D.; Mpanduji, D.; Mazet, J.A.K.; Kazwala, R.; Kiwango, H.; et al. Seasonal Movements and Habitat Use of African Buffalo in Ruaha National Park, Tanzania. BMC Ecol. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.H.F.; Ijff, S.D.; Raes, N.; Amaral, I.L.; Salomão, R.P.; Coelho, L.D.S.; Matos, F.D.D.A.; Castilho, C.V.; Filho, D.D.A.L.; López, D.C.; et al. Species Distribution Modelling: Contrasting Presence-Only Models with Plot Abundance Data. Sci. Rep. 2018, 8, 1003. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.N.; Leimgruber, P.; Werner, K.; Stabach, J.; Wittemyer, G. Identification of Landscape Features Structuring Movement Connectivity for Namibian Elephants. Landsc. Ecol. 2024, 39, 148. [Google Scholar] [CrossRef]
- Chung, O.S.; Lee, J.K. Assessing the Distribution and Richness of Mammalian Species Using a Stacking Species Distribution Model in a Temperate Forest. Animals 2024, 14, 759. [Google Scholar] [CrossRef]
- Wan, Z.; Hook, S.; Hulley, G. MOD11A2 MODIS/Aqua Land Surface Temperature and Emissivity 8-Day L3 Global 1km SIN Grid V061; NASA Land Processes Distributed Active Archive Center: Sioux Falls, SD, USA, 2021. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Roy, D.P.; Kovalskyy, V.; Zhang, H.K.; Vermote, E.F.; Yan, L.; Kumar, S.S.; Egorov, A. Characterization of Landsat-7 to Landsat-8 Reflective Wavelength and Normalized Difference Vegetation Index Continuity. Remote Sens. Environ. 2016, 185, 57–70. [Google Scholar] [CrossRef]
- Reichle, R.; De Lannoy, G.; Koster, R.; Crow, W.; Kimball, J.; Liu, Q. SMAP L4 Global 3-Hourly 9 Km EASE-Grid Surface and Root Zone Soil Moisture Analysis Update, Version 4; NASA National Snow and Ice Data Center Distributed Active Archive Center: Boulder, CO, USA, 2018. [Google Scholar]
- Karamvasis, K.; Karathanassi, V. Soil Moisture Estimation from Sentinel-1 Interferometric Observations over Arid Regions. Comput. Geosci. 2023, 178, 105410. [Google Scholar] [CrossRef]
- Mueller, N.; Lewis, A.; Roberts, D.; Ring, S.; Melrose, R.; Sixsmith, J.; Lymburner, L.; McIntyre, A.; Tan, P.; Curnow, S.; et al. Water Observations from Space: Mapping Surface Water from 25years of Landsat Imagery across Australia. Remote Sens. Environ. 2016, 174, 341–352. [Google Scholar] [CrossRef]
- Max, A.; Jackson, S.; Cimentada, J.; Kuhn, M.M. Package ‘Corrr.’. 2022. Available online: https://CRAN.R-project.org/package=corrr (accessed on 17 April 2025).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Casas, E.; Martín-García, L.; Hernández-Leal, P.; Arbelo, M. Species Distribution Models at Regional Scale: Cymodocea Nodosa Seagrasses. Remote Sens. 2022, 14, 4334. [Google Scholar] [CrossRef]
- Gao, H.; Dou, H.; Wang, K.; Zhang, Y.; Hua, Y. Ensemble SDMs Reveal the Effect of Environmental Suitability and Nature Reserves on Conserving Chinese Pangolins in Guangdong, China. J. Nat. Conserv. 2024, 79, 126617. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; Elith, J.; Dudík, M.; Ferrier, S.; Huettmann, F.; et al. Effects of Sample Size on the Performance of Species Distribution Models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Barnes, M.A.; Jerde, C.L.; Wittmann, M.E.; Chadderton, W.L.; Ding, J.; Zhang, J.; Purcell, M.; Budhathoki, M.; Lodge, D.M. Geographic Selection Bias of Occurrence Data Influences Transferability of Invasive Hydrilla Verticillata Distribution Models. Ecol. Evol. 2014, 4, 2584–2593. [Google Scholar] [CrossRef]
- Steen, B.; Broennimann, O.; Maiorano, L.; Guisan, A. How Sensitive Are Species Distribution Models to Different Background Point Selection Strategies? A Test with Species at Various Equilibrium Levels. Ecol. Modell. 2024, 493, 110754. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive Performance of Presence-Only Species Distribution Models: A Benchmark Study with Reproducible Code. Ecol. Monogr. 2022, 92, e1486. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting Species Distributions from Small Numbers of Occurrence Records: A Test Case Using Cryptic Geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Hijmans, A.R.J.; Phillips, S.; Leathwick, J.; Elith, J.; Hijmans, M.R.J. Package ‘Dismo.’. 2023. Available online: https://CRAN.R-project.org/package=dismo (accessed on 17 April 2025).
- Kass, M.J.M.; Jamie, A.; Kass, M.; Muscarella, R.; Galante, P.J.; Buitrago-pinilla, G.E.; Boria, R.A.; Anderson, R.P. Package ‘ENMeval.’. 2025. Available online: https://CRAN.R-project.org/package=ENMeval (accessed on 17 April 2025).
- Package, T.; Phillips, A.S. Package ‘Maxnet.’. 2022, pp. 1–7. Available online: https://CRAN.R-project.org/package=maxnet (accessed on 17 April 2025).
- Sanchez, J. CRAN: Package PROC. 2023. Available online: https://CRAN.R-project.org/package=pROC (accessed on 17 April 2025).
- Mazerolle, M.J. Package ‘AICcmodavg.’. 2025. Available online: https://CRAN.R-project.org/package=AICcmodavg (accessed on 17 April 2025).
- Calabrese, J.M.; Certain, G.; Kraan, C.; Dormann, C.F. Stacking Species Distribution Models and Adjusting Bias by Linking Them to Macroecological Models. Glob. Ecol. Biogeogr. 2014, 23, 99–112. [Google Scholar] [CrossRef]
- van Etten, J.; Sumner, M.; Cheng, J.; Baston, D.; Bevan, A.; Bivand, R.; Busetto, L.; Canty, M.; Fasoli, B.; Forrest, D.; et al. Package ‘Raster.’. 2025. Available online: https://CRAN.R-project.org/package=raster (accessed on 17 April 2025).
- Jiménez-Valverde, A.; Lobo, J.M. Threshold Criteria for Conversion of Probability of Species Presence to Either-or Presence-Absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Abade, L.; Macdonald, D.W.; Dickman, A.J. Using Landscape and Bioclimatic Features to Predict the Distribution of Lions, Leopards and Spotted Hyaenas in Tanzania’s Ruaha Landscape. PLoS ONE 2014, 9, e96261. [Google Scholar] [CrossRef] [PubMed]
- John, E.; Bunting, P.; Hardy, A.; Roberts, O.; Giliba, R.; Silayo, D.S. Modelling the Impact of Climate Change on Tanzanian Forests. Divers. Distrib. 2020, 26, 1663–1686. [Google Scholar] [CrossRef]
- Eustace, A.; Esser, L.F.; Mremi, R.; Malonza, P.K.; Mwaya, R.T. Protected Areas Network Is Not Adequate to Protect a Critically Endangered East Africa Chelonian: Modelling Distribution of Pancake Tortoise, Malacochersus Tornieri under Current and Future Climates. PLoS ONE 2021, 16, e0238669. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, Y. Comparing Species Distributions Modelled from Occurrence Data and from Expert-Based Range Maps. Implication for Predicting Range Shifts with Climate Change. Ecol. Inform. 2016, 36, 8–14. [Google Scholar] [CrossRef]
- Niamir, A.; Skidmore, A.K.; Muñoz, A.R.; Toxopeus, A.G.; Real, R. Incorporating Knowledge Uncertainty into Species Distribution Modelling. Biodivers. Conserv. 2019, 28, 571–588. [Google Scholar] [CrossRef]
- Wang, L.; Diao, C.; Lu, Y. The Role of Remote Sensing in Species Distribution Models: A Review. Int. J. Remote Sens. 2024, 46, 661–685. [Google Scholar] [CrossRef]
- van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum Required Number of Specimen Records to Develop Accurate Species Distribution Models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Moudrý, V.; Bazzichetto, M.; Remelgado, R.; Devillers, R.; Lenoir, J.; Mateo, R.G.; Lembrechts, J.J.; Sillero, N.; Lecours, V.; Cord, A.F.; et al. Optimising Species Distribution Models: Sample Size, Positional Error, and Sampling Bias Matter. EcoevoRxiv 2023. [Google Scholar] [CrossRef]
- Soley-Guardia, M.; Alvarado-Serrano, D.F.; Anderson, R.P. Top Ten Hazards to Avoid When Modeling Species Distributions: A Didactic Guide of Assumptions, Problems, and Recommendations. Ecography 2024, 2024, e06852. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P.; Liu, C. Modelling Species Distributions in Britain: A Hierarchical. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Koma, Z.; Seijmonsbergen, A.C.; Grootes, M.W.; Nattino, F.; Groot, J.; Sierdsema, H.; Foppen, R.P.B.; Kissling, W.D. Better Together? Assessing Different Remote Sensing Products for Predicting Habitat Suitability of Wetland Birds. Divers. Distrib. 2022, 28, 685–699. [Google Scholar] [CrossRef]
- Iralu, V.I.; Upadhaya, K. Integrating Ecological Niche Modeling and Land Use Analysis for Targeted Conservation of Elaeocarpus Prunifolius in India. Res. Sq. 2024, 1–15. [Google Scholar] [CrossRef]
- Loveland, T.R.; Reed, B.C.; Ohlen, D.O.; Brown, J.F.; Zhu, Z.; Yang, L.; Merchant, J.W. Development of a Global Land Cover Characteristics Database and IGBP DISCover from 1 Km AVHRR Data. Int. J. Remote Sens. 2000, 21, 1303–1330. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, L.; Zhang, X.; Chen, X.; Mi, J.; Xie, S. Consistency Analysis and Accuracy Assessment of Three Global 30-m Land-Cover Products over the European Union Using the Lucas Dataset. Remote Sens. 2020, 12, 3479. [Google Scholar] [CrossRef]
- Pandey, P.C.; Koutsias, N.; Petropoulos, G.P.; Srivastava, P.K.; Ben Dor, E. Land Use/Land Cover in View of Earth Observation: Data Sources, Input Dimensions, and Classifiers—A Review of the State of the Art. Geocarto Int. 2021, 36, 957–988. [Google Scholar] [CrossRef]
- Cánibe, M.; Titeux, N.; Domínguez, J.; Regos, A. Assessing the Uncertainty Arising from Standard Land-Cover Mapping Procedures When Modelling Species Distributions. Divers. Distrib. 2022, 28, 636–648. [Google Scholar] [CrossRef]
- Sanguet, A.; Wyler, N.; Petitpierre, B.; Honeck, E.; Poussin, C.; Martin, P.; Lehmann, A. Beyond Topo-Climatic Predictors: Does Habitats Distribution and Remote Sensing Information Improve Predictions of Species Distribution Models? Glob. Ecol. Conserv. 2022, 39, e02286. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Booth, T.H. Checking Bioclimatic Variables That Combine Temperature and Precipitation Data before Their Use in Species Distribution Models. Austral Ecol. 2022, 47, 1506–1514. [Google Scholar] [CrossRef]
- Bede-Fazekas, Á.; Somodi, I. Precipitation and Temperature Timings Underlying Bioclimatic Variables Rearrange under Climate Change Globally. Glob. Change Biol. 2024, 30, e17496. [Google Scholar] [CrossRef]
- Wango, T.J.L.; Musiega, D.; Mundia, C.N. Assessing the Suitability of the WorldClim Dataset for Ecological Studies in Southern Kenya. J. Geogr. Inf. Syst. 2018, 10, 643–658. [Google Scholar] [CrossRef][Green Version]
- Berio Fortini, L.; Kaiser, L.R.; Xue, L.; Wang, Y. Bioclimatic Variables Dataset for Baseline and Future Climate Scenarios for Climate Change Studies in Hawai’i. Data Br. 2022, 45, 108572. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.A. Use of Maximum Entropy Modeling in Wildlife Research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver Operating Characteristic Curve Analysis in Diagnostic Accuracy Studies: A Guide to Interpreting the Area under the Curve Value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef]
- Mpakairi, K.S.; Ndaimani, H.; Tagwireyi, P.; Gara, T.W.; Zvidzai, M.; Madhlamoto, D. Missing in Action: Species Competition Is a Neglected Predictor Variable in Species Distribution Modelling. PLoS ONE 2017, 12, e0181088. [Google Scholar] [CrossRef] [PubMed]
- Giliba, R.A.; Kiffner, C.; Fust, P.; Loos, J. Modelling Elephant Corridors over Two Decades Reveals Opportunities for Conserving Connectivity across a Large Protected Area Network. PLoS ONE 2023, 18, e0292918. [Google Scholar] [CrossRef]
- Montes, E.; Lefcheck, J.S.; Guerra-Castro, E.; Klein, E.; Kavanaugh, M.T.; de Azevedo Mazzuco, A.C.; Bigatti, G.; Cordeiro, C.A.M.M.; Simoes, N.; Macaya, E.C.; et al. Optimizing Large-Scale Biodiversity Sampling Effort Toward an Unbalanced Survey Design. Oceanography 2021, 34, 80–91. [Google Scholar] [CrossRef]
- Porfirio, L.L.; Harris, R.M.B.; Lefroy, E.C.; Hugh, S.; Gould, S.F.; Lee, G.; Bindoff, N.L.; Mackey, B. Improving the Use of Species Distribution Models in Conservation Planning and Management under Climate Change. PLoS ONE 2014, 9, e113749. [Google Scholar] [CrossRef]
- Franklin, J. Species Distribution Models in Conservation Biogeography: Developments and Challenges. Divers. Distrib. 2013, 19, 1217–1223. [Google Scholar] [CrossRef]
- Thuiller, W.; Broennimann, O.; Hughes, G.; Alkemade, J.R.M.; Midgley, G.F.; Corsi, F. Vulnerability of African Mammals to Anthropogenic Climate Change under Conservative Land Transformation Assumptions. Glob. Change Biol. 2006, 12, 424–440. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- East African Community (EAC). Conservation and Management of Natural Capital in East African Community Programme (2019–2022); EAC: Arusha, Tanzania, 2022; Available online: https://www.eac.int/environment/programmes-and-projects/management-of-natural-capital (accessed on 1 July 2025).
- Thorne, J.H.; Boynton, R.M.; Hollander, A.D.; Flint, L.E.; Flint, A.L.; Urban, D. The Contribution of Microrefugia to Landscape Thermal Inertia for Climate-Adaptive Conservation Strategies. Earth’s Future 2023, 11, e2022EF003338. [Google Scholar] [CrossRef]
- Bukombe, J.; Marealle, W.; Kimaro, J.; Kija, H.; Kavana, P.; Kakengi, V.; Nindi, J.; Keyyu, J.; Ntalwila, J.; Kilimba, N.; et al. Viability Assessment of the Wami-Mbiki Game Reserve to Nyerere National Park Wildlife Corridor in Southern Tanzania. Glob. Ecol. Conserv. 2022, 39, e02259. [Google Scholar] [CrossRef]
- Jones, T.; Bamford, A.J.; Ferrol-Schulte, D.; Hieronimo, P.; Mcwilliam, N.; Rovero, F. Vanishing Wildlife Corridors and Options for Restoration: A Case Study from Tanzania. Trop. Conserv. Sci. 2012, 5, 463–474. [Google Scholar] [CrossRef]
- Debonnet, G.; Nindi, S. Technical Study on Land Use and Tenure Options and Status of Wildlife Corridors in Tanzania; An Input to the Preparation of Corridor Regulations; United States Agency for International Development: Washington, DC, USA, 2017; 106p. [Google Scholar]
- Russell-Smith, J.; Yates, C.; Vernooij, R.; Eames, T.; Lucas, D.; Mbindo, K.; Banda, S.; Mukoma, K.; Kaluka, A.; Liseli, A.; et al. Framework for a Savanna Burning Emissions Abatement Methodology Applicable to Fire-Prone Miombo Woodlands in Southern Africa. Int. J. Wildl. Fire 2024, 33, WF23193. [Google Scholar] [CrossRef]
- Mwitalemi, S.S.; Kantoush, S.A.; Nguyen, B.Q. Effects of Cascading Dams on Streamflow within the Downstream Areas of the Rufiji River Basin in Tanzania. Hydrology 2024, 11, 69. [Google Scholar] [CrossRef]
- Ten Caten, C.; Dallas, T. Thinning Occurrence Points Does Not Improve Species Distribution Model Performance. Ecosphere 2023, 14, e4703. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).