Remote Sensing-Based Phenology of Dryland Vegetation: Contributions and Perspectives in the Southern Hemisphere
Abstract
1. Introduction
2. Methods
3. Phenology of Natural Vegetation in Drylands
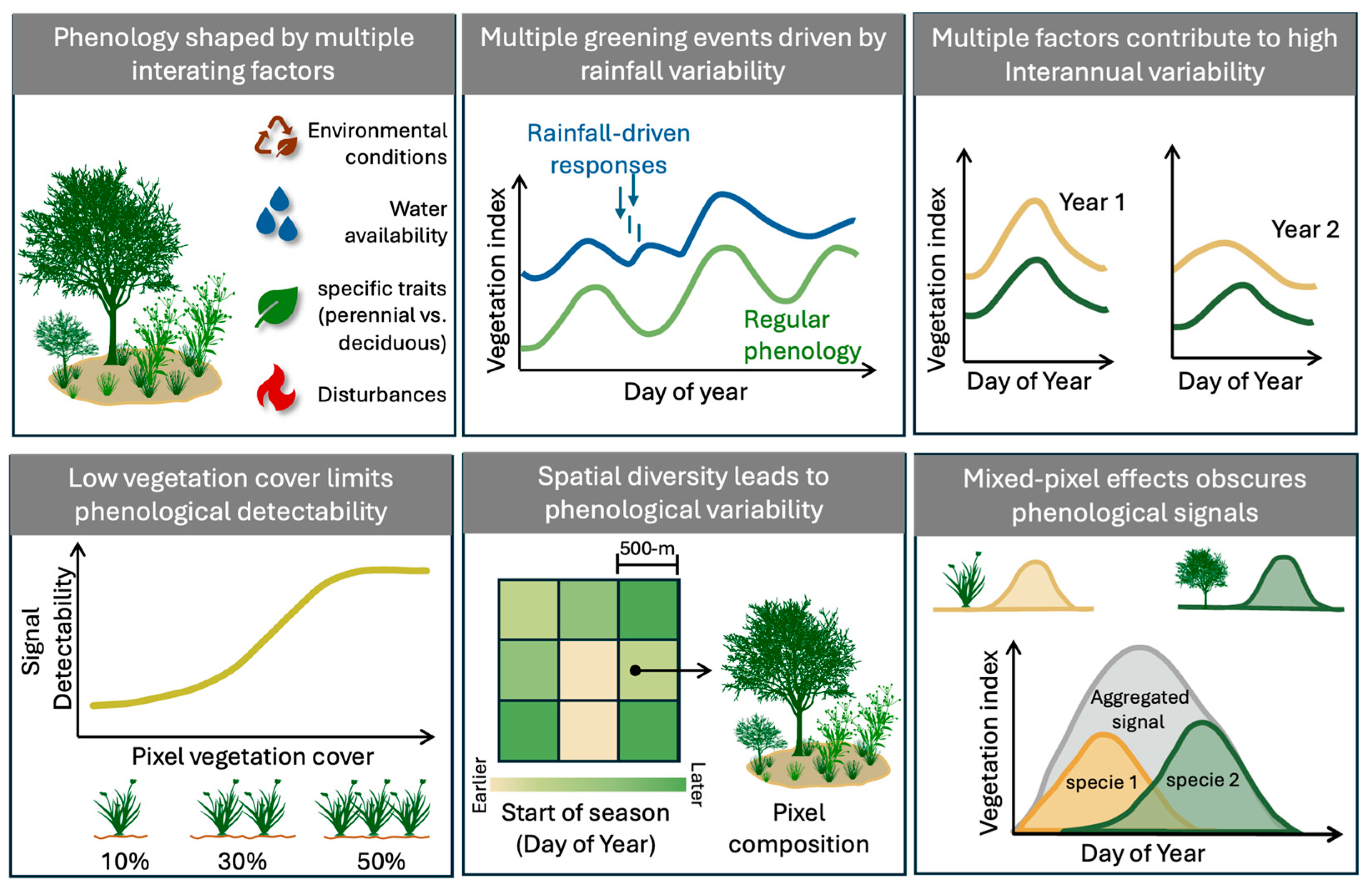
3.1. Characterization of Drylands and Vegetation Dynamics
3.2. Phenology of Natural Dryland Vegetation
4. Remote Sensing-Based Phenology: Concepts and Advances
4.1. Phenological Metrics
4.2. Time Series
4.2.1. Orbital Sensors
4.2.2. Vegetation Indices
4.2.3. Smoothing and Reconstruction of Time Series
4.2.4. Methods for Extracting Phenological Metrics
4.3. LSP Ready-to-Use Products
4.4. Ground and Near-Surface Phenology
5. Contributions, Limitations, and Perspectives of Land Surface Phenology for Dryland Ecosystems in the Southern Hemisphere
5.1. Current Developments and Limitations
5.2. Perspectives and Emerging Opportunities
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVHRR | Advanced Very High-Resolution Radiometer |
| BRDF | Bidirectional Reflectance Distribution Function |
| CCR | Curvature Change Rate |
| CIgreen | Green Chlorophyll Index |
| CIRed-edge | Red-Edge Chlorophyll Index |
| CO2 | Carbon Dioxide |
| DOY | Day of the Year |
| EOS | End of Season (end of growing season) |
| ESA | European Space Agency |
| EVI | Enhanced Vegetation Index |
| FAO | Forestry and Agriculture Organization |
| FOV | Field of View |
| fPAR | Fraction of Photosynthetically Active Radiation |
| GCC | Green Chromatic Channel |
| GEDI | Global Ecosystem Dynamics Investigation |
| GOME | Global Ozone Monitoring Experiment |
| HIMAWARI | Geostationary Meteorological Satellite |
| HLS | Harmonized Landsat Sentinel-2 |
| IVs | Vegetation Indices |
| LAI | Leaf Area Index |
| LiDAR | Light Detection and Ranging |
| LOS | Length of Season (length of growing season) |
| LSP | Land Surface Phenology |
| MAPBIOMAS | Annual Land Use and Cover Mapping Project in Brazil |
| MCD12Q2 | MODIS Land Cover Dynamics Product |
| MERIS | Medium-Resolution Imaging Spectrometer |
| MODIS | Moderate-Resolution Imaging Spectroradiometer |
| MSI | Multispectral Instrument |
| NASA | National Aeronautics and Space Administration |
| NDPI | Normalized Difference Phenology Index |
| NDRE | Normalized Difference Red Edge |
| NDVI | Normalized Difference Vegetation Index |
| NIR | Near-Infrared |
| NOAA | National Oceanic and Atmospheric Administration |
| OLI | Operational Land Imager |
| POS | Peak of Season (Peak of the growing season) |
| PRISMA | Precursor IperSpetrale della Missione Applicativa |
| RCR | Relative Change Rate |
| SAR | Synthetic Aperture Radar |
| SAVI | Soil-Adjusted Vegetation Index |
| SIF | Solar-Induced Chlorophyll Fluorescence |
| SOS | Start of Season (beginning of the growing season) |
| SPOT | Satellite Pour l’Observation of Terre Vegetation |
| SR | Remote Sensing |
| SH | Southern Hemisphere |
| SWIR | Short-Wave Infrared (Mid-Infrared) |
| TIR | Thermal Infrared |
| TM | Thematic Mapper |
| UAVs | Unmanned Aerial Vehicles |
| VIIRS | Visible Infrared Imaging Radiometer Suite |
| VIPPHEN | Global Vegetation Index and Phenology Multi-Sensor Phenology |
| VOD | Vegetation Optical Depth |
Appendix A
References
- Lieth, H. Purposes of a Phenology Book. In Phenology and Seasonality Modeling; Lieth, H., Ed.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 1974; Volume 8, pp. 3–19. ISBN 978-3-642-51865-2. [Google Scholar]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant Phenology and Global Climate Change: Current Progresses and Challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Helman, D. Land Surface Phenology: What Do We Really ‘See’ from Space? Sci. Total Environ. 2018, 618, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Mbow, C.; Brandt, M.; Ouedraogo, I.; De Leeuw, J.; Marshall, M. What Four Decades of Earth Observation Tell Us about Land Degradation in the Sahel? Remote Sens. 2015, 7, 4048–4067. [Google Scholar] [CrossRef]
- Brandt, M.; Tappan, G.; Diouf, A.; Beye, G.; Mbow, C.; Fensholt, R. Woody Vegetation Die off and Regeneration in Response to Rainfall Variability in the West African Sahel. Remote Sens. 2017, 9, 39. [Google Scholar] [CrossRef]
- Breshears, D.D. The Grassland–Forest Continuum: Trends in Ecosystem Properties for Woody Plant Mosaics? Front. Ecol. Environ. 2006, 4, 96–104. [Google Scholar] [CrossRef]
- Smith, W.K.; Dannenberg, M.P.; Yan, D.; Herrmann, S.; Barnes, M.L.; Barron-Gafford, G.A.; Biederman, J.A.; Ferrenberg, S.; Fox, A.M.; Hudson, A.; et al. Remote Sensing of Dryland Ecosystem Structure and Function: Progress, Challenges, and Opportunities. Remote Sens. Environ. 2019, 233, 111401. [Google Scholar] [CrossRef]
- Berra, E.F.; Gaulton, R. Remote Sensing of Temperate and Boreal Forest Phenology: A Review of Progress, Challenges and Opportunities in the Intercomparison of in-Situ and Satellite Phenological Metrics. For. Ecol. Manag. 2021, 480, 118663. [Google Scholar] [CrossRef]
- United Nations Convention to Combat Desertification. Part Two: The outlook. In The Global Land Outlook, 1st ed.; UNCCD: Bonn, Germany, 2017; pp. 246–269. ISBN 978-92-95110-48-9. [Google Scholar]
- FAO. Trees, Forests and Land Use in Drylands: The First Global Assessment—Full Report; FAO: Rome, Italy, 2019; ISBN 978-92-5-131999-4. [Google Scholar]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global Desertification: Building a Science for Dryland Development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Elouafi, I. Drylands under Pressure: Science and Solutions for Global Stability. Science 2025, 387, eadv6563. [Google Scholar] [CrossRef] [PubMed]
- Hanan, N.P.; Milne, E.; Aynekulu, E.; Yu, Q.; Anchang, J. A Role for Drylands in a Carbon Neutral World? Front. Environ. Sci. 2021, 9, 786087. [Google Scholar] [CrossRef]
- Marengo, J.A.; Jones, R.; Alves, L.M.; Valverde, M.C. Future Change of Temperature and Precipitation Extremes in South America as Derived from the PRECIS Regional Climate Modeling System. Int. J. Climatol. 2009, 29, 2241–2255. [Google Scholar] [CrossRef]
- Feng, S.; Fu, Q. Expansion of Global Drylands under a Warming Climate. Atmos. Chem. Phys. 2013, 13, 10081–10094. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Fu, C.; Chen, F.; Fu, Q.; Dai, A.; Shinoda, M.; Ma, Z.; Guo, W.; Li, Z.; et al. Dryland Climate Change: Recent Progress and Challenges. Rev. Geophys. 2017, 55, 719–778. [Google Scholar] [CrossRef]
- Wang, C.; Tang, Y.; Chen, J. Plant Phenological Synchrony Increases under Rapid Within-Spring Warming. Sci. Rep. 2016, 6, 25460. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B.; et al. Attributing Physical and Biological Impacts to Anthropogenic Climate Change. Nature 2008, 453, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wardlow, B.D.; Xiang, D.; Hu, S.; Li, D. A Review of Vegetation Phenological Metrics Extraction Using Time-Series, Multispectral Satellite Data. Remote Sens. Environ. 2020, 237, 111511. [Google Scholar] [CrossRef]
- Younes, N.; Joyce, K.E.; Maier, S.W. All Models of Satellite-Derived Phenology Are Wrong, but Some Are Useful: A Case Study from Northern Australia. Int. J. Appl. Earth Obs. Geoinf. 2021, 97, 102285. [Google Scholar] [CrossRef]
- De Beurs, K.M.; Henebry, G.M. Land Surface Phenology and Temperature Variation in the International Geosphere–Biosphere Program High-latitude Transects. Glob. Change Biol. 2005, 11, 779–790. [Google Scholar] [CrossRef]
- Jochner, S.; Sparks, T.H.; Laube, J.; Menzel, A. Can We Detect a Nonlinear Response to Temperature in European Plant Phenology? Int. J. Biometeorol. 2016, 60, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.E.; Altwegg, R.; Barbraud, C.; Barnard, P.; Beaumont, L.J.; Crawford, R.J.M.; Durant, J.M.; Hughes, L.; Keatley, M.R.; Low, M.; et al. Phenological Changes in the Southern Hemisphere. PLoS ONE 2013, 8, e75514. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, K.; Bush, E.R.; Forget, P.; Mendoza, I.; Morellato, L.P.C. Current Issues in Tropical Phenology: A Synthesis. Biotropica 2018, 50, 477–482. [Google Scholar] [CrossRef]
- Taylor, S.D.; Browning, D.M.; Baca, R.A.; Gao, F. Constraints and Opportunities for Detecting Land Surface Phenology in Drylands. J. Remote Sens. 2021, 2021, 9859103. [Google Scholar] [CrossRef]
- Maestre, F.T.; Benito, B.M.; Berdugo, M.; Concostrina-Zubiri, L.; Delgado-Baquerizo, M.; Eldridge, D.J.; Guirado, E.; Gross, N.; Kéfi, S.; Le Bagousse-Pinguet, Y.; et al. Biogeography of Global Drylands. New Phytol. 2021, 231, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Ganem, K.A.; Xue, Y.; Rodrigues, A.D.A.; Franca-Rocha, W.; Oliveira, M.T.D.; Carvalho, N.S.D.; Cayo, E.Y.T.; Rosa, M.R.; Dutra, A.C.; Shimabukuro, Y.E. Mapping South America’s Drylands through Remote Sensing—A Review of the Methodological Trends and Current Challenges. Remote Sens. 2022, 14, 736. [Google Scholar] [CrossRef]
- Wang, C.; Beringer, J.; Hutley, L.B.; Cleverly, J.; Li, J.; Liu, Q.; Sun, Y. Phenology Dynamics of Dryland Ecosystems Along the North Australian Tropical Transect Revealed by Satellite Solar-Induced Chlorophyll Fluorescence. Geophys. Res. Lett. 2019, 46, 5294–5302. [Google Scholar] [CrossRef]
- Revermann, R.; Finckh, M.; Stellmes, M.; Strohbach, B.J.; Frantz, D.; Oldeland, J. Linking Land Surface Phenology and Vegetation-Plot Databases to Model Terrestrial Plant α-Diversity of the Okavango Basin. Remote Sens. 2016, 8, 370. [Google Scholar] [CrossRef]
- Currier, C.M.; Sala, O.E. Precipitation versus Temperature as Phenology Controls in Drylands. Ecology 2022, 103, e3793. [Google Scholar] [CrossRef] [PubMed]
- Celleri, C.; Zapperi, G.; González Trilla, G.; Pratolongo, P. Spatial and Temporal Patterns of Rainfall Variability and Its Relationship with Land Surface Phenology in Central East Argentina. Int. J. Climatol. 2018, 38, 3963–3975. [Google Scholar] [CrossRef]
- Diogo, A.M.; de Freitas, M.W.D.; Nascimento, V.F.; Junior, C.W.M.; Barbosa, H.A. Spatial-Temporal Analysis of the Vegetation Phenology, Precipitation, and Evapotranspiration in Angola Based on Orbital Remote Sensing Images. Rev. Bras. Geogr. Fis. 2021, 14, 4096–4125. [Google Scholar] [CrossRef]
- Stan, K.D.; Sanchez-Azofeifa, A.; Duran, S.M.; Guzman, Q.J.A.; Hesketh, M.; Laakso, K.; Portillo-Quintero, C.; Rankine, C.; Doetterl, S. Tropical Dry Forest Resilience and Water Use Efficiency: An Analysis of Productivity under Climate Change. Environ. Res. Lett. 2021, 16, 054027. [Google Scholar] [CrossRef]
- Xie, Q.; Huete, A.; Hall, C.C.; Medlyn, B.E.; Power, S.A.; Davies, J.M.; Medek, D.E.; Beggs, P.J. Satellite-Observed Shifts in C3/C4 Abundance in Australian Grasslands Are Associated with Rainfall Patterns. Remote Sens. Environ. 2022, 273, 112983. [Google Scholar] [CrossRef]
- Ruiz-Díaz, S.; Pérez De Molas, L.F.; Benítez-León, E.; Almeyda Zambrano, A.M.; Johnson, D.J.; Bohlman, S.; Broadbent, E.N. Bioclimatic Predictors of Forest Structure, Composition and Phenology in the Paraguayan Dry Chaco. J. Trop. Ecol. 2024, 40, e1. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Jing, H.; Yue, L.; Zhang, Q.; Fan, L.; Yuan, Q.; Shen, H.; Zhang, L. A Global Daily Seamless 9-Km Vegetation Optical Depth (VOD) Product from 2010 to 202. Earth Syst. Sci. Data 2025, 17, 2849–2872. [Google Scholar] [CrossRef]
- Bacour, C.; Maignan, F.; Peylin, P.; MacBean, N.; Bastrikov, V.; Joiner, J.; Köhler, P.; Guanter, L.; Frankenberg, C. Differences Between OCO-2 and GOME-2 SIF Products From a Model-Data Fusion Perspective. J. Geophys. Res. Biogeosci. 2019, 124, 3143–3157. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- UNEP-WCMC. A Spatial Analysis Approach to the Global Delineation of Dryland Areas of Relevance to the CBD Programme of Work on Dry and Subhumid Lands, Cambridge. 2007. Available online: https://resources.unep-wcmc.org/products/789fcac8959943ab9ed7a225e5316f08 (accessed on 14 July 2025).
- Friedl, M.; Sulla-Menashe, D. MCD12Q1 MODIS/Terra+Aqua Land Cover Type Yearly L3 Global 500m SIN Grid V006 [Data Set]. NASA Land Processes Distributed Active Archive Center. 2019. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-mcd12q1-006 (accessed on 14 July 2025). [CrossRef]
- Didan, K. MODIS/Terra Vegetation Indices 16-Day L3 Global 1km SIN Grid V061 [Data Set]. NASA Land Processes Distributed Active Archive Center. 2021. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-mod13a2-061 (accessed on 14 July 2025). [CrossRef]
- Verbruggen, W.; Schurgers, G.; Horion, S.; Ardö, J.; Bernardino, P.N.; Cappelaere, B.; Demarty, J.; Fensholt, R.; Kergoat, L.; Sibret, T.; et al. Contrasting Responses of Woody and Herbaceous Vegetation to Altered Rainfall Characteristics in the Sahel. Biogeosciences 2021, 18, 77–93. [Google Scholar] [CrossRef]
- Whitley, R.; Beringer, J.; Hutley, L.B.; Abramowitz, G.; De Kauwe, M.G.; Evans, B.; Haverd, V.; Li, L.; Moore, C.; Ryu, Y.; et al. Challenges and Opportunities in Land Surface Modelling of Savanna Ecosystems. Biogeosciences 2017, 14, 4711–4732. [Google Scholar] [CrossRef]
- Lehmann, C.E.R.; Anderson, T.M.; Sankaran, M.; Higgins, S.I.; Archibald, S.; Hoffmann, W.A.; Hanan, N.P.; Williams, R.J.; Fensham, R.J.; Felfili, J.; et al. Savanna Vegetation-Fire-Climate Relationships Differ Among Continents. Science 2014, 343, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D. Phenology: An Integrative Environmental Science, 3rd ed.; Springer Nature: Cham, Switzerland, 2024; ISBN 978-3-031-75026-7. [Google Scholar] [CrossRef]
- Alberton, B.; Da Silva Torres, R.; Sanna Freire Silva, T.; Rocha, H.R.d.; S. B. Moura, M.; Morellato, L.P.C. Leafing Patterns and Drivers across Seasonally Dry Tropical Communities. Remote Sens. 2019, 11, 2267. [Google Scholar] [CrossRef]
- Moore, C.E.; Brown, T.; Keenan, T.F.; Duursma, R.A.; Van Dijk, A.I.J.M.; Beringer, J.; Culvenor, D.; Evans, B.; Huete, A.; Hutley, L.B.; et al. Reviews and Syntheses: Australian Vegetation Phenology: New Insights from Satellite Remote Sensingand Digital Repeat Photography. Biogeosciences 2016, 13, 5085–5102. [Google Scholar] [CrossRef]
- Clinton, N.; Yu, L.; Fu, H.; He, C.; Gong, P. Global-Scale Associations of Vegetation Phenology with Rainfall and Temperature at a High Spatio-Temporal Resolution. Remote Sens. 2014, 6, 7320–7338. [Google Scholar] [CrossRef]
- Feulner, G.; Rahmstorf, S.; Levermann, A.; Volkwardt, S. On the Origin of the Surface Air Temperature Difference between the Hemispheres in Earth’s Present-Day Climate. J. Clim. 2013, 26, 7136–7150. [Google Scholar] [CrossRef]
- Shi, S.; Yang, P.; Vrieling, A.; Tol, C.V.D. Vegetation Optimal Temperature Modulates Global Vegetation Season Onset Shifts in Response to Warming Climate. Commun. Earth Environ. 2025, 6, 203. [Google Scholar] [CrossRef]
- Nicholson, S.E. Dryland Climatology, 1st ed.; Cambridge University Press: Cambridge, UK, 2011; ISBN 978-0-521-51649-5. [Google Scholar]
- Jin, C.; Xiao, X.; Merbold, L.; Arneth, A.; Veenendaal, E.; Kutsch, W.L. Phenology and Gross Primary Production of Two Dominant Savanna Woodland Ecosystems in Southern Africa. Remote Sens. Environ. 2013, 135, 189–201. [Google Scholar] [CrossRef]
- Stevens, N.; Lehmann, C.E.R.; Murphy, B.P.; Durigan, G. Savanna Woody Encroachment Is Widespread across Three Continents. Glob. Change Biol. 2017, 23, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Paloschi, R.A.; Ramos, D.M.; Ventura, D.J.; Souza, R.; Souza, E.; Morellato, L.P.C.; Nóbrega, R.L.B.; Coutinho, Í.A.C.; Verhoef, A.; Körting, T.S.; et al. Environmental Drivers of Water Use for Caatinga Woody Plant Species: Combining Remote Sensing Phenology and Sap Flow Measurements. Remote Sens. 2021, 13, 75. [Google Scholar] [CrossRef]
- Peng, D.; Wang, Y.; Xian, G.; Huete, A.R.; Huang, W.; Shen, M.; Wang, F.; Yu, L.; Liu, L.; Xie, Q.; et al. Investigation of Land Surface Phenology Detections in Shrublands Using Multiple Scale Satellite Data. Remote Sens. Environ. 2021, 252, 112133. [Google Scholar] [CrossRef]
- Skidmore, A.K.; Coops, N.C.; Neinavaz, E.; Ali, A.; Schaepman, M.E.; Paganini, M.; Kissling, W.D.; Vihervaara, P.; Darvishzadeh, R.; Feilhauer, H.; et al. Priority List of Biodiversity Metrics to Observe from Space. Nat. Ecol. Evol. 2021, 5, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, F.; Shimabukuro, Y.; Kuplich, T. Sensoriamento Remoto da Vegetação; Oficina de Textos: São Paulo, Brasil, 2021; ISBN 978-85-7975-211-7. [Google Scholar]
- Ye, W.; Van Dijk, A.I.J.M.; Huete, A.; Yebra, M. Global Trends in Vegetation Seasonality in the GIMMS NDVI3g and Their Robustness. Int. J. Appl. Earth Obs. Geoinf. 2021, 94, 102238. [Google Scholar] [CrossRef]
- Lange, M.; Dechant, B.; Rebmann, C.; Vohland, M.; Cuntz, M.; Doktor, D. Validating MODIS and Sentinel-2 NDVI Products at a Temperate Deciduous Forest Site Using Two Independent Ground-Based Sensors. Sensors 2017, 17, 1855. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Restrepo-Coupe, N.; Huete, A.R. Multi-Scale Phenology of Temperate Grasslands: Improving Monitoring and Management with near-Surface Phenocams. Front. Environ. Sci. 2019, 7, 14. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Zhou, L.; Ciais, P.; Zhu, B. Variations in Satellite-derived Phenology in China’s Temperate Vegetation. Glob. Change Biol. 2006, 12, 672–685. [Google Scholar] [CrossRef]
- Matongera, T.N.; Mutanga, O.; Sibanda, M.; Odindi, J. Estimating and Monitoring Land Surface Phenology in Rangelands: A Review of Progress and Challenges. Remote Sens. 2021, 13, 2060. [Google Scholar] [CrossRef]
- Parplies, A.; Dubovyk, O.; Tewes, A.; Mund, J.-P.; Schellberg, J. Phenomapping of Rangelands in South Africa Using Time Series of RapidEye Data. Int. J. Appl. Earth Obs. Geoinf. 2016, 53, 90–102. [Google Scholar] [CrossRef]
- Lopes, A.P.; Nelson, B.W.; Wu, J.; Graça, P.M.L.D.A.; Tavares, J.V.; Prohaska, N.; Martins, G.A.; Saleska, S.R. Leaf Flush Drives Dry Season Green-up of the Central Amazon. Remote Sens. Environ. 2016, 182, 90–98. [Google Scholar] [CrossRef]
- Gonçalves, N.B.; Lopes, A.P.; Dalagnol, R.; Wu, J.; Pinho, D.M.; Nelson, B.W. Both Near-Surface and Satellite Remote Sensing Confirm Drought Legacy Effect on Tropical Forest Leaf Phenology after 2015/2016 ENSO Drought. Remote Sens. Environ. 2020, 237, 111489. [Google Scholar] [CrossRef]
- Wu, J.; Albert, L.P.; Lopes, A.P.; Restrepo-Coupe, N.; Hayek, M.; Wiedemann, K.T.; Guan, K.; Stark, S.C.; Christoffersen, B.; Prohaska, N.; et al. Leaf Development and Demography Explain Photosynthetic Seasonality in Amazon Evergreen Forests. Science 2016, 351, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.; Cawkwell, F.; Wingler, A. Status of Phenological Research Using Sentinel-2 Data: A Review. Remote Sens. 2020, 12, 2760. [Google Scholar] [CrossRef]
- Do Nascimento Bendini, H.; Garcia Fonseca, L.M.; Schwieder, M.; Sehn Körting, T.; Rufin, P.; Del Arco Sanches, I.; Leitão, P.J.; Hostert, P. Detailed Agricultural Land Classification in the Brazilian Cerrado Based on Phenological Information from Dense Satellite Image Time Series. Int. J. Appl. Earth Obs. Geoinf. 2019, 82, 101872. [Google Scholar] [CrossRef]
- Ding, C.; Liu, X.; Huang, F.; Li, Y.; Zou, X. Onset of Drying and Dormancy in Relation to Water Dynamics of Semi-Arid Grasslands from MODIS NDWI. Agric. For. Meteorol. 2017, 234–235, 22–30. [Google Scholar] [CrossRef]
- Bórnez, K.; Descals, A.; Verger, A.; Peñuelas, J. Land Surface Phenology from VEGETATION and PROBA-V Data. Assessment over Deciduous Forests. Int. J. Appl. Earth Obs. Geoinf. 2020, 84, 101974. [Google Scholar] [CrossRef]
- Gray, J.; Sulla-Menashe, D.; Friedl, M.A. User Guide to Collection 6 MODIS Land Cover Dynamics (MCD12Q2) Product; NASA EOSDIS Land Processes DAAC: Missoula, MT, USA, 2019. Available online: https://modis-land.gsfc.nasa.gov/pdf/MCD12Q2_Collection6_UserGuide.pdf (accessed on 14 July 2025).
- Didan, K.; Barreto, A. NASA MEaSUREs Vegetation Index and Phenology (VIP) Phenology NDVI Yearly Global 0.05Deg CMG [Data Set]. NASA Land Processes Distributed Active Archive Center. 2016. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-vipphen-ndvi-004 (accessed on 14 July 2025). [CrossRef]
- Mas, J.-F.; de Araújo, F.S. Assessing Landsat Images Availability and Its Effects on Phenological Metrics. Forests 2021, 12, 574. [Google Scholar] [CrossRef]
- Cizek, A.; Aplin, P.; Powell, I. Measuring the Timing of Woody Green-Up in African Savannas—Which Modis Data to Use? In Proceedings of the 2021 International Geoscience and Remote Sensing Symposium IGARSS, Brussels, Belgium, 11–16 July 2021; pp. 1398–1401. [Google Scholar] [CrossRef]
- Claverie, M.; Ju, J.; Masek, J.G.; Dungan, J.L.; Vermote, E.F.; Roger, J.-C.; Skakun, S.V.; Justice, C. The Harmonized Landsat and Sentinel-2 Surface Reflectance Data Set. Remote Sens. Environ. 2018, 219, 145–161. [Google Scholar] [CrossRef]
- Zhou, Q.; Rover, J.; Brown, J.; Worstell, B.; Howard, D.; Wu, Z.; Gallant, A.L.; Rundquist, B.; Burke, M. Monitoring Landscape Dynamics in Central U.S. Grasslands with Harmonized Landsat-8 and Sentinel-2 Time Series Data. Remote Sens. 2019, 11, 328. [Google Scholar] [CrossRef]
- Pastick, N.J.; Wylie, B.K.; Wu, Z. Spatiotemporal Analysis of Landsat-8 and Sentinel-2 Data to Support Monitoring of Dryland Ecosystems. Remote Sens. 2018, 10, 791. [Google Scholar] [CrossRef]
- Walker, J.J.; De Beurs, K.M.; Wynne, R.H.; Gao, F. Evaluation of Landsat and MODIS Data Fusion Products for Analysis of Dryland Forest Phenology. Remote Sens. Environ. 2012, 117, 381–393. [Google Scholar] [CrossRef]
- Liao, C.; Wang, J.; Dong, T.; Shang, J.; Liu, J.; Song, Y. Using Spatio-Temporal Fusion of Landsat-8 and MODIS Data to Derive Phenology, Biomass and Yield Estimates for Corn and Soybean. Sci. Total Environ. 2019, 650, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Masek, J.; Schwaller, M.; Hall, F. On the Blending of the Landsat and MODIS Surface Reflectance: Predicting Daily Landsat Surface Reflectance. IEEE Trans. Geosci. Remote Sens. 2006, 44, 2207–2218. [Google Scholar] [CrossRef]
- Chávez, R.O.; Moreira-Muñoz, A.; Galleguillos, M.; Olea, M.; Aguayo, J.; Latín, A.; Aguilera-Betti, I.; Muñoz, A.A.; Manríquez, H. GIMMS NDVI Time Series Reveal the Extent, Duration, and Intensity of “Blooming Desert” Events in the Hyper-Arid Atacama Desert, Northern Chile. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 193–203. [Google Scholar] [CrossRef]
- Kombani, L.; Adelabu, S.A.; Durowoju, O.S.; Jackson, C.M. Uncovering the Shifts: Land Surface Phenology in Botswana from Satellite Observations. Discov. Sustain. 2025, 6, 102. [Google Scholar] [CrossRef]
- Murwendo, T.; Murwira, A.; Masocha, M. Vegetation Phenology Patterns in Semi-Arid Savannah Woodlands of Gonarezhou National Park, Southeastern Zimbabwe. Int. J. Geoherit. Parks 2023, 11, 298–309. [Google Scholar] [CrossRef]
- Davis, C.L.; Hoffman, M.T.; Roberts, W. Long-Term Trends in Vegetation Phenology and Productivity over Namaqualand Using the GIMMS AVHRR NDVI3g Data from 1982 to 2011. S. Afr. J. Bot. 2017, 111, 76–85. [Google Scholar] [CrossRef]
- Huete, A.R.; Tucker, C.J. Investigation of Soil Influences in AVHRR Red and Near-Infrared Vegetation Index Imagery. Int. J. Remote Sens. 1991, 12, 1223–1242. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Kumari, N.; Yetemen, O.; Srivastava, A.; Rodriguez, J.F.; Saco, P.M. The Spatio-Temporal Ndvi Analysis for Two Different Australian Catchments. In Proceedings of the 23rd International Congress on Modelling and Simulation, Canberra, Australia, 1–6 December 2019; pp. 958–964. [Google Scholar]
- Kumari, N.; Saco, P.M.; Rodriguez, J.F.; Johnstone, S.A.; Srivastava, A.; Chun, K.P.; Yetemen, O. The Grass Is Not Always Greener on the Other Side: Seasonal Reversal of Vegetation Greenness in Aspect-Driven Semiarid Ecosystems. Geophys. Res. Lett. 2020, 47, e2020GL088918. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, H.; Wang, G.; Sun, H.; Fu, J. Mapping Paddy Rice Using a Convolutional Neural Network (CNN) with Landsat 8 Datasets in the Dongting Lake Area, China. Remote Sens. 2018, 10, 1840. [Google Scholar] [CrossRef]
- Friedl, M.; Gray, J.; Sulla-Menashe, D. MCD12Q2 MODIS/Terra+Aqua Land Cover Dynamics Yearly L3 Global 500m SIN Grid V006 [Data Set]. NASA Land Processes Distributed Active Archive Center. 2019. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-mcd12q2-006 (accessed on 14 July 2025). [CrossRef]
- Zhang, X. VIIRS/NPP Land Surface Phenology Yearly L3 Global 500m SIN Grid V002 [Data Set]. NASA Land Processes Distributed Active Archive Center. 2024. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-vnp22q2-002 (accessed on 14 July 2025). [CrossRef]
- Xu, D.; Wang, C.; Chen, J.; Shen, M.; Shen, B.; Yan, R.; Li, Z.; Karnieli, A.; Chen, J.; Yan, Y.; et al. The Superiority of the Normalized Difference Phenology Index (NDPI) for Estimating Grassland Aboveground Fresh Biomass. Remote Sens. Environ. 2021, 264, 112578. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M.; et al. Tracking Vegetation Phenology across Diverse North American Biomes Using PhenoCam Imagery. Sci. Data 2018, 5, 180028. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Scott, R.L.; Moore, D.J.P.; Biederman, J.A.; Smith, W.K. Understanding the Relationship between Vegetation Greenness and Productivity across Dryland Ecosystems through the Integration of PhenoCam, Satellite, and Eddy Covariance Data. Remote Sens. Environ. 2019, 223, 50–62. [Google Scholar] [CrossRef]
- Ma, M.; Veroustraete, F. Reconstructing Pathfinder AVHRR Land NDVI Time-Series Data for the Northwest of China. Adv. Space Res. 2006, 37, 835–840. [Google Scholar] [CrossRef]
- Julien, Y.; Sobrino, J.A. Comparison of Cloud-Reconstruction Methods for Time Series of Composite NDVI Data. Remote Sens. Environ. 2010, 114, 618–625. [Google Scholar] [CrossRef]
- Jonsson, P.; Eklundh, L. Seasonality Extraction by Function Fitting to Time-Series of Satellite Sensor Data. IEEE Trans. Geosci. Remote Sens. 2002, 40, 1824–1832. [Google Scholar] [CrossRef]
- Elmore, A.J.; Guinn, S.M.; Minsley, B.J.; Richardson, A.D. Landscape Controls on the Timing of Spring, Autumn, and Growing Season Length in Mid-Atlantic Forests. Glob. Change Biol. 2012, 18, 656–674. [Google Scholar] [CrossRef]
- Cao, R.; Chen, J.; Shen, M.; Tang, Y. An Improved Logistic Method for Detecting Spring Vegetation Phenology in Grasslands from MODIS EVI Time-Series Data. Agric. For. Meteorol. 2015, 200, 9–20. [Google Scholar] [CrossRef]
- Chen, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A Simple Method for Reconstructing a High-Quality NDVI Time-Series Data Set Based on the Savitzky–Golay Filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Cai, Z.; Jönsson, P.; Jin, H.; Eklundh, L. Performance of Smoothing Methods for Reconstructing NDVI Time-Series and Estimating Vegetation Phenology from MODIS Data. Remote Sens. 2017, 9, 1271. [Google Scholar] [CrossRef]
- Dixon, D.J.; Callow, J.N.; Duncan, J.M.A.; Setterfield, S.A.; Pauli, N. Satellite Prediction of Forest Flowering Phenology. Remote Sens. Environ. 2021, 255, 112197. [Google Scholar] [CrossRef]
- Ramos, D.M.; Andrade, J.M.; Alberton, B.C.; Moura, M.S.B.; Domingues, T.F.; Neves, N.; Lima, J.R.S.; Souza, R.; Souza, E.; Silva, J.R.; et al. Multiscale Phenology of Seasonally Dry Tropical Forests in an Aridity Gradient. Front. Environ. Sci. 2023, 11, 1275844. [Google Scholar] [CrossRef]
- Xin, Q.; Li, J.; Li, Z.; Li, Y.; Zhou, X. Evaluations and Comparisons of Rule-Based and Machine-Learning-Based Methods to Retrieve Satellite-Based Vegetation Phenology Using MODIS and USA National Phenology Network Data. Int. J. Appl. Earth Obs. Geoinf. 2020, 93, 102189. [Google Scholar] [CrossRef]
- Medeiros, R.; Andrade, J.; Ramos, D.; Moura, M.; Pérez-Marin, A.; Dos Santos, C.; Da Silva, B.; Cunha, J. Remote Sensing Phenology of the Brazilian Caatinga and Its Environmental Drivers. Remote Sens. 2022, 14, 2637. [Google Scholar] [CrossRef]
- Guzmán, L.M.; Villagra, P.E.; Quiroga, R.E.; Pereyra, D.I.; Pelliza, M.E.; Ricarte, A.R.; Blanco, L.J. In Search of Sustainable Livestock Management in the Dry Chaco: Effect of Different Shrub-Removal Practices on Vegetation. Rangel. J. 2022, 44, 193–202. [Google Scholar] [CrossRef]
- Landi, M.A.; Di Bella, C.M.; Bravo, S.J.; Bellis, L.M. Structural Resistance and Functional Resilience of the Chaco Forest to Wildland Fires: An Approach with MODIS Time Series. Austral Ecol. 2021, 46, 277–289. [Google Scholar] [CrossRef]
- Whitecross, M.A.; Witkowski, E.T.F.; Archibald, S. Savanna Tree-Grass Interactions: A Phenological Investigation of Green-up in Relation to Water Availability over Three Seasons. S. Afr. J. Bot. 2017, 108, 29–40. [Google Scholar] [CrossRef]
- Glade, F.E.; Miranda, M.D.; Meza, F.J.; van Leeuwen, W.J.D. Productivity and Phenological Responses of Natural Vegetation to Present and Future Inter-Annual Climate Variability across Semi-Arid River Basins in Chile. Environ. Monit. Assess. 2016, 188, 676. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huete, A.; Yu, Q.; Coupe, N.R.; Davies, K.; Broich, M.; Ratana, P.; Beringer, J.; Hutley, L.B.; Cleverly, J.; et al. Spatial Patterns and Temporal Dynamics in Savanna Vegetation Phenology across the North Australian Tropical Transect. Remote Sens. Environ. 2013, 139, 97–115. [Google Scholar] [CrossRef]
- Tan, B.; Morisette, J.T.; Wolfe, R.E.; Gao, F.; Ederer, G.A.; Nightingale, J.; Pedelty, J.A. An Enhanced TIMESAT Algorithm for Estimating Vegetation Phenology Metrics From MODIS Data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2011, 4, 361–371. [Google Scholar] [CrossRef]
- Reed, B.C.; Brown, J.F.; VanderZee, D.; Loveland, T.R.; Merchant, J.W.; Ohlen, D.O. Measuring Phenological Variability from Satellite Imagery. J. Veg. Sci. 1994, 5, 703–714. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring Vegetation Phenology Using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Chavana-Bryant, C.; Malhi, Y.; Wu, J.; Asner, G.P.; Anastasiou, A.; Enquist, B.J.; Cosio Caravasi, E.G.; Doughty, C.E.; Saleska, S.R.; Martin, R.E.; et al. Leaf Aging of Amazonian Canopy Trees as Revealed by Spectral and Physiochemical Measurements. New Phytol. 2017, 214, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Carlson, K.M.; Miura, T. Assessing the Inter-Annual Variability of Vegetation Phenological Events Observed from Satellite Vegetation Index Time Series in Dryland Sites. Ecol. Indic. 2021, 130, 108042. [Google Scholar] [CrossRef]
- Rosemartin, A.; Denny, E.G.; Gerst, K.L.; Marsh, R.L.; Posthumus, E.E.; Crimmins, T.M.; Weltzin, J.F. USA National Phenology Network observational data documentation: U.S. Geological Survey Open-File Report 2018-1060. 2018; 24p. Available online: https://pubs.usgs.gov/publication/ofr20181060 (accessed on 14 July 2025). [CrossRef]
- Ge, Q.; Dai, J.; Liu, H.; Xu, Q.; Wang, H. Typical Plant Phenological Observation Dataset of Chinese Phenological Observation Network, Beijing (1963–2012). Acta Geogr. Sin. 2014, 69, 95–97. [Google Scholar] [CrossRef]
- Templ, B.; Koch, E.; Bolmgren, K.; Ungersböck, M.; Paul, A.; Scheifinger, H.; Rutishauser, T.; Busto, M.; Chmielewski, F.-M.; Hájková, L.; et al. Pan European Phenological Database (PEP725): A Single Point of Access for European Data. Int. J. Biometeorol. 2018, 62, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Stoner, K.E.; Sánchez-Azofeifa, G.A. Ecology and Regeneration of Tropical Dry Forests in the Americas: Implications for Management. For. Ecol. Manag. 2009, 258, 903–906. [Google Scholar] [CrossRef]
- Rankine, C.; Sánchez-Azofeifa, G.A.; Guzmán, J.A.; Espirito-Santo, M.M.; Sharp, I. Comparing MODIS and Near-Surface Vegetation Indexes for Monitoring Tropical Dry Forest Phenology along a Successional Gradient Using Optical Phenology Towers. Environ. Res. Lett. 2017, 12, 105007. [Google Scholar] [CrossRef]
- Morellato, P.; Alberton, B.; Almeida, J.; Alex, J.; Mariano, G.; Torres, R. E-Phenology: Monitoring Leaf Phenology and Tracking Climate Changes in the Tropics. Geophysical Research Abstracts, 16, EGU2014-12020. EGU General Assembly 2014. Available online: https://meetingorganizer.copernicus.org/EGU2014/EGU2014-12020.pdf (accessed on 14 July 2025).
- TERN—Australia’s Terrestrial Ecosystem Research Network. Available online: https://www.tern.org.au/ (accessed on 2 June 2025).
- Cleverly, J. Alice Mulga Phenocam Images and Phenology Data Collection. 1.0 Terrestrial Ecosystem Research Network. Dataset. 2021. Available online: http://geonetwork.tern.org.au/geonetwork/srv/eng/catalog.search#/metadata/c3ebff5f-030a-48b7-ba1a-fee15a46f6b1 (accessed on 14 July 2025).
- Pinzon, J.E.; Pak, E.W.; Tucker, C.J.; Bhatt, U.S.; Frost, G.V.; Macander, M.J. Vegetation Collection Global Vegetation Greenness (NDVI) from AVHRR GIMMS-3G+, 1981–2022; ORNL DAAC: Oak Ridge, TN, USA, 2023. [CrossRef]
- Hu, P.; Sharifi, A.; Tahir, M.N.; Tariq, A.; Zhang, L.; Mumtaz, F.; Shah, S.H.I.A. Evaluation of Vegetation Indices and Phenological Metrics Using Time-Series MODIS Data for Monitoring Vegetation Change in Punjab, Pakistan. Water 2021, 13, 2550. [Google Scholar] [CrossRef]
- Ma, X.; Huete, A.; Tran, N.N.; Bi, J.; Gao, S.; Zeng, Y. Sun-Angle Effects on Remote-Sensing Phenology Observed and Modelled Using Himawari-8. Remote Sens. 2020, 12, 1339. [Google Scholar] [CrossRef]
- Ma, X.; Huete, A.; Tran, N.N. Interaction of Seasonal Sun-Angle and Savanna Phenology Observed and Modelled Using MODIS. Remote Sens. 2019, 11, 1398. [Google Scholar] [CrossRef]
- Zimba, H.M.; Coenders-Gerrits, M.; Banda, K.E.; Hulsman, P.; van de Giesen, N.; Nyambe, I.A.; Savenije, H.H.G. On the Importance of Plant Phenology in the Evaporative Process of a Semi-Arid Woodland: Could It Be Why Satellite-Based Evaporation Estimates in the Miombo Differ? Hydrol. Earth Syst. Sci. 2024, 28, 3633–3663. [Google Scholar] [CrossRef]
- Henriques, M.; McVicar, T.R.; Holland, K.L.; Daly, E. Extracting Vegetation Phenology in Heterogeneous Drylands Using LiDAR and Landsat Temporal Decomposition: A Latitudinal Assessment of Waterholes Within the Cooper Creek, Australia. J. Geophys. Res. Biogeosci. 2024, 129, e2023JG007993. [Google Scholar] [CrossRef]
- Ngubane, D.; Parrini, F.; de Lemos, H.; Ernst, Y. Matching Land Surface Phenology with the Phenology of Net Ecosystem Exchange (NEE) in the Kruger National Park, South Africa. Remote Sens. Appl. Soc. Environ. 2022, 28, 100840. [Google Scholar] [CrossRef]
- Leng, S.; Huete, A.; Cleverly, J.; Yu, Q.; Zhang, R.; Wang, Q. Spatiotemporal Variations of Dryland Vegetation Phenology Revealed by Satellite-Observed Fluorescence and Greenness across the North Australian Tropical Transect. Remote Sens. 2022, 14, 2985. [Google Scholar] [CrossRef]
- Merrick, T.; Pau, S.; Jorge, M.L.S.P.; Silva, T.S.F.; Bennartz, R. Spatiotemporal Patterns and Phenology of Tropical Vegetation Solar-Induced Chlorophyll Fluorescence across Brazilian Biomes Using Satellite Observations. Remote Sens. 2019, 11, 1746. [Google Scholar] [CrossRef]
- Cho, M.A.; Ramoelo, A.; Dziba, L. Response of Land Surface Phenology to Variation in Tree Cover during Green-up and Senescence Periods in the Semi-Arid Savanna of Southern Africa. Remote Sens. 2017, 9, 689. [Google Scholar] [CrossRef]
- Cui, L.; Shi, J. Evaluation and Comparison of Growing Season Metrics in Arid and Semi-Arid Areas of Northern China under Climate Change. Ecol. Indic. 2021, 121, 107055. [Google Scholar] [CrossRef]
- Cheng, Y.; Vrieling, A.; Fava, F.; Meroni, M.; Marshall, M.; Gachoki, S. Phenology of Short Vegetation Cycles in a Kenyan Rangeland from PlanetScope and Sentinel-2. Remote Sens. Environ. 2020, 248, 112004. [Google Scholar] [CrossRef]
- Harkort, L.; Okujeni, A.; Amputu, V.; Mahler, J.; Nill, L.; Pflugmacher, D.; Röder, A.; Hostert, P. Mapping Fractional Vegetation Cover in Sub-Saharan Rangelands Using Phenological Feature Spaces. Remote Sens. Environ. 2025, 319, 114646. [Google Scholar] [CrossRef]
- Marchesini, V.A.; Fernández, R.J.; Reynolds, J.F.; Sobrino, J.A.; Di Bella, C.M. Changes in Evapotranspiration and Phenology as Consequences of Shrub Removal in Dry Forests of Central Argentina. Ecohydrology 2015, 8, 1304–1311. [Google Scholar] [CrossRef]
- Steinaker, D.F.; Jobbágy, E.G.; Martini, J.P.; Arroyo, D.N.; Pacheco, J.L.; Marchesini, V.A. Vegetation Composition and Structure Changes Following Roller-Chopping Deforestation in Central Argentina Woodlands. J. Arid Environ. 2016, 133, 19–24. [Google Scholar] [CrossRef]
- Robinson, T.P.; Trotter, L.; Wardell-Johnson, G.W. Uncertainty Modelling of Groundwater-Dependent Vegetation. Land 2024, 13, 2208. [Google Scholar] [CrossRef]
- Xie, Q.; Moore, C.E.; Cleverly, J.; Hall, C.C.; Ding, Y.; Ma, X.; Leigh, A.; Huete, A. Land Surface Phenology Indicators Retrieved across Diverse Ecosystems Using a Modified Threshold Algorithm. Ecol. Indic. 2023, 147, 110000. [Google Scholar] [CrossRef]
- Walker, J.; De Beurs, K.; Wynne, R. Phenological Response of an Arizona Dryland Forest to Short-Term Climatic Extremes. Remote Sens. 2015, 7, 10832–10855. [Google Scholar] [CrossRef]
- Xie, Q.; Cleverly, J.; Moore, C.E.; Ding, Y.; Hall, C.C.; Ma, X.; Brown, L.A.; Wang, C.; Beringer, J.; Prober, S.M.; et al. Land Surface Phenology Retrievals for Arid and Semi-Arid Ecosystems. ISPRS J. Photogramm. Remote Sens. 2022, 185, 129–145. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Chen, J.; Wang, C.; Shen, M. The Mixed Pixel Effect in Land Surface Phenology: A Simulation Study. Remote Sens. Environ. 2018, 211, 338–344. [Google Scholar] [CrossRef]
- Dronova, I.; Taddeo, S. Remote Sensing of Phenology: Towards the Comprehensive Indicators of Plant Community Dynamics from Species to Regional Scales. J. Ecol. 2022, 110, 1460–1484. [Google Scholar] [CrossRef]
- van Blerk, J.J.; Slingsby, J.A.; West, A.G. Unpacking Satellite Pixels: UAVs Reveal Fine-Scale Drivers of Land Surface Phenology in a Winter Rainfall Shrubland. Environ. Res. Lett. 2024, 19, 084008. [Google Scholar] [CrossRef]
- Godlee, J.L.; Ryan, C.M.; Siampale, A.; Dexter, K.G. Tree Species Diversity Drives the Land Surface Phenology of Seasonally Dry Tropical Woodlands. J. Ecol. 2024, 112, 1978–1991. [Google Scholar] [CrossRef]
- White, M.A.; De Beurs, K.M.; Didan, K.; Inouye, D.W.; Richardson, A.D.; Jensen, O.P.; O’Keefe, J.; Zhang, G.; Nemani, R.R.; Van Leeuwen, W.J.D.; et al. Intercomparison, Interpretation, and Assessment of Spring Phenology in North America Estimated from Remote Sensing for 1982–2006. Glob. Change Biol. 2009, 15, 2335–2359. [Google Scholar] [CrossRef]
- Warter, M.M.; Singer, M.B.; Cuthbert, M.O.; Roberts, D.; Caylor, K.K.; Sabathier, R.; Stella, J. Modeling Seasonal Vegetation Phenology from Hydroclimatic Drivers for Contrasting Plant Functional Groups within Drylands of the Southwestern USA. Environ. Res. Ecol. 2023, 2, 025001. [Google Scholar] [CrossRef]
- Ganem, K.; Xue, Y.; Dutra, A.C.; Pareyn, F.G.C.; Shimabukuro, Y.E. From Rainforests to Drylands: A Context-Specific Framework for Mapping Land Use and Land Cover Dynamics in Northeast Brazil (2000–2020). GISci. Remote Sens. 2025, 62. [Google Scholar] [CrossRef]
- Souza, C.M.; Z. Shimbo, J.; Rosa, M.R.; Parente, L.L.; A. Alencar, A.; Rudorff, B.F.T.; Hasenack, H.; Matsumoto, M.; G. Ferreira, L.; Souza-Filho, P.W.M.; et al. Reconstructing Three Decades of Land Use and Land Cover Changes in Brazilian Biomes with Landsat Archive and Earth Engine. Remote Sens. 2020, 12, 2735. [Google Scholar] [CrossRef]
- Friedl, M.A.; Sulla-Menashe, D.; Tan, B.; Schneider, A.; Ramankutty, N.; Sibley, A.; Huang, X. MODIS Collection 5 Global Land Cover: Algorithm Refinements and Characterization of New Datasets. Remote Sens. Environ. 2010, 114, 168–182. [Google Scholar] [CrossRef]
- Yu, L.; Wang, J.; Gong, P. Improving 30 m Global Land-Cover Map FROM-GLC with Time Series MODIS and Auxiliary Data Sets: A Segmentation-Based Approach. Int. J. Remote Sens. 2013, 34, 5851–5867. [Google Scholar] [CrossRef]
- Buitenwerf, R.; Rose, L.; Higgins, S.I. Three Decades of Multi-Dimensional Change in Global Leaf Phenology. Nat. Clim. Change 2015, 5, 364–368. [Google Scholar] [CrossRef]
- Bontempo, E.; Dalagnol, R.; Ponzoni, F.; Valeriano, D. Adjustments to SIF Aid the Interpretation of Drought Responses at the Caatinga of Northeast Brazil. Remote Sens. 2020, 12, 3264. [Google Scholar] [CrossRef]
- Tian, F.; Brandt, M.; Liu, Y.Y.; Verger, A.; Tagesson, T.; Diouf, A.A.; Rasmussen, K.; Mbow, C.; Wang, Y.; Fensholt, R. Remote Sensing of Vegetation Dynamics in Drylands: Evaluating Vegetation Optical Depth (VOD) Using AVHRR NDVI and in Situ Green Biomass Data over West African Sahel. Remote Sens. Environ. 2016, 177, 265–276. [Google Scholar] [CrossRef]
- Brandt, M.; Wigneron, J.-P.; Chave, J.; Tagesson, T.; Penuelas, J.; Ciais, P.; Rasmussen, K.; Tian, F.; Mbow, C.; Al-Yaari, A.; et al. Satellite Passive Microwaves Reveal Recent Climate-Induced Carbon Losses in African Drylands. Nat. Ecol. Evol. 2018, 2, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Pan, B.; Li, N.; Wang, W.; Zhang, J.; Zhang, X. A Systematic Method for Spatio-Temporal Phenology Estimation of Paddy Rice Using Time Series Sentinel-1 Images. Remote Sens. Environ. 2021, 259, 112394. [Google Scholar] [CrossRef]
- Pontes, G.M.; Wainer, I.; Taschetto, A.S.; Sen Gupta, A.; Abe-Ouchi, A.; Brady, E.C.; Chan, W.-L.; Chandan, D.; Contoux, C.; Feng, R.; et al. Drier Tropical and Subtropical Southern Hemisphere in the Mid-Pliocene Warm Period. Sci. Rep. 2020, 10, 13458. [Google Scholar] [CrossRef] [PubMed]
- Daramola, M.T.; Xu, M. Recent Changes in Global Dryland Temperature and Precipitation. Int. J. Climatol. 2022, 42, 1267–1282. [Google Scholar] [CrossRef]
- Cai, W.; Cowan, T.; Thatcher, M. Rainfall Reductions over Southern Hemisphere Semi-Arid Regions: The Role of Subtropical Dry Zone Expansion. Sci. Rep. 2012, 2, 702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jia, G.; Xu, X.; Zhang, A.; Wang, H. Divergent Effects of Intensified Precipitation on Primary Production in Global Drylands. Sci. Total Environ. 2023, 892, 164736. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huete, A.; Moran, S.; Ponce-Campos, G.; Eamus, D. Abrupt Shifts in Phenology and Vegetation Productivity under Climate Extremes. J. Geophys. Res. Biogeosci. 2015, 120, 2036–2052. [Google Scholar] [CrossRef]
- Lian, X.; Piao, S.; Chen, A.; Huntingford, C.; Fu, B.; Li, L.Z.X.; Huang, J.; Sheffield, J.; Berg, A.M.; Keenan, T.F.; et al. Multifaceted Characteristics of Dryland Aridity Changes in a Warming World. Nat. Rev. Earth Environ. 2021, 2, 232–250. [Google Scholar] [CrossRef]

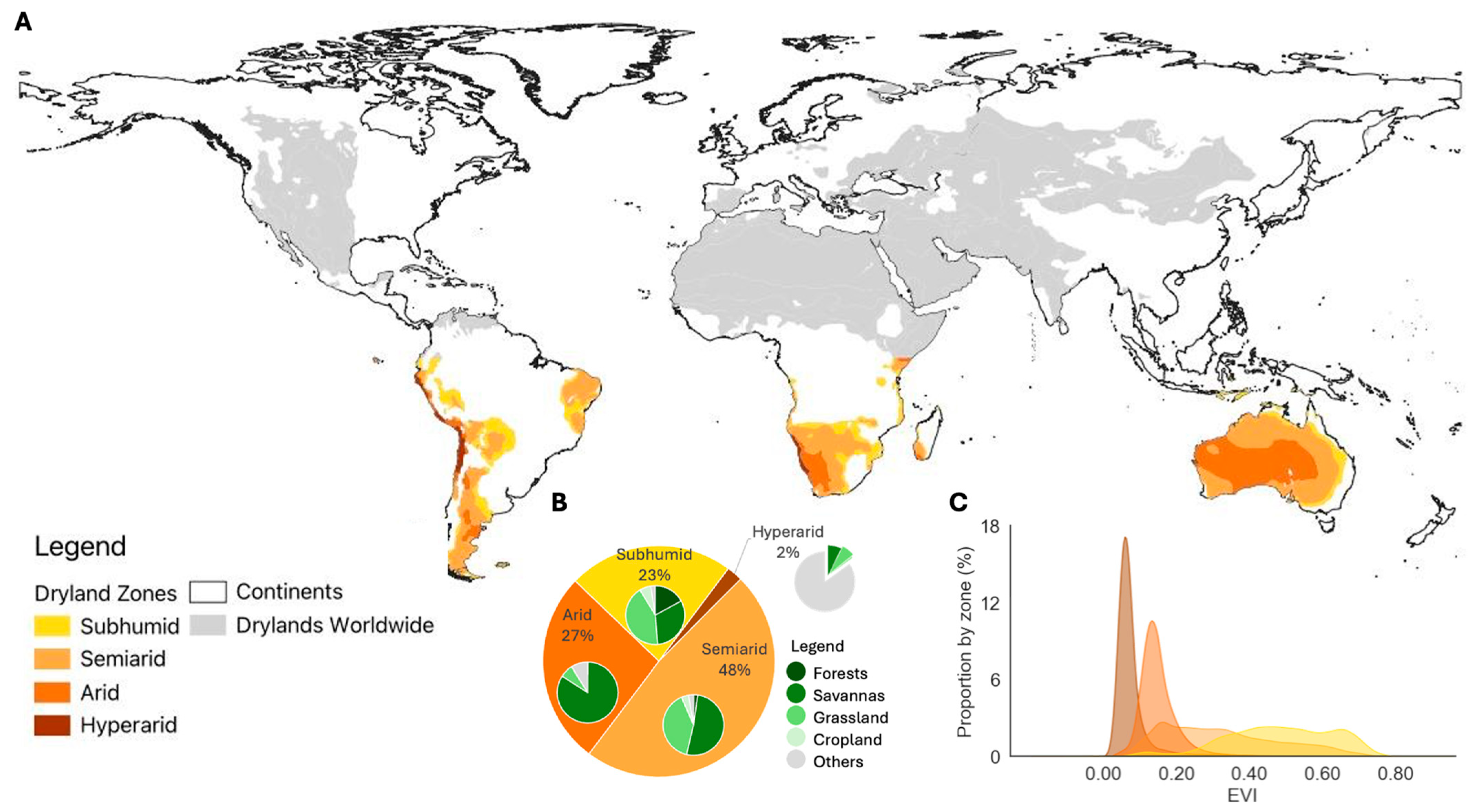
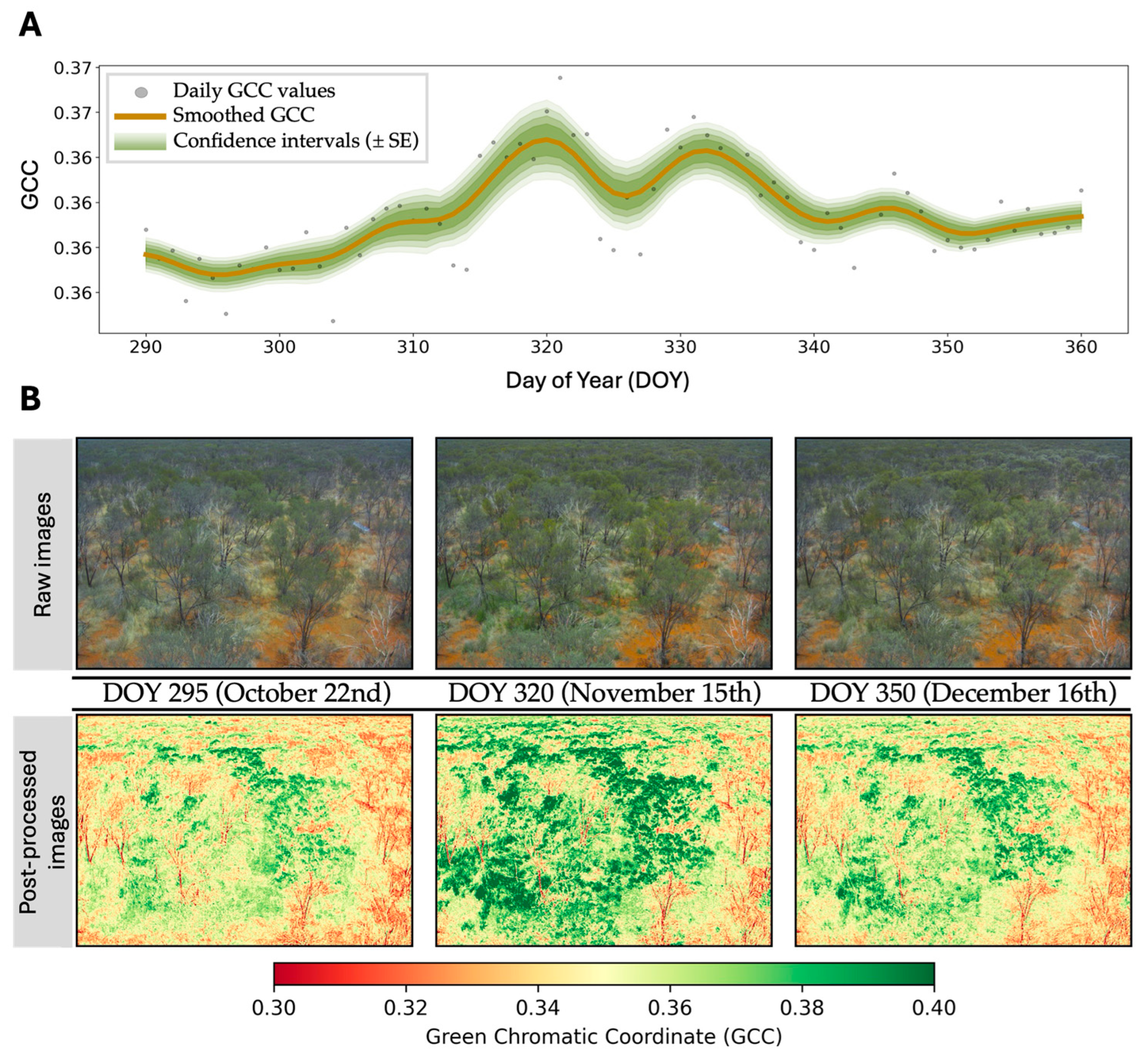
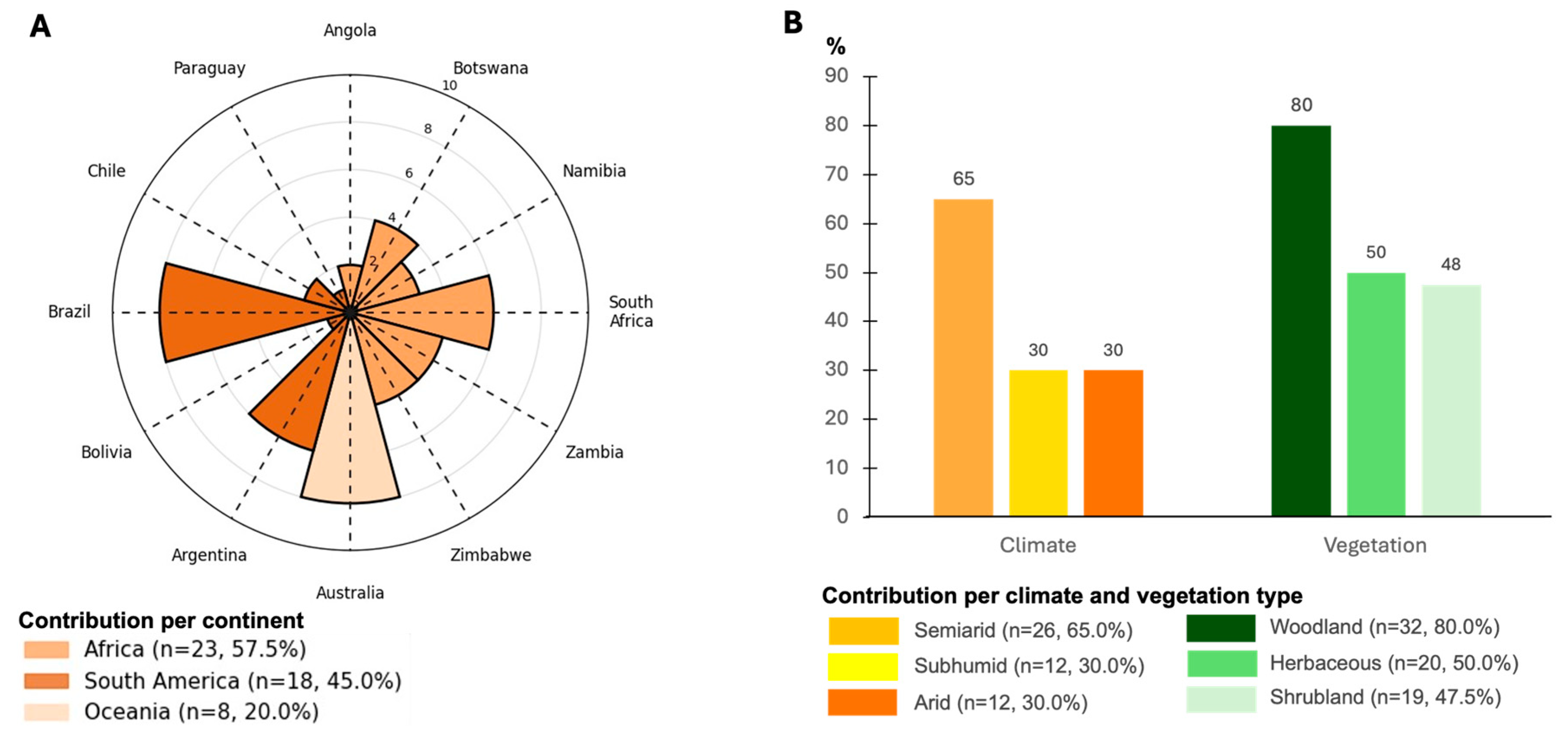
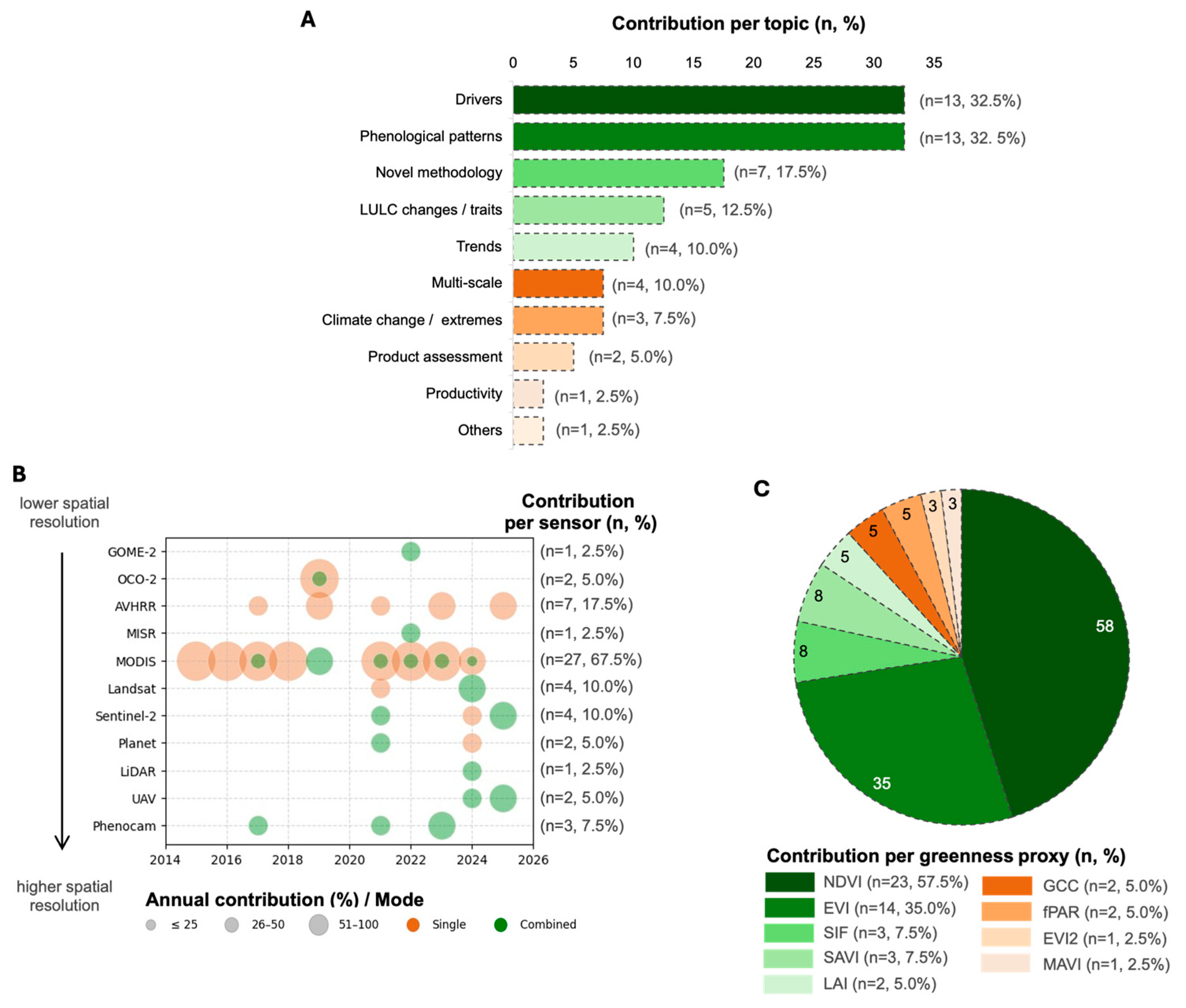
| Phenological Metrics | Definition | Unit of Measurement |
|---|---|---|
| SOS | Start of the growing season (i.e., greening) | DOY 1 |
| Maturity | Complete development (i.e., end of greening phase) | DOY |
| POS | Peak of the growing season (i.e., when the maximum season value is reached) | DOY |
| Senescence | Decline in photosynthetic activity towards the end of the growing season (i.e., leaf fall) | DOY |
| EOS | End of the growing season (i.e., dormancy) | DOY |
| Amplitude | Magnitude of variation in the growing season (e.g., POS value–minimum value) | value of observation 2 |
| LOS | Season length (EOS–SOS) | value of observation or number of days |
| Cycles | Number of valid seasons detectable in a year | integer |
| Satellite/Sensor | Spatial Resolution (m) | Temporal Resolution (Frequency) | Spectral Bands | Start/End of Mission |
|---|---|---|---|---|
| PlanetScope/PS2 | 3–5 | Daily | 4 | 2018/- |
| Sentinel-2A and B/MSI | 10–60 | ~5 days | 13 | 2015/- |
| Landsat constellation 1 | 15–120 | 16 days | 4–11 | 1972/- |
| TERRA and AQUA/MODIS | 250–1000 | Daily | 36 | 1999/- |
| S-NPP/VIIRS | 375–750 | 12 h | 22 | 2011/- |
| Himawari-8 and 9/AHI | 500–2000 | 10 min | 16 | 2016/- |
| SPOT-4 and 5/VGT | 1015 | Daily | 4 | 1998/2014 2 |
| NOAA and EUMETSAT/AVHRR series | 1100 | 12 h | 6 | 1978/2019 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutra, A.C.; Srivastava, A.; Ganem, K.A.; Arai, E.; Huete, A.; Shimabukuro, Y.E. Remote Sensing-Based Phenology of Dryland Vegetation: Contributions and Perspectives in the Southern Hemisphere. Remote Sens. 2025, 17, 2503. https://doi.org/10.3390/rs17142503
Dutra AC, Srivastava A, Ganem KA, Arai E, Huete A, Shimabukuro YE. Remote Sensing-Based Phenology of Dryland Vegetation: Contributions and Perspectives in the Southern Hemisphere. Remote Sensing. 2025; 17(14):2503. https://doi.org/10.3390/rs17142503
Chicago/Turabian StyleDutra, Andeise Cerqueira, Ankur Srivastava, Khalil Ali Ganem, Egidio Arai, Alfredo Huete, and Yosio Edemir Shimabukuro. 2025. "Remote Sensing-Based Phenology of Dryland Vegetation: Contributions and Perspectives in the Southern Hemisphere" Remote Sensing 17, no. 14: 2503. https://doi.org/10.3390/rs17142503
APA StyleDutra, A. C., Srivastava, A., Ganem, K. A., Arai, E., Huete, A., & Shimabukuro, Y. E. (2025). Remote Sensing-Based Phenology of Dryland Vegetation: Contributions and Perspectives in the Southern Hemisphere. Remote Sensing, 17(14), 2503. https://doi.org/10.3390/rs17142503








