Unlocking Potato Phenology: Harnessing Sentinel-1 and Sentinel-2 Synergy for Precise Crop Stage Detection
Abstract
1. Introduction
2. Materials
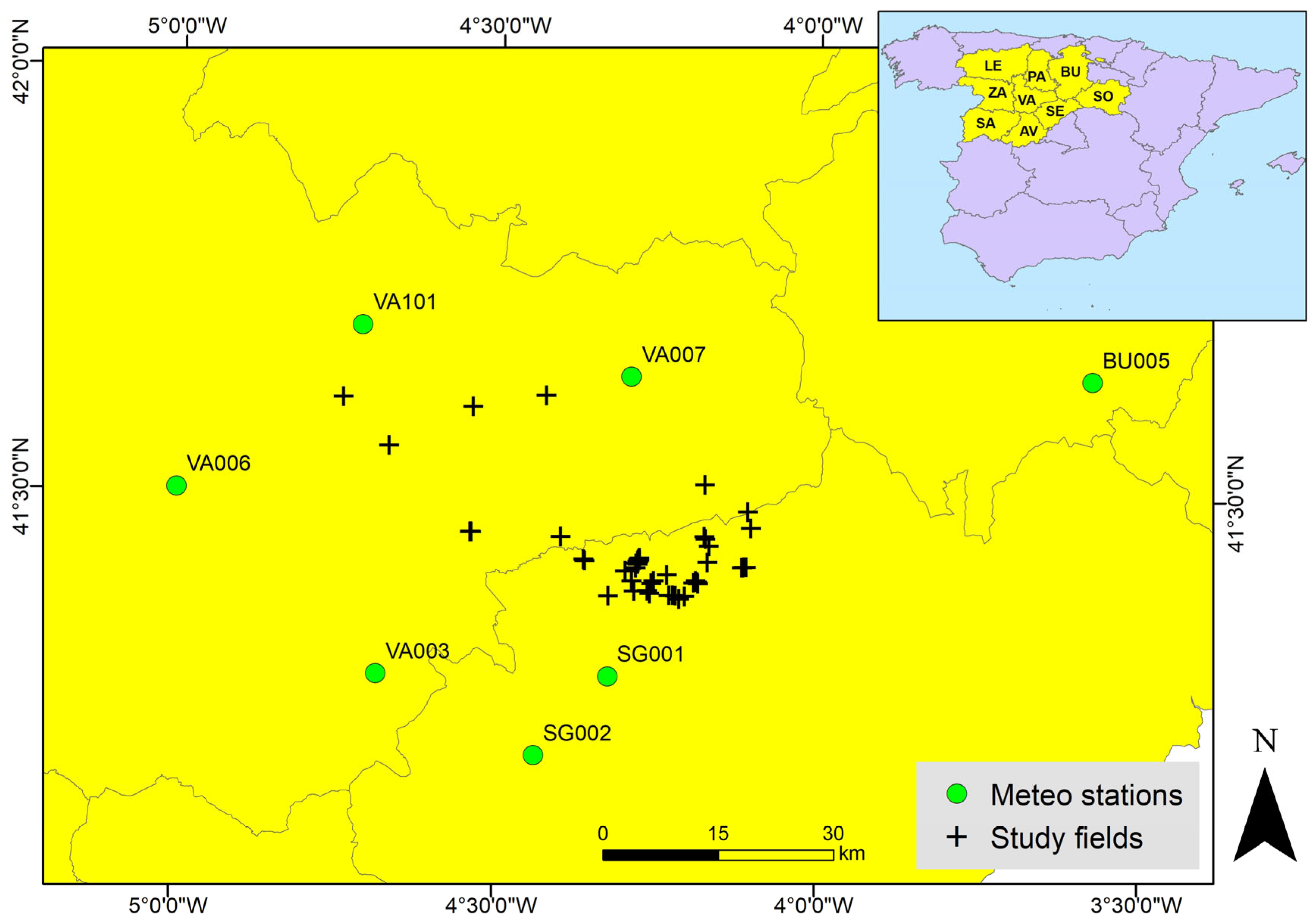
2.1. Study Area and In Situ Data
2.2. Satellite Data
2.2.1. Sentinel-1 (S1)
2.2.2. Sentinel-2 (S2)
2.3. Meteorological Data
3. Methods
3.1. Assumptions
3.2. Phenology Detection
4. Results
4.1. Description of In Situ Observations
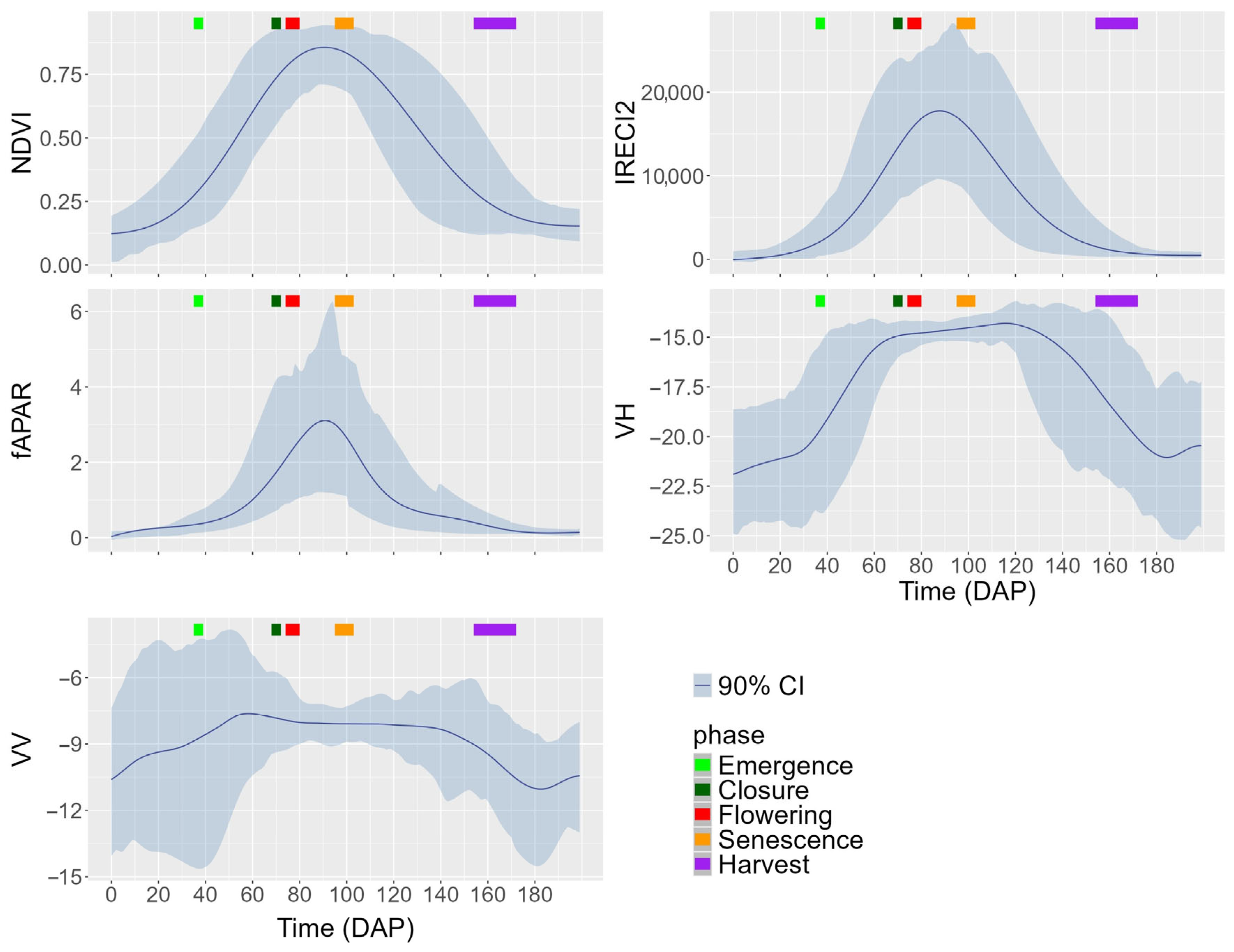
4.2. Description of Curves and Their Variability Across DAPs
4.3. Performance of the Detection Algorithm per Phase and Variable
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fanzo, J.; Davis, C.; McLaren, R.; Choufani, J. The Effect of Climate Change across Food Systems: Implications for Nutrition Outcomes. Glob. Food Secur. 2018, 18, 12–19. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing Climate Change Adaptation Needs for Food Security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate Change and Food Systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- Hall, C.; Dawson, T.P.; Macdiarmid, J.I.; Matthews, R.B.; Smith, P. The Impact of Population Growth and Climate Change on Food Security in Africa: Looking Ahead to 2050. Int. J. Agric. Sustain. 2017, 15, 124–135. [Google Scholar] [CrossRef]
- IPCC. Climate Change and Land; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Kundzewicz, Z.W.; Mata, L.J.; Arnell, N.W.; Döll, P.; Jimenez, B.; Miller, K.; Oki, T.; Şen, Z.; Shiklomanov, I. The Implications of Projected Climate Change for Freshwater Resources and Their Management. Hydrol. Sci. J. 2008, 53, 3–10. [Google Scholar] [CrossRef]
- IPCC. AR5 Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- EPA. NOAA Climate Change Indicators-Us and Global; EPA: Washington, DC, USA, 2016. [Google Scholar]
- Webber, H.; Gaiser, T.; Ewert, F. What Role Can Crop Models Play in Supporting Climate Change Adaptation Decisions to Enhance Food Security in Sub-Saharan Africa? Agric. Syst. 2014, 127, 161–177. [Google Scholar] [CrossRef]
- Gonzalez Piqueras, J.; Jara, J.; López, H.; Villodre, J.; Hernández, D.; Calera, A.; López-Urrea, R.; Sánchez, J.M. Determining Crop Phenology for Different Varieties of Barley and Wheat on Intensive Plots Using Proximal Remote Sensing. In Proceedings of the Remote Sensing for Agriculture, Ecosystems, and Hydrology XXI, Strasbourg, France, 21 October 2019; Neale, C.M., Maltese, A., Eds.; SPIE: Bellingham, WA, USA, 2019; p. 19. [Google Scholar]
- Ruml, M.; Vulic, T. Importance of Phenological Observations and Predictions in Agriculture. J. Agric. Sci. Belgrade 2005, 50, 217–225. [Google Scholar] [CrossRef]
- Aslam, M.A.; Ahmed, M.; Stöckle, C.O.; Higgins, S.S.; Hassan, F.U.; Hayat, R. Can Growing Degree Days and Photoperiod Predict Spring Wheat Phenology? Front. Environ. Sci. 2017, 5, 57. [Google Scholar] [CrossRef]
- Setiyono, T.D.; Weiss, A.; Specht, J.; Bastidas, A.M.; Cassman, K.G.; Dobermann, A. Understanding and Modeling the Effect of Temperature and Daylength on Soybean Phenology under High-Yield Conditions. Field Crops Res. 2007, 100, 257–271. [Google Scholar] [CrossRef]
- Shaykewich, C.F.; Bullock, P.R. Modeling Soybean Phenology. In Agronomy Monographs; Hatfield, J.L., Sivakumar, M.V.K., Prueger, J.H., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Inc.: Madison, WI, USA, 2018; pp. 279–302. ISBN 978-0-89118-358-7. [Google Scholar]
- Soltani, A.; Hammer, G.L.; Torabi, B.; Robertson, M.J.; Zeinali, E. Modeling Chickpea Growth and Development: Phenological Development. Field Crops Res. 2006, 99, 1–13. [Google Scholar] [CrossRef]
- Hufkens, K.; Melaas, E.K.; Mann, M.L.; Foster, T.; Ceballos, F.; Robles, M.; Kramer, B. Monitoring Crop Phenology Using a Smartphone Based Near-Surface Remote Sensing Approach. Agric. For. Meteorol. 2019, 265, 327–337. [Google Scholar] [CrossRef]
- ESA. New Satellite for Unprecedented Earth Monitoring; ESA: Paris, France, 2015. [Google Scholar]
- Zheng, Y.; Wu, B.; Zhang, M.; Zeng, H. Crop Phenology Detection Using High Spatio-Temporal Resolution Data Fused from SPOT5 and MODIS Products. Sensors 2016, 16, 2099. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Meng, J.; Zhang, M.; Dong, T. Remote Sensing Based Detection of Crop Phenology for Agricultural Zones in China Using a New Threshold Method. Remote Sens. 2013, 5, 3190–3211. [Google Scholar] [CrossRef]
- USGS NDVI. The Foundation for Remote Sensing Phenology; USGS: Sunrise Valley Drive Reston, VA, USA, 2020. [Google Scholar]
- Joshi, N.; Baumann, M.; Ehammer, A.; Fensholt, R.; Grogan, K.; Hostert, P.; Jepsen, M.; Kuemmerle, T.; Meyfroidt, P.; Mitchard, E.; et al. A Review of the Application of Optical and Radar Remote Sensing Data Fusion to Land Use Mapping and Monitoring. Remote Sens. 2016, 8, 70. [Google Scholar] [CrossRef]
- Della Vecchia, A.; Ferrazzoli, P.; Guerriero, L.; Ninivaggi, L.; Strozzi, T.; Wegmuller, U. Observing and Modeling Multifrequency Scattering of Maize During the Whole Growth Cycle. IEEE Trans. Geosci. Remote Sens. 2008, 46, 3709–3718. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakaiya, E.; Wang, C. Capability of C-Band Backscattering Coefficients from High-Resolution Satellite SAR Sensors to Assess Biophysical Variables in Paddy Rice. Remote Sens. Environ. 2014, 140, 257–266. [Google Scholar] [CrossRef]
- Vreugdenhil, M.; Wagner, W.; Bauer-Marschallinger, B.; Pfeil, I.; Teubner, I.; Rüdiger, C.; Strauss, P. Sensitivity of Sentinel-1 Backscatter to Vegetation Dynamics: An Austrian Case Study. Remote Sens. 2018, 10, 1396. [Google Scholar] [CrossRef]
- Schlund, M.; Erasmi, S. Sentinel-1 Time Series Data for Monitoring the Phenology of Winter Wheat. Remote Sens. Environ. 2020, 246, 111814. [Google Scholar] [CrossRef]
- Khabbazan, S.; Vermunt, P.; Steele-Dunne, S.; Ratering Arntz, L.; Marinetti, C.; Van Der Valk, D.; Iannini, L.; Molijn, R.; Westerdijk, K.; Van Der Sande, C. Crop Monitoring Using Sentinel-1 Data: A Case Study from The Netherlands. Remote Sens. 2019, 11, 1887. [Google Scholar] [CrossRef]
- Mercier, A.; Betbeder, J.; Baudry, J.; Le Roux, V.; Spicher, F.; Lacoux, J.; Roger, D.; Hubert-Moy, L. Evaluation of Sentinel-1 & 2 Time Series for Predicting Wheat and Rapeseed Phenological Stages. ISPRS J. Photogramm. Remote Sens. 2020, 163, 231–256. [Google Scholar] [CrossRef]
- Nasrallah, A.; Baghdadi, N.; El Hajj, M.; Darwish, T.; Belhouchette, H.; Faour, G.; Darwich, S.; Mhawej, M. Sentinel-1 Data for Winter Wheat Phenology Monitoring and Mapping. Remote Sens. 2019, 11, 2228. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J. Mapping Winter Wheat Planting Area and Monitoring Its Phenology Using Sentinel-1 Backscatter Time Series. Remote Sens. 2019, 11, 449. [Google Scholar] [CrossRef]
- Stendardi, L.; Karlsen, S.R.; Niedrist, G.; Gerdol, R.; Zebisch, M.; Rossi, M.; Notarnicola, C. Exploiting Time Series of Sentinel-1 and Sentinel-2 Imagery to Detect Meadow Phenology in Mountain Regions. Remote Sens. 2019, 11, 542. [Google Scholar] [CrossRef]
- Veloso, A.; Mermoz, S.; Bouvet, A.; Le Toan, T.; Planells, M.; Dejoux, J.-F.; Ceschia, E. Understanding the Temporal Behavior of Crops Using Sentinel-1 and Sentinel-2-like Data for Agricultural Applications. Remote Sens. Environ. 2017, 199, 415–426. [Google Scholar] [CrossRef]
- FAO. Promotion of Underutilized Indigenous Food Resources for Food Security and Nutrition in Asia and the Pacific; FAO: Rome, Italy, 2014. [Google Scholar]
- Wijesinha-Bettoni, R.; Mouillé, B. The Contribution of Potatoes to Global Food Security, Nutrition and Healthy Diets. Am. J. Potato Res. 2019, 96, 139–149. [Google Scholar] [CrossRef]
- Hack, H.; Gall, H.; Klemke, T.; Klose, R.; Meier, U.; Stauss, R.; Witzenberger, A. Phänologische Entwicklungsstadien der Kartoffel (Solanum tuberosum L.). Codierung und Beschreibung nach der erweiterten BBCH-Skala mit Abbildungen. Das Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 1993, 45, 11–19. [Google Scholar]
- Aien, A.; Chaturvedi, A.K.; Bahuguna, R.N.; Pal, M. Phenological Sensitivity to High Temperature Stress Determines Dry Matter Partitioning and Yield in Potato. Indian J. Plant Physiol. 2017, 22, 63–69. [Google Scholar] [CrossRef]
- Rykaczewska, K. The Effect of High Temperature Occurring in Subsequent Stages of Plant Development on Potato Yield and Tuber Physiological Defects. Am. J. Potato Res. 2015, 92, 339–349. [Google Scholar] [CrossRef]
- Arana, C.; Orlando, R.; Laercio, Z.; Claudio, L. Potato early blight epidemics and comparison of methods to determine its initial symptoms in a potato field. Rev. Fac. Nac. Agron. 2007, 60, 3877–3890. [Google Scholar]
- Gent, D.H.; Schwartz, H.F. Validation of Potato Early Blight Disease Forecast Models for Colorado Using Various Sources of Meteorological Data. Plant Dis. 2003, 87, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hoelle, J.; Asch, F.; Khan, A.; Bonierbale, M. Phenology-Adjusted Stress Severity Index to Assess Genotypic Responses to Terminal Drought in Field Grown Potato. Agronomy 2020, 10, 1298. [Google Scholar] [CrossRef]
- JCyL. Patata de Siembra; Junta de Castilla y Leon: Valladolid, Spain, 2021. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- MAPA. Sigpac; MAPA: Ankara, Turkey, 2020. [Google Scholar]
- Sands, P.J.; Hackett, C.; Nix, H.A. A Model of the Development and Bulking of Potatoes (Solanum tuberosum L.) I. Derivation from Well-Managed Field Crops. Field Crops Res. 1979, 2, 309–331. [Google Scholar] [CrossRef]
- ESA. Mission Ends for Copernicus Sentinel-1B Satellite; ESA: Paris, France, 2022. [Google Scholar]
- Mullissa, A.; Vollrath, A.; Odongo-Braun, C.; Slagter, B.; Balling, J.; Gou, Y.; Gorelick, N.; Reiche, J. Sentinel-1 SAR Backscatter Analysis Ready Data Preparation in Google Earth Engine. Remote Sens. 2021, 13, 1954. [Google Scholar] [CrossRef]
- Lee, J.-S. Digital Image Enhancement and Noise Filtering by Use of Local Statistics. IEEE Trans. Pattern Anal. Mach. Intell. 1980, PAMI-2, 165–168. [Google Scholar] [CrossRef]
- Lopes, A.; Touzi, R.; Nezry, E. Adaptive Speckle Filters and Scene Heterogeneity. IEEE Trans. Geosci. Remote Sens. 1990, 28, 992–1000. [Google Scholar] [CrossRef]
- Lee, J.-S.; Grunes, M.R.; De Grandi, G. Polarimetric SAR Speckle Filtering and Its Implication for Classification. IEEE Trans. Geosci. Remote Sens. 1999, 37, 2363–2373. [Google Scholar] [CrossRef]
- Vollrath, A.; Mullissa, A.; Reiche, J. Angular-Based Radiometric Slope Correction for Sentinel-1 on Google Earth Engine. Remote Sens. 2020, 12, 1867. [Google Scholar] [CrossRef]
- Lavreniuk, M.; Kussul, N.; Meretsky, M.; Lukin, V.; Abramov, S.; Rubel, O. Impact of SAR Data Filtering on Crop Classification Accuracy. In Proceedings of the 2017 IEEE First Ukraine Conference on Electrical and Computer Engineering (UKRCON), Kyiv, Ukraine, 29 May–2 June 2017; IEEE: New York, NY, USA, 2017; pp. 912–917. [Google Scholar]
- Marghany, M.; Sabu, Z.; Hashim, M. Mapping Coastal Geomorphology Changes Using Synthetic Aperture Radar Data. Int. J. Phys. Sci. 2010, 5, 1890–1896. [Google Scholar]
- Qiu, F.; Berglund, J.; Jensen, J.R.; Thakkar, P.; Ren, D. Speckle Noise Reduction in SAR Imagery Using a Local Adaptive Median Filter. GIScience Remote Sens. 2004, 41, 244–266. [Google Scholar] [CrossRef]
- Hoekman, D.H.; Reiche, J. Multi-Model Radiometric Slope Correction of SAR Images of Complex Terrain Using a Two-Stage Semi-Empirical Approach. Remote Sens. Environ. 2015, 156, 1–10. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing, R version 4.1.3; R Core Team R: Vienna, Austria, 2022. [Google Scholar]
- Graesser, J.; Stanimirova, R.; Friedl, M.A. Reconstruction of Satellite Time Series with a Dynamic Smoother. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2022, 15, 1803–1813. [Google Scholar] [CrossRef]
- S2 Products. Available online: https://sentiwiki.copernicus.eu/web/s2-products (accessed on 28 June 2025).
- GEE Harmonized Sentinel-2 MSI: MultiSpectral Instrument, Level-2A (SR)|Earth Engine Data Catalog|Google for Developers. Available online: https://developers.google.com/earth-engine/datasets/catalog/COPERNICUS_S2_SR_HARMONIZED (accessed on 24 March 2025).
- Hassan, M.A.; Yang, M.; Rasheed, A.; Yang, G.; Reynolds, M.; Xia, X.; Xiao, Y.; He, Z. A Rapid Monitoring of NDVI across the Wheat Growth Cycle for Grain Yield Prediction Using a Multi-Spectral UAV Platform. Plant Sci. 2019, 282, 95–103. [Google Scholar] [CrossRef]
- Luo, Q.; Song, J.; Yang, L.; Wang, J. Improved Spring Vegetation Phenology Calculation Method Using a Coupled Model and Anomalous Point Detection. Remote Sens. 2019, 11, 1432. [Google Scholar] [CrossRef]
- Shammi, S.A.; Meng, Q. Use Time Series NDVI and EVI to Develop Dynamic Crop Growth Metrics for Yield Modeling. Ecol. Indic. 2021, 121, 107124. [Google Scholar] [CrossRef]
- Frampton, W.J.; Dash, J.; Watmough, G.; Milton, E.J. Evaluating the Capabilities of Sentinel-2 for Quantitative Estimation of Biophysical Variables in Vegetation. ISPRS J. Photogramm. Remote Sens. 2013, 82, 83–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.-B.; Lyapustin, A.I.; Wang, Y.; Gao, F.; Suyker, A.; Verma, S.; Middleton, E.M. Estimation of Crop Gross Primary Production (GPP): FAPARchl versus MOD15A2 FPAR. Remote Sens. Environ. 2014, 153, 1–6. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, Z.; Li, J.; Yang, H.; Song, J. Evaluation of Global Fraction of Absorbed Photosynthetically Active Radiation (FAPAR) Products at 500 m Spatial Resolution. Remote Sens. 2022, 14, 3304. [Google Scholar] [CrossRef]
- Badawy, B.; Rödenbeck, C.; Reichstein, M.; Carvalhais, N.; Heimann, M. Technical Note: The Simple Diagnostic Photosynthesis and Respiration Model (SDPRM). Biogeosciences 2013, 10, 6485–6508. [Google Scholar] [CrossRef]
- Los, S.O.; Pollack, N.H.; Parris, M.T.; Collatz, G.J.; Tucker, C.J.; Sellers, P.J.; Malmström, C.M.; DeFries, R.S.; Bounoua, L.; Dazlich, D.A. A Global 9-Yr Biophysical Land Surface Dataset from NOAA AVHRR Data. J. Hydrometeorol. 2000, 1, 183–199. [Google Scholar] [CrossRef]
- Russell, E.S. Effect of Dew and Intercepted Precipitation on Radar Backscatter of a Soybean Canopy. Master’s Thesis, Iowa State University, Digital Repository, Ames, IA, USA, 2011; p. 2808315. [Google Scholar]
- Vermunt, P.C.; Khabbazan, S.; Steele-Dunne, S.C.; Judge, J.; Monsivais-Huertero, A.; Guerriero, L.; Liu, P.-W. Response of Subdaily L-Band Backscatter to Internal and Surface Canopy Water Dynamics. IEEE Trans. Geosci. Remote Sens. 2021, 59, 7322–7337. [Google Scholar] [CrossRef]
- SIAR. WebSiar; SIAR: Mexico City, Mexico, 2020. [Google Scholar]
- Ouaadi, N.; Jarlan, L.; Ezzahar, J.; Zribi, M.; Khabba, S.; Bouras, E.; Bousbih, S.; Frison, P.-L. Monitoring of Wheat Crops Using the Backscattering Coefficient and the Interferometric Coherence Derived from Sentinel-1 in Semi-Arid Areas. Remote Sens. Environ. 2020, 251, 112050. [Google Scholar] [CrossRef]
- SRUC. Haulm Destruction in Potato Crops; SRUC: Edinburgh, UK, 2021. [Google Scholar]
- Oregon University Chemical Potato Vine Kill (Desiccation). Available online: https://pnwhandbooks.org/weed/agronomic/potato/chemical-potato-vine-kill-desiccation (accessed on 8 April 2025).
- Guo, Y.; Chen, S.; Fu, Y.H.; Xiao, Y.; Wu, W.; Wang, H.; Beurs, K. de Comparison of Multi-Methods for Identifying Maize Phenology Using PhenoCams. Remote Sens. 2022, 14, 244. [Google Scholar] [CrossRef]
- Pei, J.; Tan, S.; Zou, Y.; Liao, C.; He, Y.; Wang, J.; Huang, H.; Wang, T.; Tian, H.; Fang, H.; et al. The Role of Phenology in Crop Yield Prediction: Comparison of Ground-Based Phenology and Remotely Sensed Phenology. Agric. For. Meteorol. 2025, 361, 110340. [Google Scholar] [CrossRef]
- Liao, C.; Wang, J.; Shan, B.; Shang, J.; Dong, T.; He, Y. Near Real-Time Detection and Forecasting of within-Field Phenology of Winter Wheat and Corn Using Sentinel-2 Time-Series Data. ISPRS J. Photogramm. Remote Sens. 2023, 196, 105–119. [Google Scholar] [CrossRef]
- Gobin, A.; Sallah, A.-H.M.; Curnel, Y.; Delvoye, C.; Weiss, M.; Wellens, J.; Piccard, I.; Planchon, V.; Tychon, B.; Goffart, J.-P.; et al. Crop Phenology Modelling Using Proximal and Satellite Sensor Data. Remote Sens. 2023, 15, 2090. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Xie, Z.; Zhan, P.; Huang, X.; Sun, L.; Duan, Z. Assessing the Effects of Time Interpolation of NDVI Composites on Phenology Trend Estimation. Remote Sens. 2021, 13, 5018. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Liu, Y.; Jayavelu, S.; Wang, J.; Moon, M.; Henebry, G.M.; Friedl, M.A.; Schaaf, C.B. Generation and Evaluation of the VIIRS Land Surface Phenology Product. Remote Sens. Environ. 2018, 216, 212–229. [Google Scholar] [CrossRef]
- Diao, C. Remote Sensing Phenological Monitoring Framework to Characterize Corn and Soybean Physiological Growing Stages. Remote Sens. Environ. 2020, 248, 111960. [Google Scholar] [CrossRef]
- Diao, C.; Li, G. Near-Surface and High-Resolution Satellite Time Series for Detecting Crop Phenology. Remote Sens. 2022, 14, 1957. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, X.; Chen, J.; Wang, C.; Shen, M.; Yang, W.; Tan, X.; Xu, S.; Li, Z. Improving the Accuracy of Spring Phenology Detection by Optimally Smoothing Satellite Vegetation Index Time Series Based on Local Cloud Frequency. ISPRS J. Photogramm. Remote Sens. 2021, 180, 29–44. [Google Scholar] [CrossRef]
- Liu, L.; Cao, R.; Chen, J.; Shen, M.; Wang, S.; Zhou, J.; He, B. Detecting Crop Phenology from Vegetation Index Time-Series Data by Improved Shape Model Fitting in Each Phenological Stage. Remote Sens. Environ. 2022, 277, 113060. [Google Scholar] [CrossRef]
- Kawakita, S.; Takahashi, H.; Moriya, K. Prediction and Parameter Uncertainty for Winter Wheat Phenology Models Depend on Model and Parameterization Method Differences. Agric. For. Meteorol. 2020, 290, 107998. [Google Scholar] [CrossRef]
- Yang, C.; Menz, C.; Reis, S.; Machado, N.; Santos, J.A.; Torres-Matallana, J.A. Calibration for an Ensemble of Grapevine Phenology Models under Different Optimization Algorithms. Agronomy 2023, 13, 679. [Google Scholar] [CrossRef]
- Yang, C.; Lei, N.; Menz, C.; Ceglar, A.; Torres-Matallana, J.A.; Li, S.; Jiang, Y.; Tan, X.; Tao, L.; He, F.; et al. Regional Uncertainty Analysis between Crop Phenology Model Structures and Optimal Parameters. Agric. For. Meteorol. 2024, 355, 110137. [Google Scholar] [CrossRef]
- Kganyago, M.; Adjorlolo, C.; Mhangara, P.; Tsoeleng, L. Optical Remote Sensing of Crop Biophysical and Biochemical Parameters: An Overview of Advances in Sensor Technologies and Machine Learning Algorithms for Precision Agriculture. Comput. Electron. Agric. 2024, 218, 108730. [Google Scholar] [CrossRef]
- Sah, S.; Haldar, D.; Chandra, S.; Nain, A.S. Discrimination and Monitoring of Rice Cultural Types Using Dense Time Series of Sentinel-1 SAR Data. Ecol. Inform. 2023, 76, 102136. [Google Scholar] [CrossRef]
- Allies, A.; Roumiguié, A.; Dejoux, J.-F.; Fieuzal, R.; Jacquin, A.; Veloso, A.; Champolivier, L.; Baup, F. Evaluation of Multiorbital SAR and Multisensor Optical Data for Empirical Estimation of Rapeseed Biophysical Parameters. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 7268–7283. [Google Scholar] [CrossRef]
- Bhatti, M.T.; Gilani, H.; Ashraf, M.; Iqbal, M.S.; Munir, S. Field Validation of NDVI to Identify Crop Phenological Signatures. Precis. Agric. 2024, 25, 2245–2270. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, X. Mapping Crop Phenology in Near Real-Time Using Satellite Remote Sensing: Challenges and Opportunities. J. Remote Sens. 2021, 2021, 8379391. [Google Scholar] [CrossRef]
- Wei, W.; Wu, W.; Li, Z.; Yang, P.; Zhou, Q. Selecting the Optimal NDVI Time-Series Reconstruction Technique for Crop Phenology Detection. Intell. Autom. Soft Comput. 2016, 22, 237–247. [Google Scholar] [CrossRef]
- Gu, Y.; Wylie, B.K.; Howard, D.M.; Phuyal, K.P.; Ji, L. NDVI Saturation Adjustment: A New Approach for Improving Cropland Performance Estimates in the Greater Platte River Basin, USA. Ecol. Indic. 2013, 30, 1–6. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; Wing, I.S.; et al. Net Carbon Uptake Has Increased through Warming-Induced Changes in Temperate Forest Phenology. Nat. Clim. Change 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Bala, S.K.; Islam, A.S. Correlation between Potato Yield and MODIS-derived Vegetation Indices. Int. J. Remote Sens. 2009, 30, 2491–2507. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, R.; Qiao, L.; Zhang, Y.; Li, M.; Sun, H.; Xing, Z.; Wang, X. Growth Stages Classification of Potato Crop Based on Analysis of Spectral Response and Variables Optimization. Sensors 2020, 20, 3995. [Google Scholar] [CrossRef]
- Löw, J.; Hill, S.; Otte, I.; Thiel, M.; Ullmann, T.; Conrad, C. How Phenology Shapes Crop-Specific Sentinel-1 PolSAR Features and InSAR Coherence across Multiple Years and Orbits. Remote Sens. 2024, 16, 2791. [Google Scholar] [CrossRef]
- Pasternak, M.; Pawłuszek-Filipiak, K. Evaluation of C and X-Band Synthetic Aperture Radar Derivatives for Tracking Crop Phenological Development. Remote Sens. 2023, 15, 4996. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, S.; Zhao, L.; Huang, X.; Jiang, X. Parcel-Based Summer Maize Mapping and Phenology Estimation Combined Using Sentinel-2 and Time Series Sentinel-1 Data. Int. J. Appl. Earth Obs. Geoinf. 2022, 108, 102720. [Google Scholar] [CrossRef]
- Zhao, Z.; Dong, J.; Zhang, G.; Yang, J.; Liu, R.; Wu, B.; Xiao, X. Improved Phenology-Based Rice Mapping Algorithm by Integrating Optical and Radar Data. Remote Sens. Environ. 2024, 315, 114460. [Google Scholar] [CrossRef]
- Lobert, F.; Löw, J.; Schwieder, M.; Gocht, A.; Schlund, M.; Hostert, P.; Erasmi, S. A Deep Learning Approach for Deriving Winter Wheat Phenology from Optical and SAR Time Series at Field Level. Remote Sens. Environ. 2023, 298, 113800. [Google Scholar] [CrossRef]
- d’Andrimont, R.; Taymans, M.; Lemoine, G.; Ceglar, A.; Yordanov, M.; van der Velde, M. Detecting Flowering Phenology in Oil Seed Rape Parcels with Sentinel-1 and -2 Time Series. Remote Sens. Environ. 2020, 239, 111660. [Google Scholar] [CrossRef] [PubMed]
- Lokupitiya, E.; Denning, S.; Paustian, K.; Baker, I.; Schaefer, K.; Verma, S.; Meyers, T.; Bernacchi, C.J.; Suyker, A.; Fischer, M. Incorporation of Crop Phenology in Simple Biosphere Model (SiBcrop) to Improve Land-Atmosphere Carbon Exchanges from Croplands. Biogeosciences 2009, 6, 969–986. [Google Scholar] [CrossRef]
- Bolton, D.K.; Friedl, M.A. Forecasting Crop Yield Using Remotely Sensed Vegetation Indices and Crop Phenology Metrics. Agric. For. Meteorol. 2013, 173, 74–84. [Google Scholar] [CrossRef]
- Sakamoto, T.; Gitelson, A.A.; Arkebauer, T.J. MODIS-Based Corn Grain Yield Estimation Model Incorporating Crop Phenology Information. Remote Sens. Environ. 2013, 131, 215–231. [Google Scholar] [CrossRef]




| RMSE (Days) | Emergence | Closure | Flowering | Onset Senescence | Harvest |
|---|---|---|---|---|---|
| NDVI | 12 | 14 | - | 17 | 29 |
| IRECI2 | 13 | 19 | - | 19 | 44 |
| fAPAR | 13 | 22 | - | 17 | 52 |
| VH | 15 | 19 | 22 | 33 | 29 |
| VV | 15 | 24 | 23 | 30 | 28 |
| NDVI_VH | 12 | 15 | 22 | 19 | 23 |
| Baseline | 20 | 11 | 11 | 26 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, D.; Salvador, P.; Gil, J.; Rodrigo, J.F. Unlocking Potato Phenology: Harnessing Sentinel-1 and Sentinel-2 Synergy for Precise Crop Stage Detection. Remote Sens. 2025, 17, 2336. https://doi.org/10.3390/rs17142336
Gomez D, Salvador P, Gil J, Rodrigo JF. Unlocking Potato Phenology: Harnessing Sentinel-1 and Sentinel-2 Synergy for Precise Crop Stage Detection. Remote Sensing. 2025; 17(14):2336. https://doi.org/10.3390/rs17142336
Chicago/Turabian StyleGomez, Diego, Pablo Salvador, Jorge Gil, and Juan Fernando Rodrigo. 2025. "Unlocking Potato Phenology: Harnessing Sentinel-1 and Sentinel-2 Synergy for Precise Crop Stage Detection" Remote Sensing 17, no. 14: 2336. https://doi.org/10.3390/rs17142336
APA StyleGomez, D., Salvador, P., Gil, J., & Rodrigo, J. F. (2025). Unlocking Potato Phenology: Harnessing Sentinel-1 and Sentinel-2 Synergy for Precise Crop Stage Detection. Remote Sensing, 17(14), 2336. https://doi.org/10.3390/rs17142336







