Canola Yield Estimation Using Remotely Sensed Images and M5P Model Tree Algorithm
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Data
2.2. Method
2.2.1. Spectral Profile of Canola Flowers
2.2.2. Estimating Canola Yield Using Remote Sensing
3. Results
3.1. Spectral Profile of Flowers
3.2. Spectral Variations of Canola Pods
3.3. Estimating Canola Yield Using Time Series of Remote Sensing Images
4. Discussion
4.1. Spectral Profile of Flowers and Pods
4.2. Estimating Canola Yield Using Remote Sensing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canola Council of Canada. Industry Overview. Available online: https://www.canolacouncil.org/about-canola/industry/ (accessed on 27 September 2024).
- Canola Council of Canada. Canadian Canola Production Statistics. Available online: https://www.canolacouncil.org/markets-stats/production/ (accessed on 2 December 2024).
- New Horizons Ontario’s Agricultural Soil Health and Conservation Strategy. 2022. Available online: https://www.researchgate.net/publication/322730732_NEW_HORIZONS_Ontario’s_Agricultural_Soil_Health_and_Conservation_Strategy (accessed on 27 September 2024).
- OMAFRA Field Crop Team. Canola 2024 Seasonal Summary. Available online: https://fieldcropnews.com/2024/11/canola-sexiest-crop-of-the-year/ (accessed on 24 February 2025).
- Government of Canada. The Biology of Brassica napus L. (Canola/Rapeseed). Available online: https://inspection.canada.ca/en/plant-varieties/plants-novel-traits/applicants/directive-94-08/biology-documents/brassica-napus (accessed on 10 May 2025).
- Jin, X.; Kumar, L.; Li, Z.; Feng, H.; Xu, X.; Yang, G.; Wang, J. A Review of Data Assimilation of Remote Sensing and Crop Models. Eur. J. Agron. 2018, 92, 141–152. [Google Scholar] [CrossRef]
- Luo, L.; Sun, S.; Xue, J.; Gao, Z.; Zhao, J.; Yin, Y.; Gao, F.; Luan, X. Crop Yield Estimation Based on Assimilation of Crop Models and Remote Sensing Data: A Systematic Evaluation. Agric. Syst. 2023, 210, 103711. [Google Scholar] [CrossRef]
- Li, A.; Liang, S.; Wang, A.; Qin, J. Estimating Crop Yield from Multi-Temporal Satellite Data Using Multivariate Regression and Neural Network Techniques. Photogramm. Eng. Remote Sens. 2007, 73, 1149–1157. [Google Scholar] [CrossRef]
- Lin, P.; Lee, W.S.; Chen, Y.M.; Peres, N.; Fraisse, C. A Deep-Level Region-Based Visual Representation Architecture for Detecting Strawberry Flowers in an Outdoor Field. Precis. Agric. 2020, 21, 387–402. [Google Scholar] [CrossRef]
- Wang, N.; Cao, H.; Huang, X.; Ding, M. Rapeseed Flower Counting Method Based on GhP2-YOLO and StrongSORT Algorithm. Plants 2024, 13, 2388. [Google Scholar] [CrossRef]
- Ma, C.; Liu, M.; Ding, F.; Li, C.; Cui, Y.; Chen, W.; Wang, Y. Wheat Growth Monitoring and Yield Estimation Based on Remote Sensing Data Assimilation into the SAFY Crop Growth Model. Sci. Rep. 2022, 12, 5473. [Google Scholar] [CrossRef]
- Getahun, S.; Kefale, H.; Gelaye, Y. Application of Precision Agriculture Technologies for Sustainable Crop Production and Environmental Sustainability: A Systematic Review. Sci. World J. 2024, 2024, 2126734. [Google Scholar] [CrossRef]
- Sulik, J.J.; Long, D.S. Automated Detection of Phenological Transitions for Yellow Flowering Plants Such as Brassica Oilseeds. Agrosystems Geosci. Environ. 2020, 3, e20125. [Google Scholar] [CrossRef]
- Wan, L.; Li, Y.; Cen, H.; Zhu, J.; Yin, W.; Wu, W.; Zhu, H.; Sun, D.; Zhou, W.; He, Y. Combining UAV-Based Vegetation Indices and Image Classification to Estimate Flower Number in Oilseed Rape. Remote Sens. 2018, 10, 1484. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Robinson, S.; Galpern, P. Medium-Resolution Multispectral Satellite Imagery in Precision Agriculture: Mapping Precision Canola (Brassica napus L.) Yield Using Sentinel-2 Time Series. Precis. Agric. 2022, 23, 1051–1071. [Google Scholar] [CrossRef]
- Tian, H.; Chen, T.; Li, Q.; Mei, Q.; Wang, S.; Yang, M.; Wang, Y.; Qin, Y. A Novel Spectral Index for Automatic Canola Mapping by Using Sentinel-2 Imagery. Remote Sens. 2022, 14, 1113. [Google Scholar] [CrossRef]
- Zhang, T.; Vail, S.; Duddu, H.S.N.; Parkin, I.A.P.; Guo, X.; Johnson, E.N.; Shirtliffe, S.J. Phenotyping Flowering in Canola (Brassica napus L.) and Estimating Seed Yield Using an Unmanned Aerial Vehicle-Based Imagery. Front. Plant Sci. 2021, 12, 686332. [Google Scholar] [CrossRef] [PubMed]
- Nasteski, V. An Overview of the Supervised Machine Learning Methods. Horiz. B 2017, 4, 51–62. [Google Scholar] [CrossRef]
- Fernando, H.; Ha, T.; Attanayake, A.; Benaragama, D.; Nketia, K.A.; Kanmi-Obembe, O.; Shirtliffe, S.J. High-Resolution Flowering Index for Canola Yield Modelling. Remote Sens. 2022, 14, 4464. [Google Scholar] [CrossRef]
- Lukas, V.; Huňady, I.; Kintl, A.; Mezera, J.; Hammerschmiedt, T.; Sobotková, J.; Brtnický, M.; Elbl, J. Using UAV to Identify the Optimal Vegetation Index for Yield Prediction of Oil Seed Rape (Brassica napus L.) at the Flowering Stage. Remote Sens. 2022, 14, 4953. [Google Scholar] [CrossRef]
- Rai, N.; Pathak, H.; Mahecha, M.V.; Buckmaster, D.R.; Huang, Y.; Overby, P.; Sun, X. A Case Study on Canola (Brassica napus L.) Potential Yield Prediction Using Remote Sensing Imagery and Advanced Data Analytics. Smart Agric. Technol. 2024, 9, 100698. [Google Scholar] [CrossRef]
- Ahmed, S.; Nicholson, C.E.; Rutter, S.R.; Marshall, J.R.; Perry, J.J.; Dean, J.R. Use of an Unmanned Aerial Vehicle for Monitoring and Prediction of Oilseed Rape Crop Performance. PLoS ONE 2023, 18, e0294184. [Google Scholar] [CrossRef]
- Holzapfel, C.B.; Lafond, G.P.; Brandt, S.A.; Bullock, P.R.; Irvine, R.B.; Morrison, M.J.; May, W.E.; James, D.C. Estimating Canola (Brassica napus L.) Yield Potential Using an Active Optical Sensor. Can. J. Plant Sci. 2009, 89, 1149–1160. [Google Scholar] [CrossRef]
- Muruganantham, P.; Wibowo, S.; Grandhi, S.; Samrat, N.H.; Islam, N. A Systematic Literature Review on Crop Yield Prediction with Deep Learning and Remote Sensing. Remote Sens. 2022, 14, 1990. [Google Scholar] [CrossRef]
- Dolado, J.J.; Rodríguez, D.; Riquelme, J.; Ferrer-Troyano, F.; Cuadrado, J.J. A Two-Stage Zone Regression Method for Global Characterization of a Project Database. In Advances in Machine Learning Applications in Software Engineering; IGI Global: Hershey, PA, USA, 2007; pp. 1–13. [Google Scholar]
- Abdelkader, S.S.; Grolinger, K.; Capretz, M.A.M. Predicting Energy Demand Peak Using M5 Model Trees. In Proceedings of the 2015 IEEE 14th International Conference on Machine Learning and Applications (ICMLA), Miami, FL, USA, 9–11 December 2015. [Google Scholar]
- Quinlan Basser, J.R. Learning With Continuous Classes; World Scientific: London, UK, 1992. [Google Scholar]
- Rahimikhoob, A.; Asadi, M.; Mashal, M. A Comparison Between Conventional and M5 Model Tree Methods for Converting Pan Evaporation to Reference Evapotranspiration for Semi-Arid Region. Water Resour. Manag. 2013, 27, 4815–4826. [Google Scholar] [CrossRef]
- Keshtegar, B.; Piri, J.; Hussan, W.U.; Ikram, K.; Yaseen, M.; Kisi, O.; Adnan, R.M.; Adnan, M.; Waseem, M. Prediction of Sediment Yields Using a Data-Driven Radial M5 Tree Model. Water 2023, 15, 1437. [Google Scholar] [CrossRef]
- Witten, I.H.; Frank, E.; Hall, M.A. Data Mining: Practical Machine Learning Tools and Techniques, 3rd ed.; Morgan Kaufmann Publishers: Burlington, NJ, USA, 2011. [Google Scholar]
- Taghi Sattari, M.; Pal, M.; Apaydin, H.; Ozturk, F. M5 Model Tree Application in Daily River Flow Forecasting in Sohu Stream, Turkey. Water Resour. 2013, 40, 233–242. [Google Scholar] [CrossRef]
- Alipour, A.; Yarahmadi, J.; Mahdavi, M. Comparative Study of M5 Model Tree and Artificial Neural Network in Estimating Reference Evapotranspiration Using MODIS Products. J. Climatol. 2014, 2014, 839205. [Google Scholar] [CrossRef]
- Karthikeyan, J.; Murugan, A. Analysis and Prediction for Crop Yield Variations across States in India Using Machine Learning Approaches. NeuroQuantology 2022, 20, 4850. [Google Scholar]
- Gonzalez-Sanchez, A.; Frausto-Solis, J.; Ojeda-Bustamante, W. Attribute Selection Impact on Linear and Nonlinear Regression Models for Crop Yield Prediction. Sci. World J. 2014, 2014, 509429. [Google Scholar] [CrossRef]
- Lakehead University. Who We Are. Available online: https://www.lakeheadu.ca/centre/luars/who (accessed on 21 November 2024).
- MicaSense. RedEdge-MX Integration Guide. Available online: https://support.micasense.com/hc/en-us/articles/360011389334-RedEdge-MX-Integration-Guide (accessed on 16 November 2024).
- DJI. Matrice 300 RTK. Available online: https://www.dji.com/ca/support/product/matrice-300 (accessed on 16 November 2024).
- Canola Council of Canada. Canola Growth Stages. Available online: https://www.canolacouncil.org/canola-encyclopedia/growth-stages/#overview-of-canola-growth-stages (accessed on 17 November 2024).
- PIX4D. PIX4Dmapper. Available online: https://www.pix4d.com/product/pix4dmapper-photogrammetry-software/ (accessed on 21 November 2024).
- Therneau, T.; Atkinson, B.; Ripley, B. Package “rpart”, version 4.1.24; UTC: Washington, DC, USA, 2025.
- Xu, M.; Watanachaturaporn, P.; Varshney, P.; Arora, M. Decision Tree Regression for Soft Classification of Remote Sensing Data. Remote Sens. Environ. 2005, 97, 322–336. [Google Scholar] [CrossRef]
- Ahmad, A. Decision Tree Ensembles Based on Kernel Features. Appl. Intell. 2014, 41, 855–869. [Google Scholar] [CrossRef]
- Sulik, J.J.; Long, D.S. Spectral Indices for Yellow Canola Flowers. Int. J. Remote Sens. 2015, 36, 2751–2765. [Google Scholar] [CrossRef]
- Sulik, J.J.; Long, D.S. Spectral Considerations for Modeling Yield of Canola. Remote Sens. Environ. 2016, 184, 161–174. [Google Scholar] [CrossRef]
- Ashourloo, D.; Shahrabi, H.S.; Azadbakht, M.; Aghighi, H.; Nematollahi, H.; Alimohammadi, A.; Matkan, A.A. Automatic Canola Mapping Using Time Series of Sentinel 2 Images. ISPRS J. Photogramm. Remote Sens. 2019, 156, 63–76. [Google Scholar] [CrossRef]
- Fernando, H. Remote Sensing Approaches in Canola Seed Yield Estimation Using Reproductive Spectral Signature. Ph.D. Dissertation, University of Saskatchewan, Saskatoon, SK, Canada, 2022. [Google Scholar]
- Leandro, E.R.; Heenkenda, M.K.; Romero, K.F. Estimating Sugarcane Maturity Using High Spatial Resolution Remote Sensing Images. Crops 2024, 4, 333–347. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote Estimation of Chlorophyll Content in Higher Plant Leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Thompson, C.N.; Guo, W.; Sharma, B.; Ritchie, G.L. Using Normalized Difference Red Edge Index to Assess Maturity in Cotton. Crop Sci. 2019, 59, 2167–2177. [Google Scholar] [CrossRef]
- Rouse, J.W. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA Technical Reports Server: Houston, TX, USA, 1974.
- Penuelas, J.; Baret, F.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Fernando, H.; Ha, T.; Duddu, H.; Attanayake, A.; Olakorede, K.-O.; Shirtliffe, S. Canola Yield Simulation through Digitalized Flower Number Using High-Resolution UAV-RGB Imagery 2021. 2022. Available online: https://essopenarchive.org/doi/full/10.1002/essoar.10508314.3 (accessed on 24 September 2024).
- Esri. ArcGIS PRO. Available online: https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview (accessed on 28 November 2024).
- Hornik, K.; Buchta, C.; Karatzoglou, A.; Meyer, D.; Zeileis, A. Package “RWeka”, version 0.4-46; UTC: Washington, DC, USA, 2023.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 28 September 2024).
- Roth, G.W.; Hunter, J. Winter Canola in Pennsylvania: Production and Agronomic Recommendations; Penn State Extension: State College, PA, USA, 2010. [Google Scholar]
- Ontario. Climate Zones and Planting Dates for Vegetables in Ontario. Available online: https://www.ontario.ca/page/climate-zones-and-planting-dates-vegetables-ontario (accessed on 24 February 2025).
- Chapagain, T. Farming in Northern Ontario: Untapped Potential for the Future. Agronomy 2017, 7, 59. [Google Scholar] [CrossRef]
- Shao, C.; Shuai, Y.; Wu, H.; Deng, X.; Zhang, X.; Xu, A. Development of a Spectral Index for the Detection of Yellow-Flowering Vegetation. Remote Sens. 2023, 15, 1725. [Google Scholar] [CrossRef]
- Shen, M.; Chen, J.; Zhu, X.; Tang, Y. Yellow Flowers Can Decrease NDVI and EVI Values: Evidence from a Field Experiment in an Alpine Meadow. Can. J. Remote Sens. 2009, 35, 99–106. [Google Scholar] [CrossRef]
- Singh, K.D.; Duddu, H.S.N.; Vail, S.; Parkin, I.; Shirtliffe, S.J. UAV-Based Hyperspectral Imaging Technique to Estimate Canola (Brassica napus L.) Seedpods Maturity. Can. J. Remote Sens. 2021, 47, 33–47. [Google Scholar] [CrossRef]
- Collins, W. Remote Sensing of Crop Type and Maturity. Photogramm. Eng. Remote Sens. 1978, 44, 43–55. [Google Scholar]
- Fayaz, S.A.; Zaman, M.; Kaul, S.; Butt, M.A. How M5 Model Trees (M5-MT) on Continuous Data Are Used in Rainfall Prediction: An Experimental Evaluation. Rev. D’intelligence Artif. 2022, 36, 409–415. [Google Scholar] [CrossRef]
- Edwards, J. Canola Growth & Development; NSW Department of Primary Industries: Sydney, Australia, 2011; ISBN 9781742562124.
- Yantai, G.; Harker, K.N.; Kutcher, H.R.; Gulden, R.H.; Irvine, B.; May, W.E.; O’Donovan, J.T. Canola Seed Yield and Phenological Responses to Plant Density. Can. J. Plant Sci. 2016, 96, 151–159. [Google Scholar] [CrossRef]
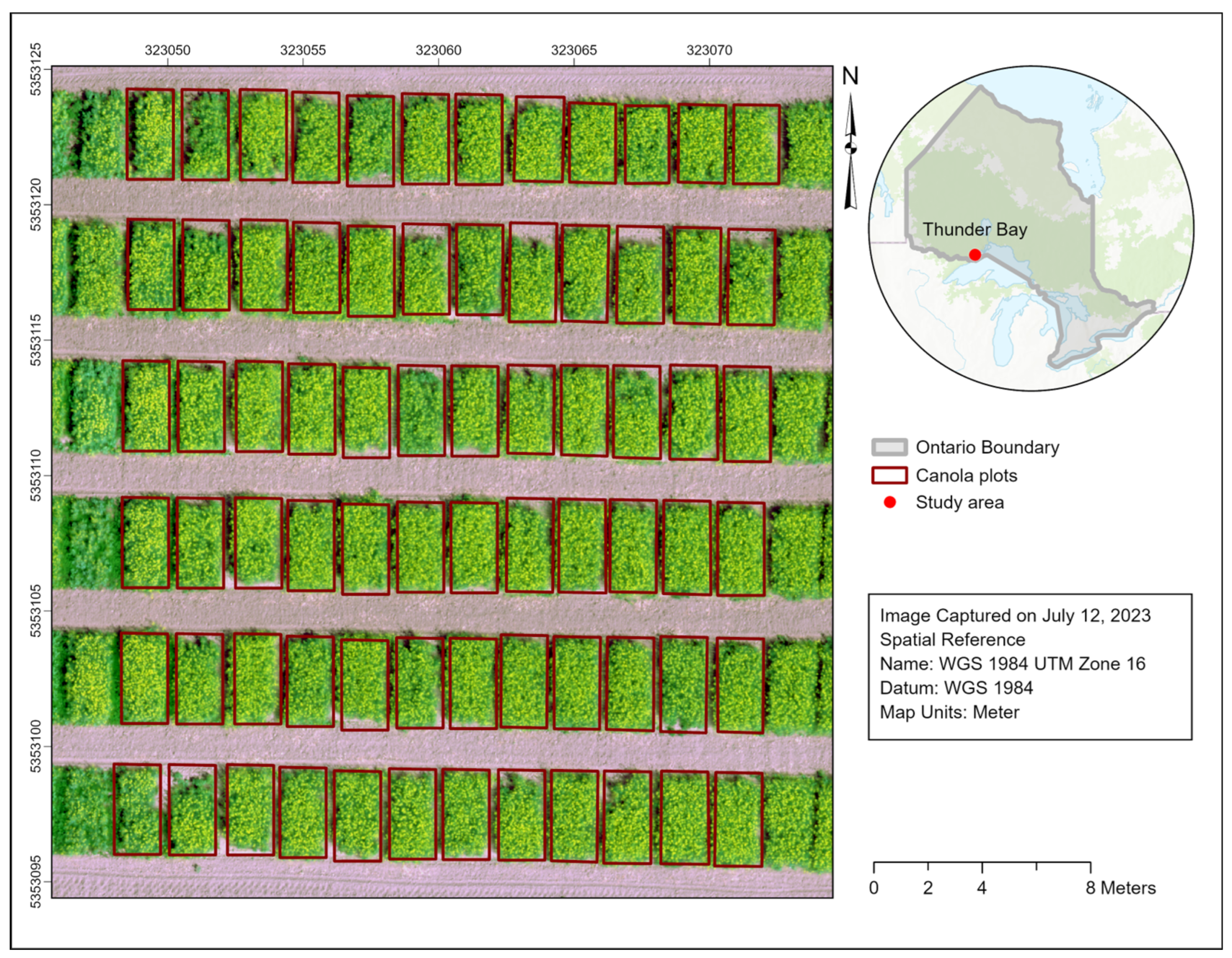
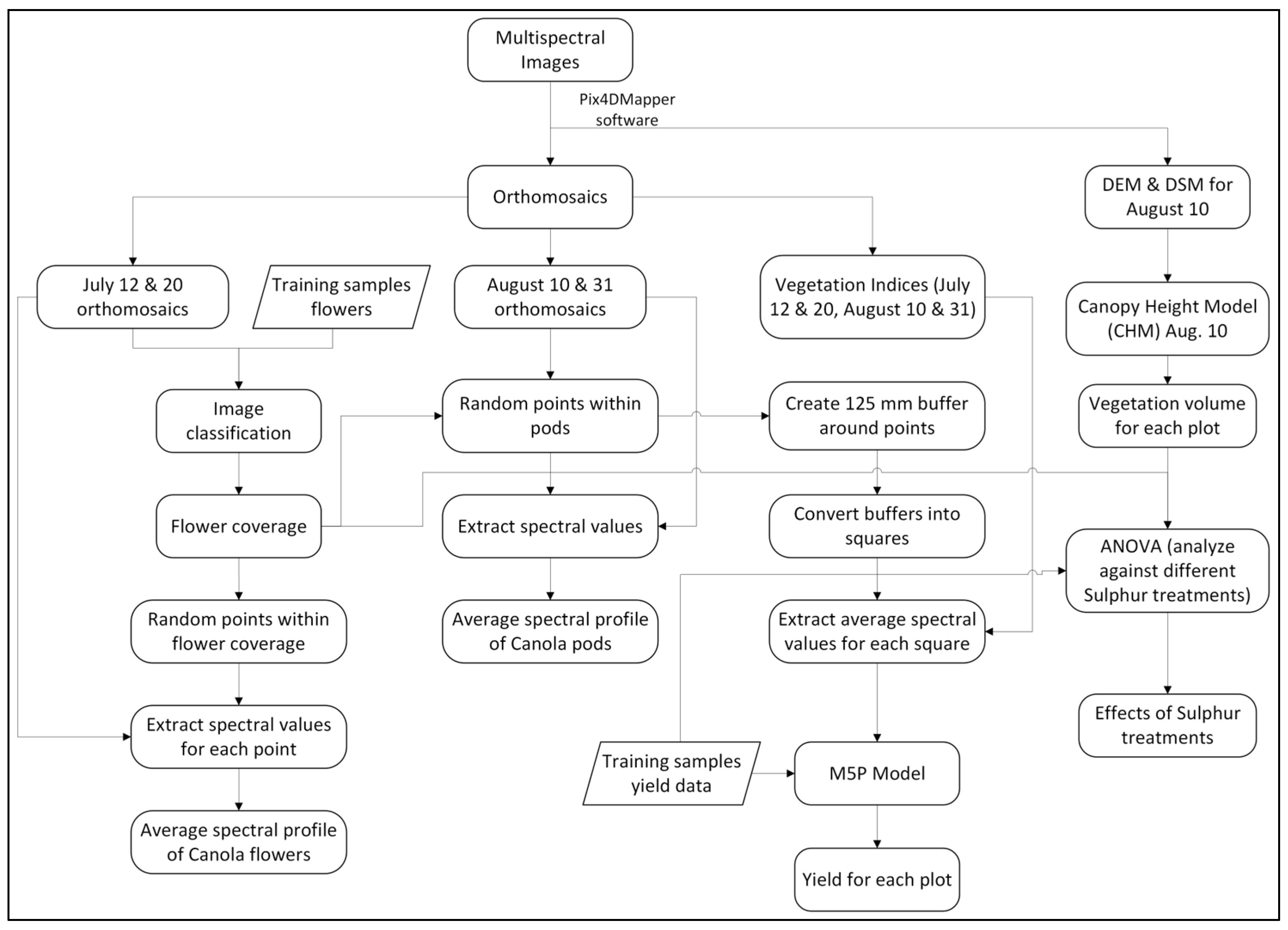


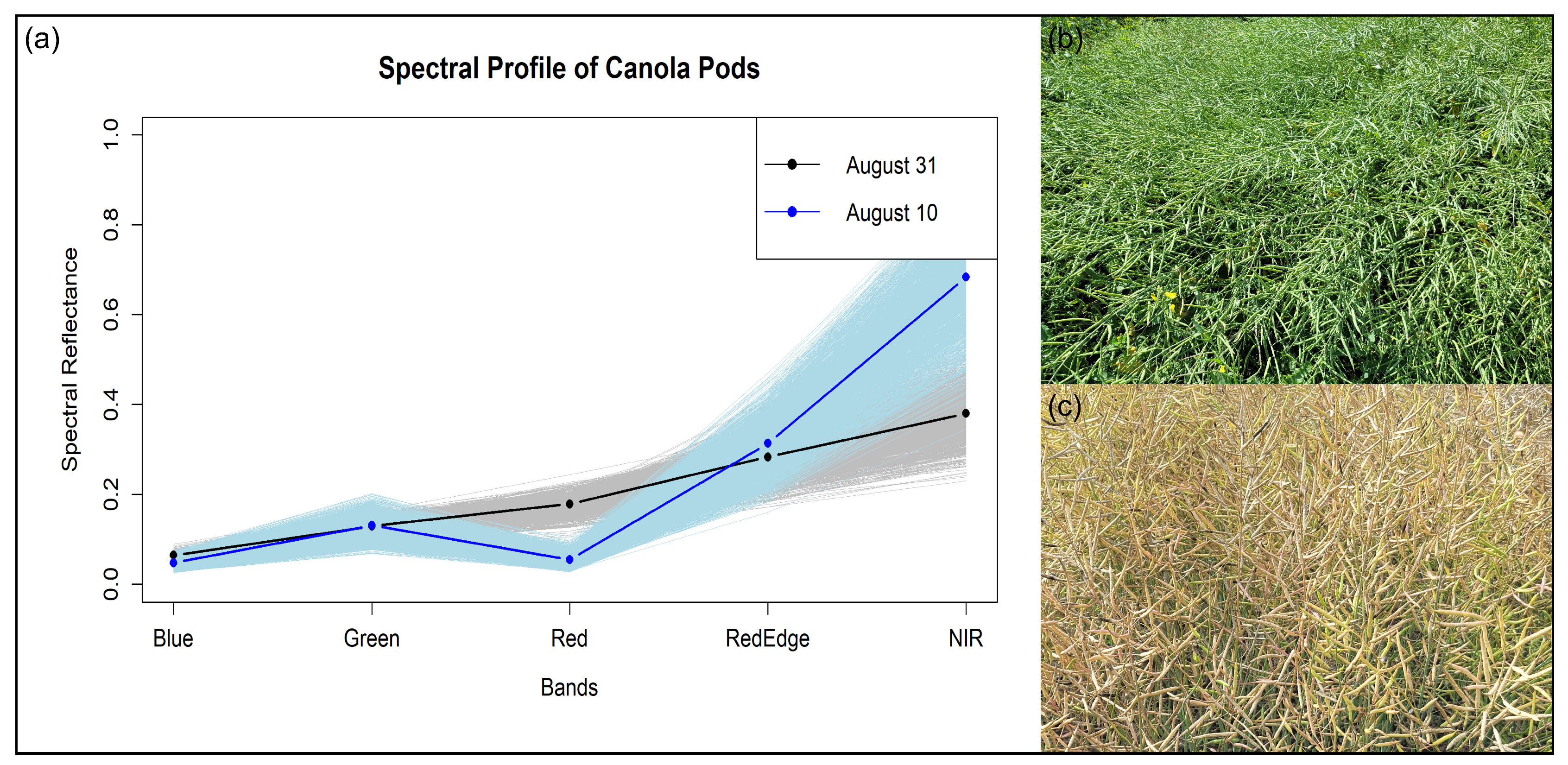

| Treatment No. | Treatment |
|---|---|
| T1 | No Sulfur |
| T2 | Ammonium Sulphate at 36 kg S/ha and Phosphorous as per soil test |
| T3 | Ammonium Sulphate at 36 kg S/ha |
| T4 | Ammonium Sulphate at 24 kg S/ha and SymTRX S10 * at 12 kg S/ha |
| T5 | Ammonium Sulphate at 18 kg S/ha and SymTRX S10 * at 18 kg S/ha |
| T6 | Ammonium Sulphate at 12 kg S/ha and SymTRX S10 * at 24 kg S/ha |
| T7 | SymTRX S10 * at 36 kg S/ha |
| Stages | Image Date |
|---|---|
| Bare soil/seeding | 12 May 2023 |
| Germination | 26 May 2023 |
| Leaf development | 8 and 16 June 2023 |
| Rosette | 23 June 2023 |
| Bolting | 4 July 2023 |
| Flowering | 12 July 2023 |
| Podding | 20 July 2023 |
| Ripening | 10 August 2023 |
| Senescence/harvesting | 31 August 2023 |
| Vegetation Index | Equation |
|---|---|
| Normalized Difference Yellowness Index [44] | NDYI = (G − B)/(G + B) |
| Difference Yellowness Index [44] | DYI = G − B |
| Canola Index [45] | CI = NIR × (R + G) |
| Canola Ratio Index [43] | CRI = G/B |
| Canola Flower Index [16] | CFI = NDVI × (R + G) |
| Normalized Difference Red Edge [48] | NDRE = (NIR − RE)/(NIR + RE) |
| Green Normalized Difference Vegetation Index [49] | GNDRE = (RE − G)/(RE + G) |
| Normalized Difference Vegetation Index [50] | NDVI = (NIR − R)/(NIR + R) |
| Blue Normalized Difference Vegetation Index [44] | BNDVI = (NIR − B)/(NIR + B) |
| Structure Insensitive Pigment Index [51] | SIPI = (NIR − R)/(NIR − B) |
| Normalized Difference Red-Blue Index [52] | NDRB = (R − B)/(R + B) |
| Image Date | Vegetation Index |
|---|---|
| 20 July | CI, CRI, CFI, DYI, NDYI, NDVI, SIPI, GNDRE, and NDRB |
| 31 August | CRI, CFI, and NDYI |
| 12 and 20 July | Percentage of flower coverage |
| Image Date | Class * | Producer’s Accuracy | User’s Accuracy |
|---|---|---|---|
| 12 July 2023 | Flowers 1 | 84.62% | 88.00% |
| Flowers 2 | 91.67% | 84.62% | |
| 20 July 2023 | Flowers 1 | 100.00% | 98.28% |
| Metric | Model Sensitivity (Cross-Validation) |
|---|---|
| Correlation coefficient (r) | 0.88 |
| Mean Absolute Error (MAE) | 0.50 mt |
| Root Mean Squared Error (RMSE) | 0.68 mt |
| Relative Absolute Error (RAE) | 51% |
| Root Relative Squared Error (RRSE) | 57% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallas Calderón, I.D.l.Á.; Heenkenda, M.K.; Sahota, T.S.; Serrano, L.S. Canola Yield Estimation Using Remotely Sensed Images and M5P Model Tree Algorithm. Remote Sens. 2025, 17, 2127. https://doi.org/10.3390/rs17132127
Fallas Calderón IDlÁ, Heenkenda MK, Sahota TS, Serrano LS. Canola Yield Estimation Using Remotely Sensed Images and M5P Model Tree Algorithm. Remote Sensing. 2025; 17(13):2127. https://doi.org/10.3390/rs17132127
Chicago/Turabian StyleFallas Calderón, Ileana De los Ángeles, Muditha K. Heenkenda, Tarlok S. Sahota, and Laura Segura Serrano. 2025. "Canola Yield Estimation Using Remotely Sensed Images and M5P Model Tree Algorithm" Remote Sensing 17, no. 13: 2127. https://doi.org/10.3390/rs17132127
APA StyleFallas Calderón, I. D. l. Á., Heenkenda, M. K., Sahota, T. S., & Serrano, L. S. (2025). Canola Yield Estimation Using Remotely Sensed Images and M5P Model Tree Algorithm. Remote Sensing, 17(13), 2127. https://doi.org/10.3390/rs17132127






