Investigating the Mechanisms of Hyperspectral Remote Sensing for Belowground Yield Traits in Potato Plants
Abstract
1. Introduction
2. Field Experiment and Data Collection
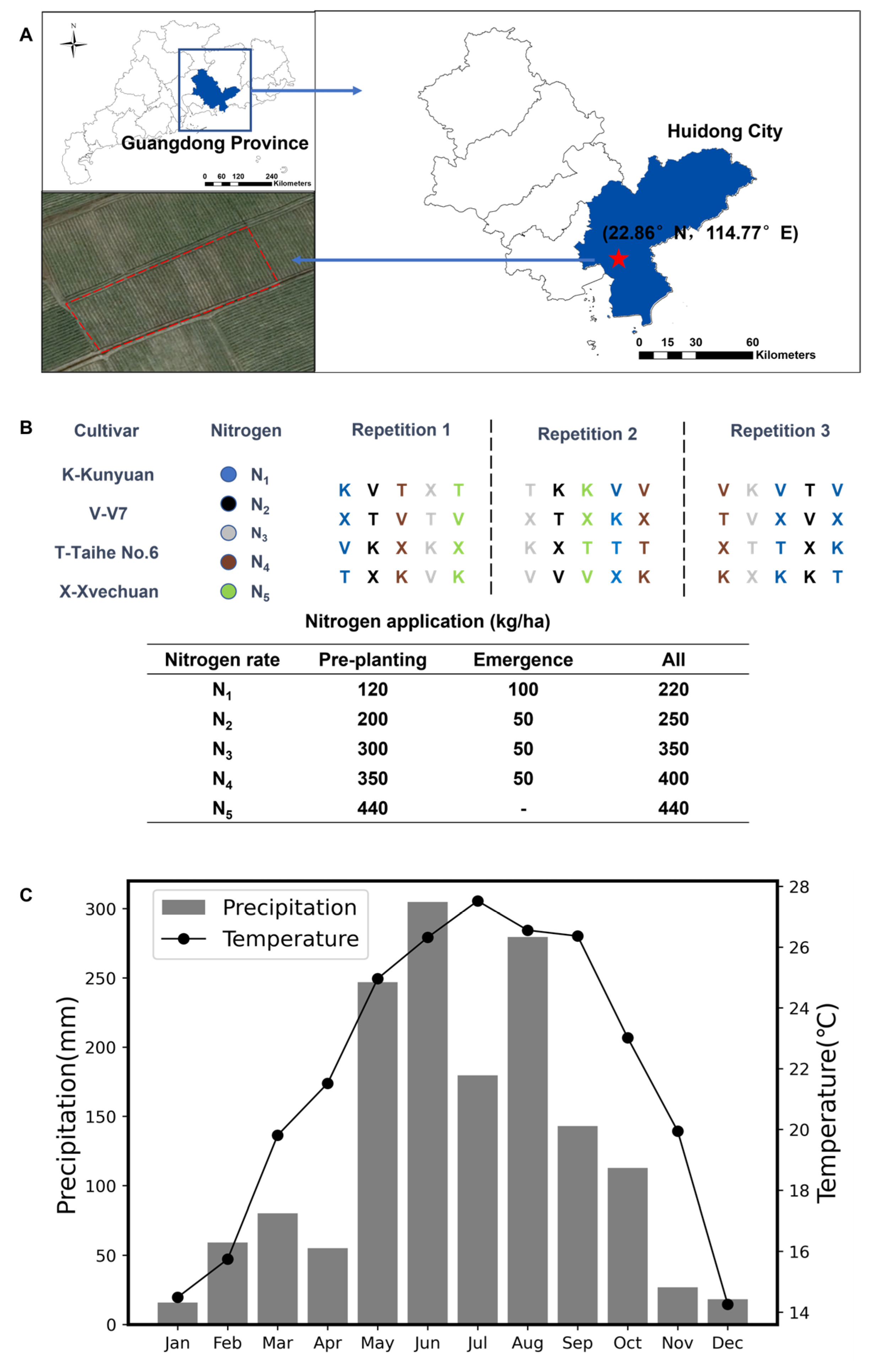
2.1. Field Experiment
2.2. Data Collection
3. Methods
3.1. The Direct Inversion Approach
3.2. The Indirect Inversion Approach
3.3. Model Evaluation
4. Results
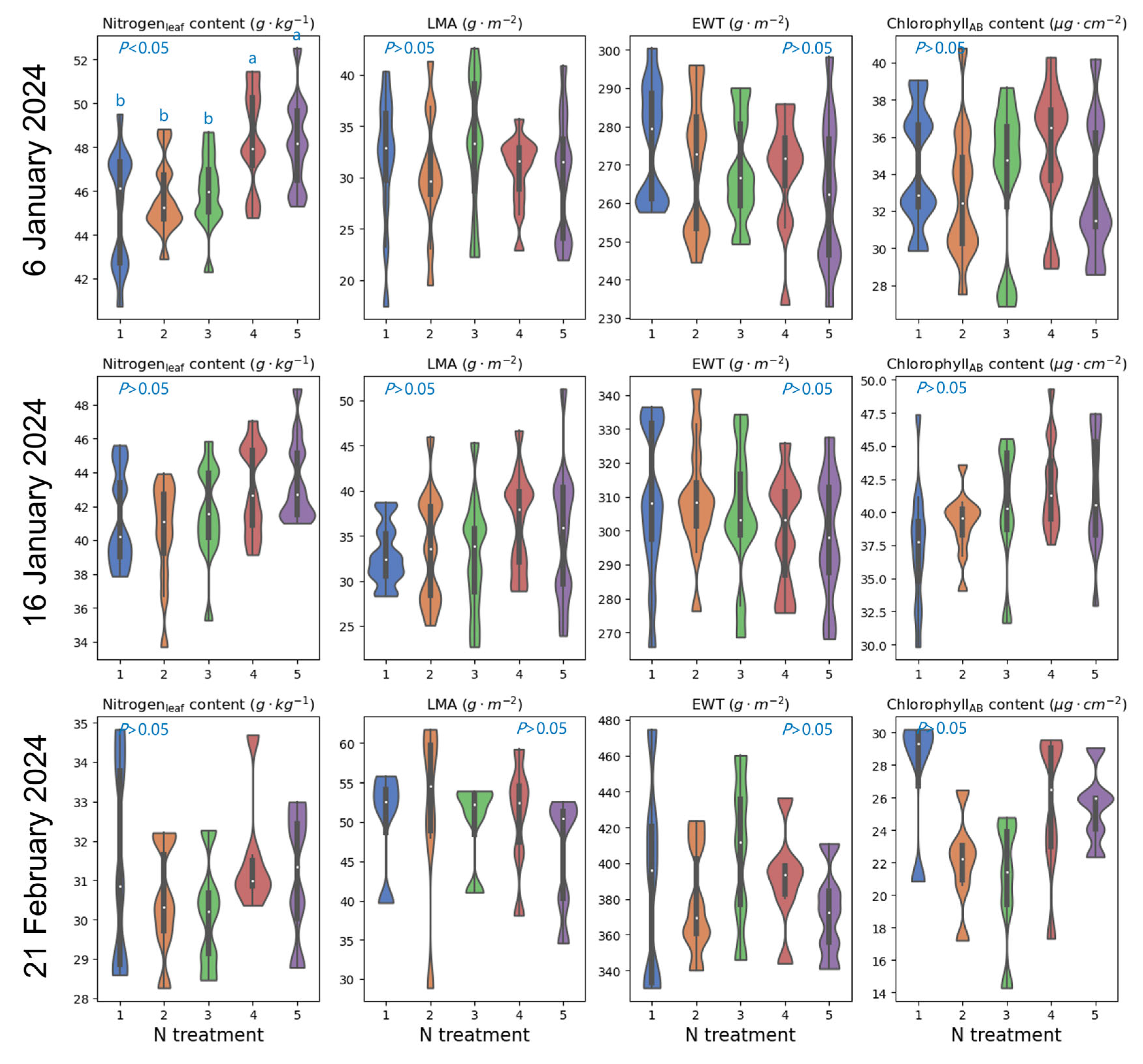
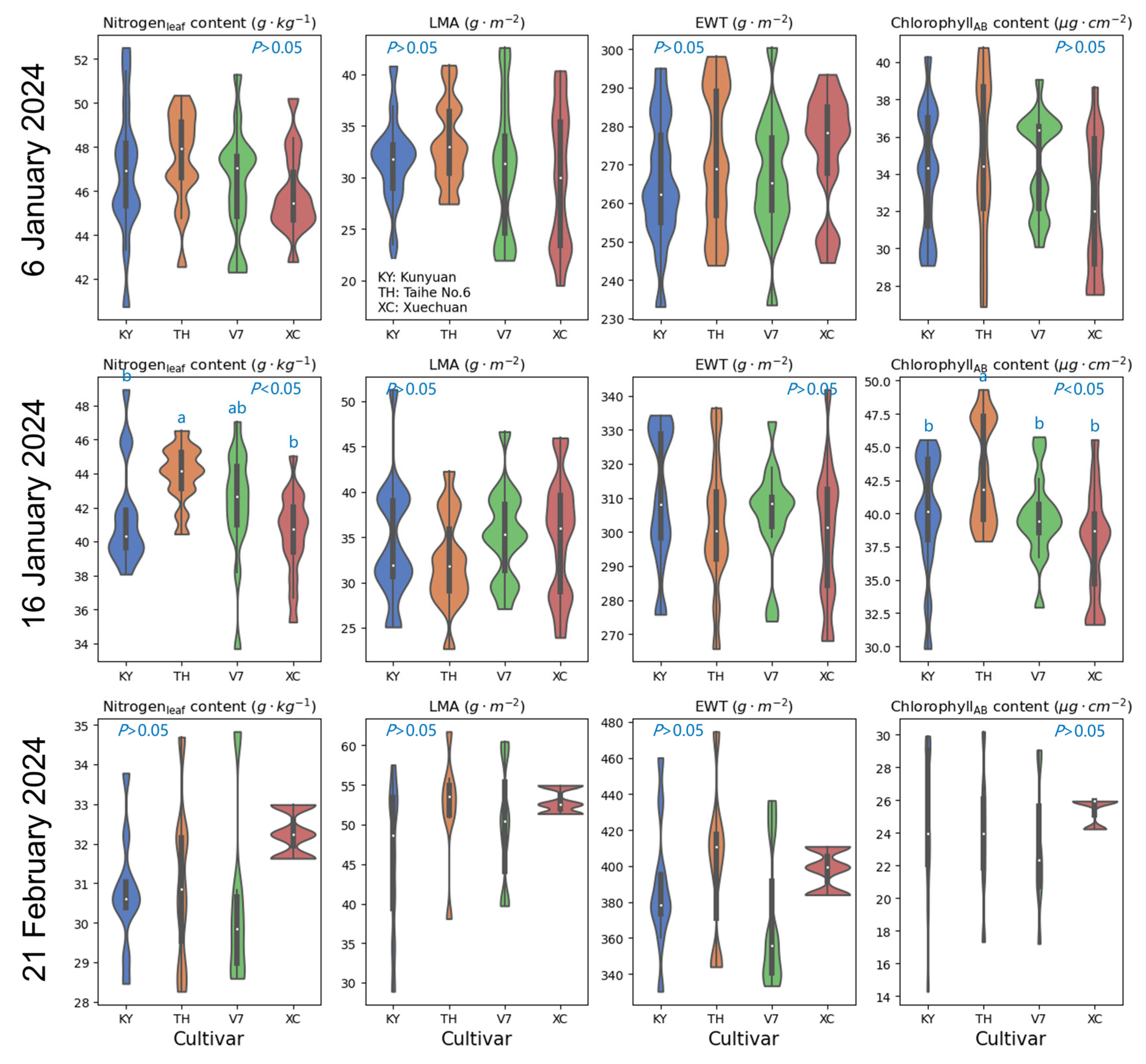
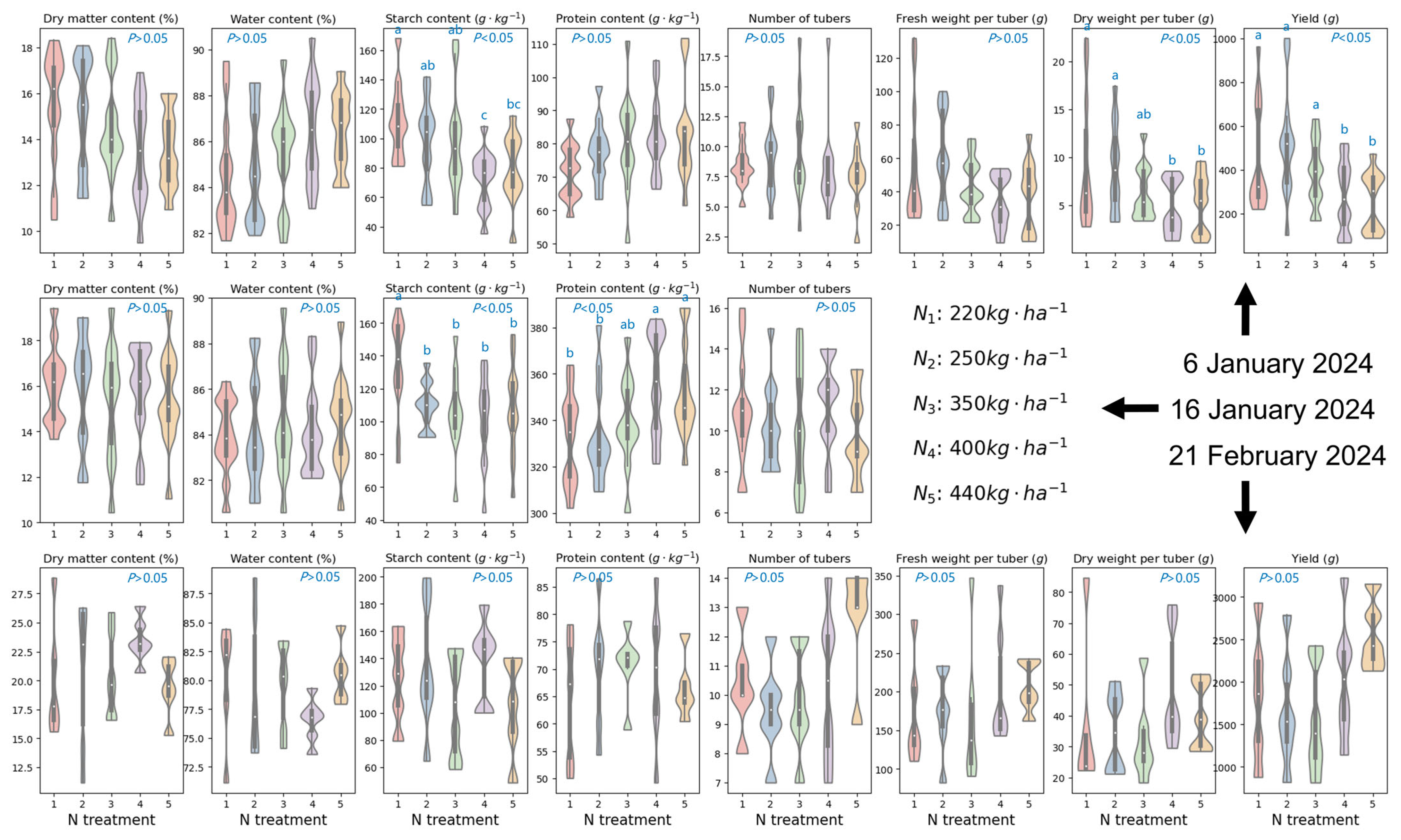
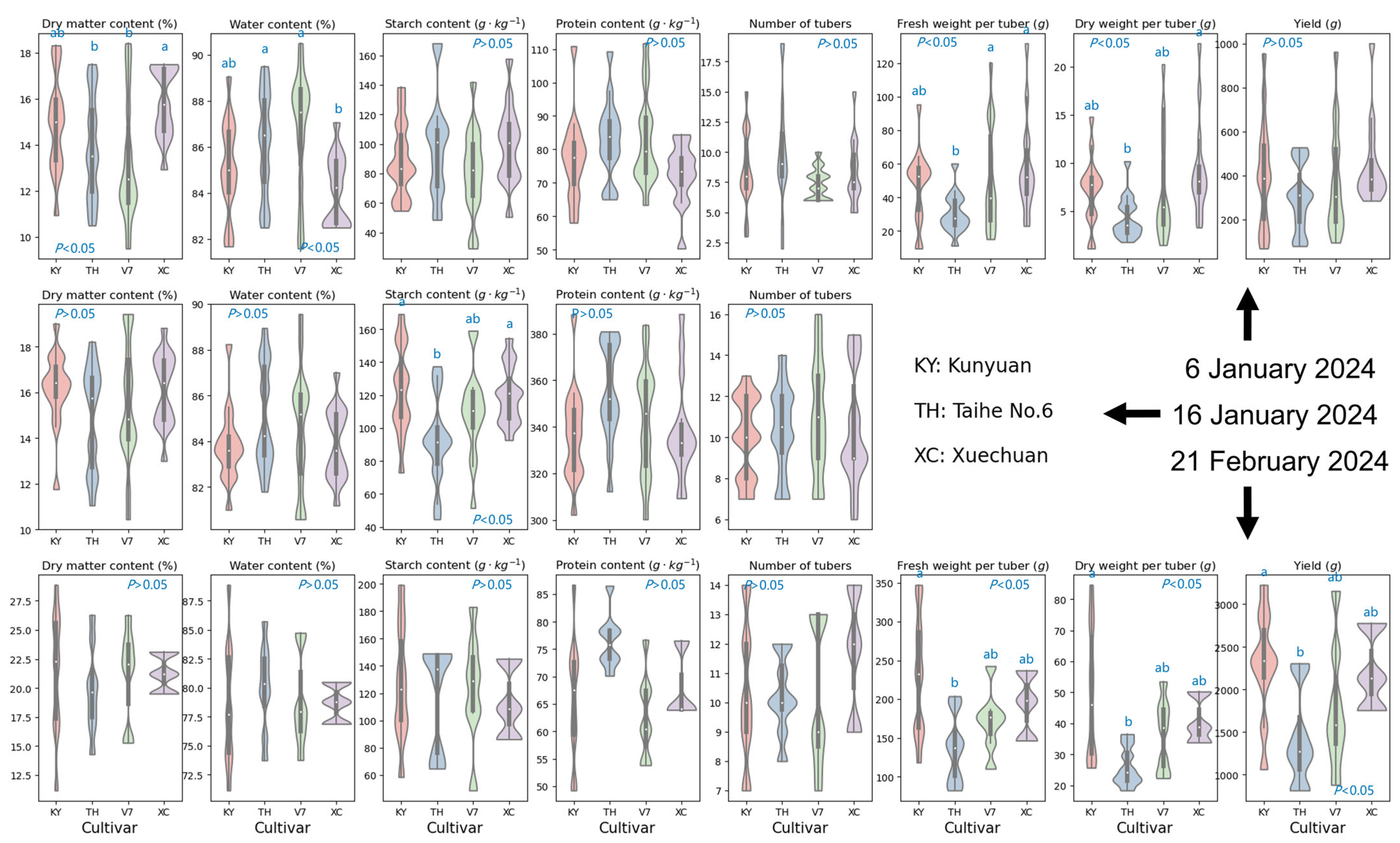
4.1. Influence of Nitrogen Application Rates and Cultivar Selection on Foliar Biochemistries
4.2. Influence of Nitrogen Application Rates and Cultivar Selection on Yield Traits
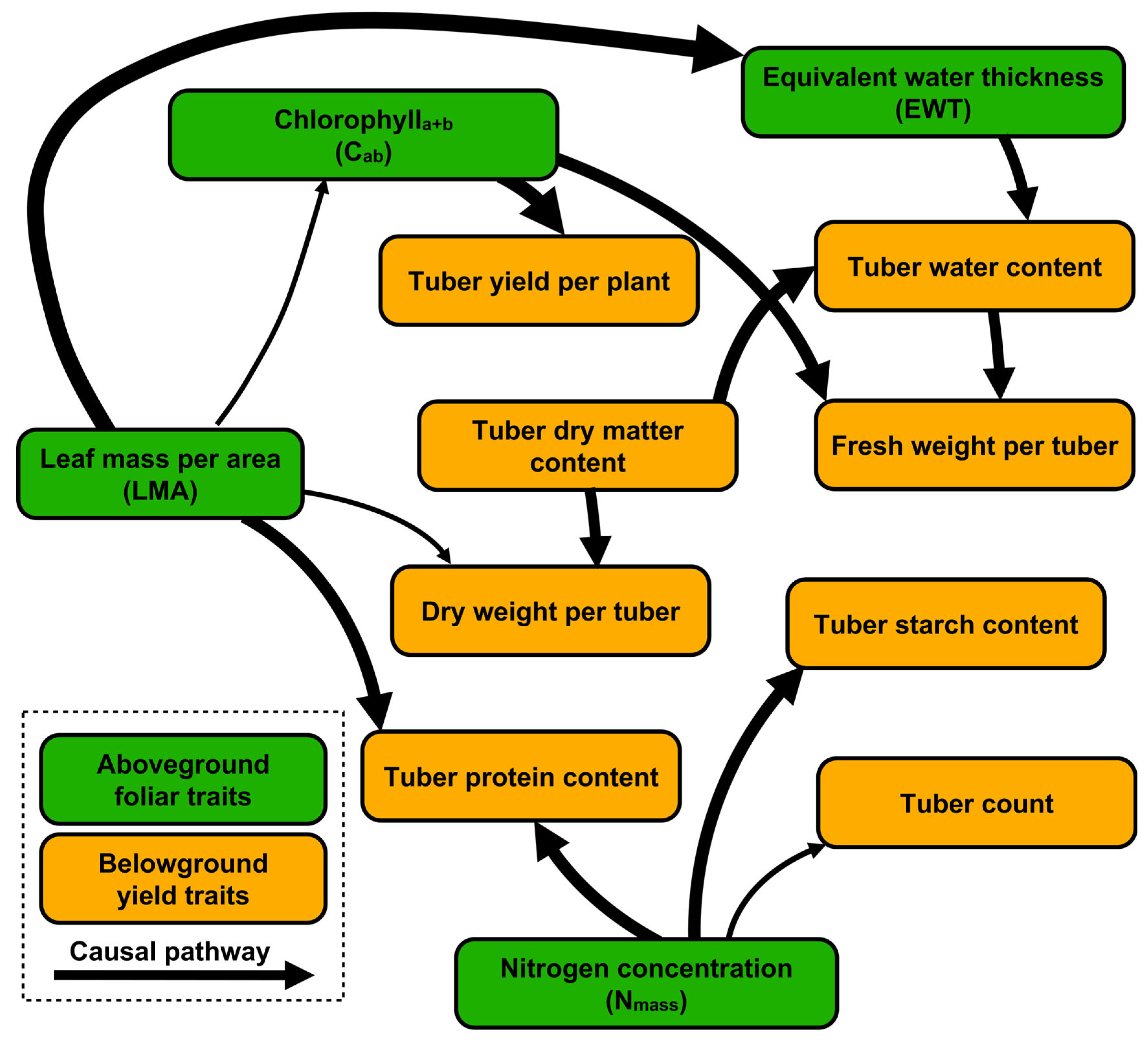
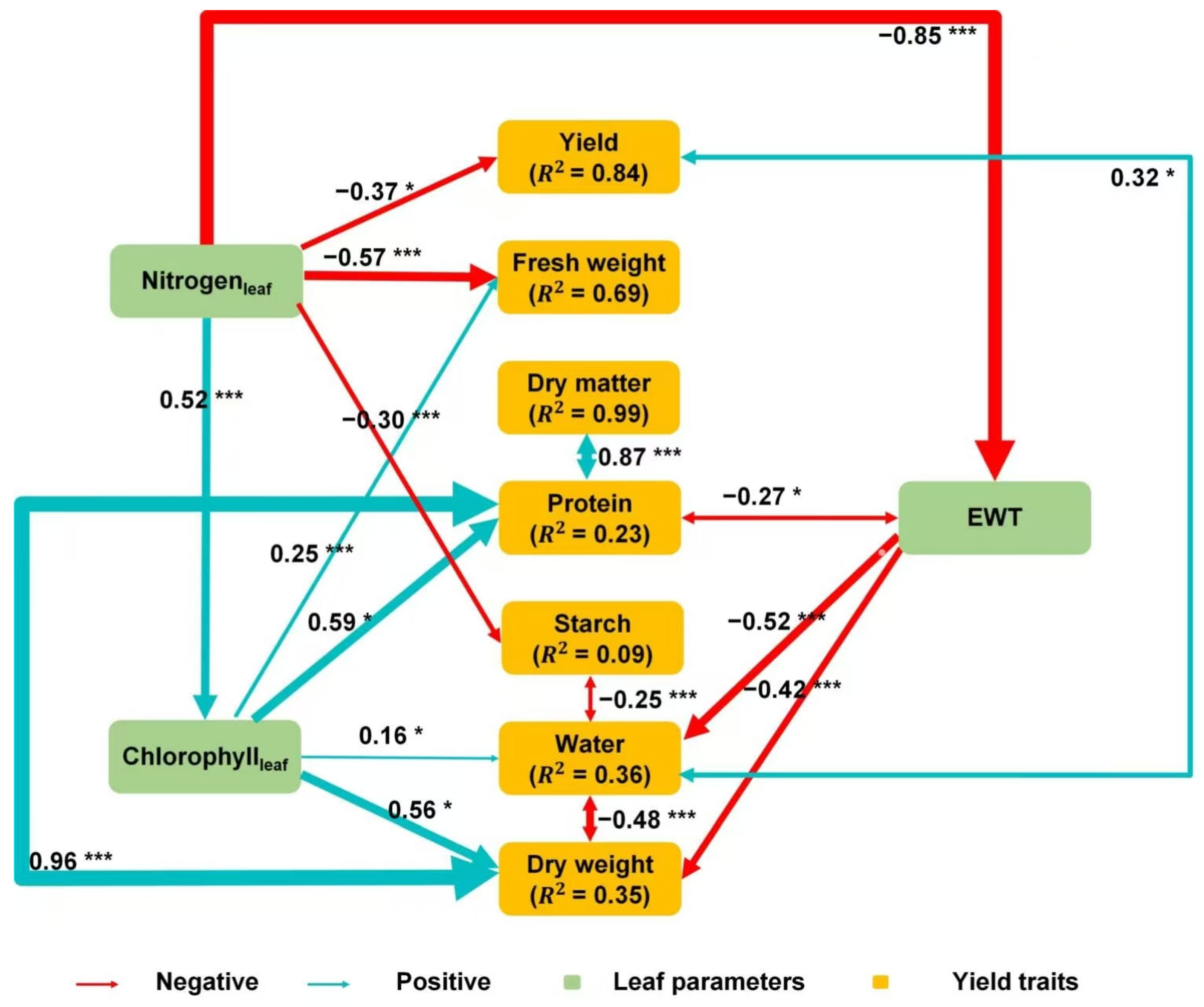
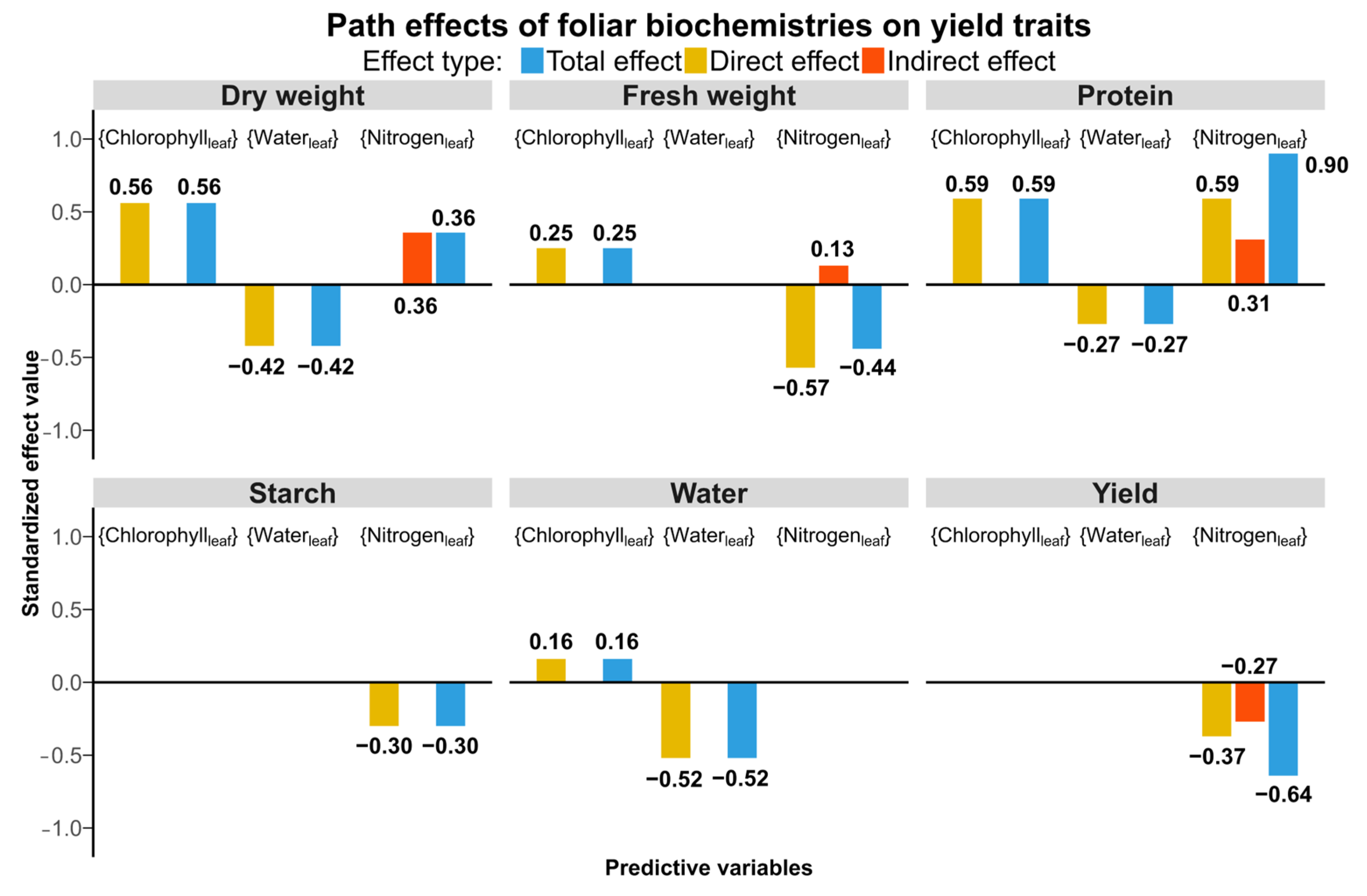
4.3. Relationships Between Aboveground Foliar Traits and Belowground Yield Traits
4.4. Direct Inversion of Belowground Yield Traits
4.5. Indirect Inversion of Belowground Yield Traits
5. Discussion
5.1. Effects of Aboveground Foliar Biochemistries on Belowground Yield Traits
5.2. Comparison of Direct and Indirect Inversion Approaches
5.3. Implications for Precision Agriculture
5.4. Future Research
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- FAO. A Guide to the International Day of Potato 2024; Foods and Agriculture Organization of the United Nations: Rome, Italy, 2024. [Google Scholar]
- Chivasa, W.; Mutanga, O.; Biradar, C. Application of Remote Sensing in Estimating Maize Grain Yield in Heterogeneous African Agricultural Landscapes: A Review. Int. J. Remote Sens. 2017, 38, 6816–6845. [Google Scholar] [CrossRef]
- Chang, D.C.; Hur, O.S.; Park, C.S.; Kim, S.Y. Field Performance, Yield Components and Nitrogen Utilization Efficiency of Potato Plants Grown from Hydroponic Small Tubers. Hortic. Environ. Biotechnol. 2011, 52, 369–375. [Google Scholar] [CrossRef]
- Kammlade, S.M. Potato Tuber Yield, Quality, Mineral Nutrient Concentration, Soil Health and Soil Food Web in Conventional and Organic Potato Systems. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2015. [Google Scholar]
- Liu, N.; Townsend, P.A.; Naber, M.R.; Bethke, P.C.; Hills, W.B.; Wang, Y. Hyperspectral Imagery to Monitor Crop Nutrient Status Within and Across Growing Seasons. Remote Sens. Environ. 2021, 255, 112303. [Google Scholar] [CrossRef]
- Bala, S.K.; Islam, A.S. Correlation Between Potato Yield and MODIS-derived Vegetation Indices. Int. J. Remote Sens. 2009, 30, 2491–2507. [Google Scholar] [CrossRef]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, J.L. Potato Yield Prediction Using Machine Learning Techniques and Sentinel 2 Data. Remote Sens. 2019, 11, 1745. [Google Scholar] [CrossRef]
- Li, B.; Xu, X.; Zhang, L.; Han, J.; Bian, C.; Li, G.; Liu, J.; Jin, L. Above-Ground Biomass Estimation and Yield Prediction in Potato by Using UAV-Based RGB and Hyperspectral Imaging. ISPRS J. Photogramm. Remote Sens. 2020, 162, 161–172. [Google Scholar] [CrossRef]
- Luo, S.; He, Y.; Li, Q.; Jiao, W.; Zhu, Y.; Zhao, X. Nondestructive Estimation of Potato Yield Using Relative Variables Derived from Multi-Period LAI and Hyperspectral Data Based on Weighted Growth Stage. Plant Methods 2020, 16, 150. [Google Scholar] [CrossRef]
- Nigon, T.J. Aerial Imagery and Other Non-Invasive Approaches to Detect Nitrogen and Water Stress in A Potato Crop; University of Minnesota: Minneapolis, MN, USA, 2012; ISBN 1-267-86282-3. [Google Scholar]
- Salvador, P.; Gómez, D.; Sanz, J.; Casanova, J.L. Estimation of Potato Yield Using Satellite Data at a Municipal Level: A Machine Learning Approach. Int. J. Geo-Inf. 2020, 9, 343. [Google Scholar] [CrossRef]
- Maimaitijiang, M.; Sagan, V.; Sidike, P.; Hartling, S.; Esposito, F.; Fritschi, F.B. Soybean Yield Prediction from UAV Using Multimodal Data Fusion and Deep Learning. Remote Sens. Environ. 2020, 237, 111599. [Google Scholar] [CrossRef]
- Torgbor, B.A.; Rahman, M.M.; Brinkhoff, J.; Sinha, P.; Robson, A. Integrating Remote Sensing and Weather Variables for Mango Yield Prediction Using a Machine Learning Approach. Remote Sens. 2023, 15, 3075. [Google Scholar] [CrossRef]
- Guan, K.; Wu, J.; Kimball, J.S.; Anderson, M.C.; Frolking, S.; Li, B.; Hain, C.R.; Lobell, D.B. The Shared and Unique Values of Optical, Fluorescence, Thermal and Microwave Satellite Data for Estimating Large-Scale Crop Yields. Remote Sens. Environ. 2017, 199, 333–349. [Google Scholar] [CrossRef]
- Sun, C.; Feng, L.; Zhang, Z.; Ma, Y.; Crosby, T.; Naber, M.; Wang, Y. Prediction of End-Of-Season Tuber Yield and Tuber Set in Potatoes Using In-Season UAV-Based Hyperspectral Imagery and Machine Learning. Sensors 2020, 20, 5293. [Google Scholar] [CrossRef] [PubMed]
- Abrougui, K.; Gabsi, K.; Mercatoris, B.; Khemis, C.; Amami, R.; Chehaibi, S. Prediction of Organic Potato Yield Using Tillage Systems and Soil Properties by Artificial Neural Network (ANN) and Multiple Linear Regressions (MLR). Soil Tillage Res. 2019, 190, 202–208. [Google Scholar] [CrossRef]
- Ebrahimy, H.; Wang, Y.; Zhang, Z. Utilization of Synthetic Minority Oversampling Technique for Improving Potato Yield Prediction Using Remote Sensing Data and Machine Learning Algorithms with Small Sample Size of Yield Data. ISPRS J. Photogramm. Remote Sens. 2023, 201, 12–25. [Google Scholar] [CrossRef]
- Sagan, V.; Maimaitijiang, M.; Bhadra, S.; Maimaitiyiming, M.; Brown, D.R.; Sidike, P.; Fritschi, F.B. Field-Scale Crop Yield Prediction Using Multi-Temporal WorldView-3 and PlanetScope Satellite Data and Deep Learning. ISPRS J. Photogramm. Remote Sens. 2021, 174, 265–281. [Google Scholar] [CrossRef]
- Tedesco, D.; Almeida Moreira, B.R.D.; Barbosa Júnior, M.R.; Papa, J.P.; Silva, R.P.D. Predicting on Multi-Target Regression for the Yield of Sweet Potato by the Market Class of Its Roots upon Vegetation Indices. Comput. Electron. Agric. 2021, 191, 106544. [Google Scholar] [CrossRef]
- Rattanasopa, K.; Saengprachatanarug, K.; Wongpichet, S.; Posom, J.; Saikaew, K.; Ungsathittavorn, K.; Pilawut, S.; Chinapas, A.; Taira, E. UAV-Based Multispectral Imagery for Estimating Cassava Tuber Yields. Eng. Agric. Environ. Food 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Njane, S.N.; Tsuda, S.; Sugiura, R.; Katayama, K.; Goto, K.; Tsuchiya, S.; Tsuji, H. Phenotyping System for Precise Monitoring of Potato Crops during Growth. Eng. Agric. Environ. Food 2023, 16, 24–36. [Google Scholar] [CrossRef]
- Hoque, M.J.; Islam, M.S.; Uddin, J.; Samad, M.A.; De Abajo, B.S.; Vargas, D.L.R.; Ashraf, I. Incorporating Meteorological Data and Pesticide Information to Forecast Crop Yields Using Machine Learning. IEEE Access 2024, 12, 47768–47786. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sharma, D.P.; Verma, J.K. Study on Machine-Learning Algorithms in Crop Yield Predictions Specific to Indian Agricultural Contexts. In Proceedings of the 2021 International Conference on Computational Performance Evaluation (ComPE), Shillong, India, 1 December 2021; pp. 155–166. [Google Scholar]
- Nnadozie, E.C.; Iloanusi, O.N.; Ani, O.A.; Yu, K. Detecting Cassava Plants under Different Field Conditions Using UAV-Based RGB Images and Deep Learning Models. Remote Sens. 2023, 15, 2322. [Google Scholar] [CrossRef]
- Wellens, J.; Raes, D.; Fereres, E.; Diels, J.; Coppye, C.; Adiele, J.G.; Ezui, K.S.G.; Becerra, L.-A.; Selvaraj, M.G.; Dercon, G.; et al. Calibration and Validation of the FAO AquaCrop Water Productivity Model for Cassava (Manihot Esculenta Crantz). Agric. Water Manag. 2022, 263, 107491. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Yue, J.; Jin, X.; Chen, R.; Bian, M.; Ma, Y.; Yang, G.; Feng, H. Estimation of Potato Yield Using a Semi-Mechanistic Model Developed by Proximal Remote Sensing and Environmental Variables. Comput. Electron. Agric. 2024, 223, 109117. [Google Scholar] [CrossRef]
- Singh, K.; Huang, Y.; Young, W.; Harvey, L.; Hall, M.; Zhang, X.; Lobaton, E.; Jenkins, J.; Shankle, M. Sweet Potato Yield Prediction Using Machine Learning Based on Multispectral Images Acquired from a Small Unmanned Aerial Vehicle. Agriculture 2025, 15, 420. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, M.; Liao, Z.; Guo, J.; Zhang, F.; Fan, J.; Feng, H.; Yang, Q.; Wu, L.; Wang, X. Performance Evaluation of AquaCrop and DSSAT-SUBSTOR-Potato Models in Simulating Potato Growth, Yield and Water Productivity under Various Drip Fertigation Regimes. Agric. Water Manag. 2023, 276, 108076. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Tang, J.; Wang, E.; Pan, Z.; Pan, X.; Hu, Q. Optimum Planting Date and Cultivar Maturity to Optimize Potato Yield and Yield Stability in North China. Field Crops Res. 2021, 269, 108179. [Google Scholar] [CrossRef]
- Camino, C.; Araño, K.; Berni, J.A.; Dierkes, H.; Trapero-Casas, J.L.; León-Ropero, G.; Montes-Borrego, M.; Roman-Écija, M.; Velasco-Amo, M.P.; Landa, B.B.; et al. Detecting Xylella Fastidiosa in a Machine Learning Framework Using Vcmax and Leaf Biochemistry Quantified with Airborne Hyperspectral Imagery. Remote Sens. Environ. 2022, 282, 113281. [Google Scholar] [CrossRef]
- Dechant, B.; Cuntz, M.; Vohland, M.; Schulz, E.; Doktor, D. Estimation of Photosynthesis Traits from Leaf Reflectance Spectra: Correlation to Nitrogen Content as the Dominant Mechanism. Remote Sens. Environ. 2017, 196, 279–292. [Google Scholar] [CrossRef]
- Lopatin, J.; Kattenborn, T.; Galleguillos, M.; Perez-Quezada, J.F.; Schmidtlein, S. Using Aboveground Vegetation Attributes as Proxies for Mapping Peatland Belowground Carbon Stocks. Remote Sens. Environ. 2019, 231, 111217. [Google Scholar] [CrossRef]
- Watt, M.S.; Buddenbaum, H.; Leonardo, E.M.C.; Estarija, H.J.; Bown, H.E.; Gomez-Gallego, M.; Hartley, R.J.L.; Pearse, G.D.; Massam, P.; Wright, L.; et al. Monitoring Biochemical Limitations to Photosynthesis in N and P-Limited Radiata Pine Using Plant Functional Traits Quantified from Hyperspectral Imagery. Remote Sens. Environ. 2020, 248, 112003. [Google Scholar] [CrossRef]
- Hu, L.; Bentler, P.M. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria versus New Alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Warren, C.R.; Dreyer, E.; Adams, M.A. Photosynthesis-Rubisco Relationships in Foliage of Pinus Sylvestris in Response to Nitrogen Supply and the Proposed Role of Rubisco and Amino Acids as Nitrogen Stores. Trees 2003, 17, 359–366. [Google Scholar] [CrossRef]
- Nurmanov, Y.T.; Chernenok, V.G.; Kuzdanova, R.S. Potato in Response to Nitrogen Nutrition Regime and Nitrogen Fertilization. Field Crops Res. 2019, 231, 115–121. [Google Scholar] [CrossRef]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Fahnenstich, H.; Sonnewald, U. Maize Source Leaf Adaptation to Nitrogen Deficiency Affects Not Only Nitrogen and Carbon Metabolism But Also Control of Phosphate Homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, M.; Shi, M.; Kang, Y.; Zhang, R.; Wang, Y.; Zhang, W.; Qin, S. Effects of Potassium Fertilizer Base/Topdressing Ratio on Dry Matter Quality, Photosynthetic Fluorescence Characteristics and Carbon and Nitrogen Metabolism of Potato. Potato Res. 2024, 68, 835–853. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, K.; Li, S.; Zhou, Y.; Ran, S.; Xu, R.; Lin, Y.; Shen, L.; Huang, W.; Zhong, F. Carbon and Nitrogen Metabolism in Tomato (Solanum Lycopersicum L.) Leaves Response to Nitrogen Treatment. Plant Growth Regul 2023, 100, 747–756. [Google Scholar] [CrossRef]
- Loreti, E.; Van Veen, H.; Perata, P. Plant Responses to Flooding Stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Burritt, D.J.; Tran, L.-S.P. Plant Responses to Low-Oxygen Stress: Interplay between ROS and NO Signaling Pathways. Environ. Exp. Bot. 2019, 161, 134–142. [Google Scholar] [CrossRef]
- Mommer, L.; Visser, E.J.W. Underwater Photosynthesis in Flooded Terrestrial Plants: A Matter of Leaf Plasticity. Ann. Bot. 2005, 96, 581–589. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, Z.; Tong, R.; Hu, X.; Du, K. Anatomy and Ultrastructure Adaptations to Soil Flooding of Two Full-Sib Poplar Clones Differing in Flood-Tolerance. Flora 2017, 233, 90–98. [Google Scholar] [CrossRef]
- Sharkey, T.D. The End Game(s) of Photosynthetic Carbon Metabolism. Plant Physiol. 2024, 195, 67–78. [Google Scholar] [CrossRef]
- Li, D.; Miao, Y.; Gupta, S.K.; Rosen, C.J.; Yuan, F.; Wang, C.; Wang, L.; Huang, Y. Improving Potato Yield Prediction by Combining Cultivar Information and UAV Remote Sensing Data Using Machine Learning. Remote Sens. 2021, 13, 3322. [Google Scholar] [CrossRef]
- El-Kenawy, E.-S.M.; Alhussan, A.A.; Khodadadi, N.; Mirjalili, S.; Eid, M.M. Predicting Potato Crop Yield with Machine Learning and Deep Learning for Sustainable Agriculture. Potato Res. 2024, 68, 759–792. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, H.; Fan, Y.; Yue, J.; Yang, F.; Fan, J.; Ma, Y.; Chen, R.; Bian, M.; Yang, G. Utilizing UAV-Based Hyperspectral Remote Sensing Combined with Various Agronomic Traits to Monitor Potato Growth and Estimate Yield. Comput. Electron. Agric. 2025, 231, 109984. [Google Scholar] [CrossRef]
- Lin, Y.; Li, S.; Duan, S.; Ye, Y.; Li, B.; Li, G.; Lyv, D.; Jin, L.; Bian, C.; Liu, J. Methodological Evolution of Potato Yield Prediction: A Comprehensive Review. Front. Plant Sci. 2023, 14, 1214006. [Google Scholar] [CrossRef]
- Wei, Q.; Yin, Y.; Tong, Q.; Gong, Z.; Shi, Y. Multi-Omics Analysis of Excessive Nitrogen Fertilizer Application: Assessing Environmental Damage and Solutions in Potato Farming. Ecotoxicol. Environ. Saf. 2024, 284, 116916. [Google Scholar] [CrossRef]
- Cooper, M.; Voss-Fels, K.P.; Messina, C.D.; Tang, T.; Hammer, G.L. Tackling G × E × M Interactions to Close On-Farm Yield-Gaps: Creating Novel Pathways for Crop Improvement by Predicting Contributions of Genetics and Management to Crop Productivity. Theor. Appl. Genet. 2021, 134, 1625–1644. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.; Yan, L.; Yan, K.; Zeng, Y.; Yang, B. Improving the Estimation of Canopy Structure Using Spectral Invariants: Theoretical Basis and Validation. Remote Sens. Environ. 2023, 284, 113368. [Google Scholar] [CrossRef]
- Yang, P. Exploring the Interrelated Effects of Soil Background, Canopy Structure and Sun-Observer Geometry on Canopy Photochemical Reflectance Index. Remote Sens. Environ. 2022, 279, 113133. [Google Scholar] [CrossRef]

| (A) Aboveground Foliar Biochemistries | ||||||
| Parameters | Abbr. | Unit | Min | Max | Mean | Std. |
| Chlorophyll-a and -b | Chlorophyllab | mg·cm−2 | 14.28 | 94.28 | 35.64 | 9.15 |
| Equivalent water thickness | EWT | g·m−2 | 233.05 | 474.59 | 304.49 | 48.95 |
| Leaf mass per area | LMA | g·m−2 | 17.44 | 76.31 | 36.07 | 9.81 |
| Nitrogen content | Nitrogenleaf | g·kg−1 | 28.26 | 52.52 | 41.98 | 6.24 |
| (B) Belowground Tuber Yield Traits | ||||||
| Parameters | Abbr. | Unit | Min | Max | Mean | Std. |
| Total yield per plant | Yield | g | 66.60 | 3224.50 | 871.45 | 849.41 |
| Fresh weight per tuber | FWtuber | g | 9.51 | 347.00 | 91.09 | 79.21 |
| Dry weight per tuber | DWtuber | g | 1.13 | 84.55 | 17.08 | 18.27 |
| Number of tubers | Count | - | 2 | 19 | 9.70 | 2.90 |
| Water content | Watertuber | % | 71.14 | 90.50 | 83.87 | 3.57 |
| Dry matter content | DMtuber | % | 9.50 | 28.86 | 16.13 | 3.57 |
| Starch content | Starchtuber | g·kg−1 | 29.11 | 199.09 | 105.57 | 32.75 |
| Protein content | Proteintuber | g·kg−1 | 49.23 | 388.41 | 184.51 | 132.87 |
| Models | Parameters | Optimization Range/Setting |
|---|---|---|
| PLSR | Latent variables | 1, 2, …, 30 |
| SVR | Kernel function | RBF |
| Regularization parameter | 2−15, 2−14, …, 216 | |
| Kernel coefficient | 2−5, 2−4, …, 26 | |
| Penalty coefficient | 10−5, 10−4, …,104 | |
| RFR | Number of trees | 100 |
| Number of regression features | 1, 6, 11, …, one third of the number of spectral bands | |
| Maximum tree depth | 10, 20, …, 90 |
| Indices | Criteria for Good Fitting | Value |
|---|---|---|
| RMSEA | <0.05 | 0.041 |
| χ2/df | <3 | 1.245 |
| TLI | >0.9 | 0.996 |
| CFI | >0.9 | 0.999 |
| NFI | >0.9 | 0.993 |
| IFI | >0.9 | 0.999 |
| AIC | - | 613.276 |
| BIC | - | 747.229 |
| Model | Metrics | Fresh Weight | Dry weight | Yield | Count | Dry Matter | Protein | Starch | Water |
|---|---|---|---|---|---|---|---|---|---|
| PLSR | R2 | 0.63 | 0.69 | 0.58 | 0.02 | 0.15 | 0.84 | 0.01 | 0.26 |
| RMSE | 53.26 | 9.88 | 591.43 | 2.87 | 3.49 | 51.34 | 37.61 | 3.39 | |
| NRMSE | 48.30 | 48.36 | 58.09 | 31.57 | 22.20 | 30.85 | 35.50 | 4.08 | |
| SVR | R2 | 0.60 | 0.70 | 0.59 | 0.03 | 0.12 | 0.69 | 0.00 | 0.19 |
| RMSE | 56.38 | 9.66 | 588.13 | 2.73 | 3.56 | 68.62 | 33.17 | 3.51 | |
| NRMSE | 51.12 | 45.47 | 57.76 | 30.03 | 22.58 | 41.37 | 31.31 | 4.22 | |
| RFR | R2 | 0.63 | 0.71 | 0.69 | 0.21 | 0.11 | 0.65 | 0.03 | 0.26 |
| RMSE | 52.40 | 9.34 | 507.85 | 2.50 | 3.36 | 73.12 | 36.94 | 3.40 | |
| NRMSE | 47.52 | 45.70 | 49.88 | 27.56 | 21.37 | 44.75 | 34.87 | 4.09 |
| Parameters | PLSR | SVR | RFR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | NRMSE | R2 | RMSE | NRMSE | R2 | RMSE | NRMSE | |
| Chlorophyll | 0.30 | 6.99 | 17.00 | 0.02 | 8.30 | 20.00 | 0.29 | 7.00 | 17.00 |
| Nitrogen | 0.76 | 3.15 | 14.00 | 0.05 | 6.42 | 28.00 | 0.76 | 3.34 | 14.00 |
| EWT | 0.78 | 26.55 | 13.00 | 0.08 | 53.08 | 26.00 | 0.75 | 29.10 | 14.00 |
| LMA | 0.47 | 6.98 | 19.00 | 0.01 | 9.62 | 26.00 | 0.46 | 7.24 | 19.00 |
| Model | Metrics | Fresh Weight | Dry Weight | Dry Matter | Yield | Protein | Starch | Water |
|---|---|---|---|---|---|---|---|---|
| PLSR + SEM | R2 | 0.36 | 0.41 | 0.29 | 0.43 | 0.19 | 0.07 | 0.29 |
| RMSE | 33.75 | 10.52 | 17.55 | 494.04 | 35.33 | 20.36 | 38.80 | |
| NRMSE | 10.00 | 14.00 | 119.00 | 33.00 | 11.00 | 16.00 | 263.00 | |
| RFR + SEM | R2 | 0.31 | 0.29 | 0.35 | 0.45 | 0.16 | 0.04 | 0.35 |
| RMSE | 28.37 | 6.08 | 7.85 | 538.21 | 22.82 | 100.61 | 36.03 | |
| NRMSE | 9.00 | 8.00 | 53.00 | 23.00 | 7.00 | 77.00 | 244.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Huang, Y.; Tan, W.; Deng, Y.; Yang, C.; Zhu, X.; Shen, J.; Liu, N. Investigating the Mechanisms of Hyperspectral Remote Sensing for Belowground Yield Traits in Potato Plants. Remote Sens. 2025, 17, 2097. https://doi.org/10.3390/rs17122097
Chen W, Huang Y, Tan W, Deng Y, Yang C, Zhu X, Shen J, Liu N. Investigating the Mechanisms of Hyperspectral Remote Sensing for Belowground Yield Traits in Potato Plants. Remote Sensing. 2025; 17(12):2097. https://doi.org/10.3390/rs17122097
Chicago/Turabian StyleChen, Wenqian, Yurong Huang, Wei Tan, Yujia Deng, Cuihong Yang, Xiguang Zhu, Jian Shen, and Nanfeng Liu. 2025. "Investigating the Mechanisms of Hyperspectral Remote Sensing for Belowground Yield Traits in Potato Plants" Remote Sensing 17, no. 12: 2097. https://doi.org/10.3390/rs17122097
APA StyleChen, W., Huang, Y., Tan, W., Deng, Y., Yang, C., Zhu, X., Shen, J., & Liu, N. (2025). Investigating the Mechanisms of Hyperspectral Remote Sensing for Belowground Yield Traits in Potato Plants. Remote Sensing, 17(12), 2097. https://doi.org/10.3390/rs17122097








